Abstract
Background
The current gold standard for diagnostic classification of many solid-tissue neoplasms is immunohistochemistry (IHC) performed on formalin-fixed, paraffin-embedded (FFPE) tissue. Although IHC is commonly used, there remain important issues related to preanalytic variability, nonstandard methods, and operator bias that may contribute to clinically significant error. To increase the quantitative accuracy and reliability of FFPE tissue-based diagnosis, we sought to develop a clinical proteomic method to characterize protein expression in pathologic tissue samples rapidly and quantitatively.
Methods
We subclassified FFPE tissue from 136 clinical pituitary adenoma samples according to hormone translation with IHC and then extracted tissue proteins and quantified pituitary hormones with multiplex bead-based immunoassays. Hormone concentrations were normalized and compared across diagnostic groups. We developed a quantitative classification scheme for pituitary adenomas on archived samples and validated it on prospectively collected clinical samples.
Results
The most abundant relative hormone concentrations differentiated sensitively and specifically between IHC-classified hormone-expressing adenoma types, correctly predicting IHC-positive diagnoses in 85% of cases overall, with discrepancies found only in cases of clinically nonfunctioning adenomas. Several adenomas with clinically relevant hormone-expressing phenotypes were identified with this assay yet called “null” by IHC, suggesting that multiplex immunoassays may be more sensitive than IHC for detecting clinically meaningful protein expression.
Conclusions
Multiplex immunoassays performed on FFPE tissue extracts can provide diagnostically relevant information and may exceed the performance of IHC in classifying some pituitary neoplasms. This technique is simple, largely amenable to automation, and likely applicable to other diagnostic problems in molecular pathology.
INTRODUCTION
Immunohistochemistry (IHC) is used for in situ protein analysis of formalin-fixed, paraffin-embedded (FFPE) tissue in diagnostic pathology. For some diagnostic problems, such as determining the site of origin of a carcinoma presenting as a metastasis from an unknown primary, IHC is the current diagnostic gold standard (1). In studying pathologic entities where subcellular localization of a protein is essential, such as nuclear localization of -catenin in some soft-tissue neoplasms (2), disruptive proteomic techniques that obliterate cytoarchitecture are not useful, and IHC is mandatory.
Preanalytical variability in IHC, largely stemming from the processes used for preparing FFPE tissue or “retrieving” antigens after FFPE treatment, can be an important source of false-negative and false-positive results (3). Use of different antibody clones to identify the same target in different clinical laboratories can also cause substantial laboratory-to-laboratory variation and has been responsible for highly publicized and potentially life-threatening tumor misclassification (4). Absolute quantification of IHC signals may reduce this variability, but quantitative IHC requires automated image analysis systems (5) that can outperform the human eye in measuring subtle staining differences. In addition, a quantitative IHC study performed on pituitary tissue (6) demonstrated that accurate quantification was possible only under rigorously controlled conditions that are not normally maintained in the clinical IHC laboratory, with even small variations in reagent concentrations leading to gross misrepresentations of apparent target quantities.
To address these shortcomings, we have been investigating the use of multiplex immunoassays on tissue homogenates as an alternative to IHC. There are several diagnostic contexts in which the simple quantity of an analyte in bulk tissue, even when disrupted, is known to be diagnostically relevant. Historically, charcoal-binding assays for estrogen receptor status in breast carcinoma were known to be accurate predictors of hormone sensitivity (7), and a newer multiplex, bead-based immunoassay has recently been shown to aid in molecularly subtyping breast carcinoma biopsy specimens (8), although this assay requires fresh tissue, not the fixed tissue that is usually obtained in the pathology laboratory. Another application of this methodology is a demonstration that T-cell counts can be analyzed quantitatively by immunoassays for surface proteins in dried blood spot material (9), indicating that single discrete cells need not be counted or even identified to arrive at reliable estimates of quantities of cells. Additionally, we have demonstrated that multiplex immunoassays of muscle tissue homogenates or cytokines could aid in diagnosing inflammatory myopathies (10).
In this study, we focused on pituitary adenomas (PAs). PAs are among the most common intracranial neoplasms in humans, believed to be present in 15%–20% of humans based on autopsy and radiologic studies (11), although symptomatic PAs are much less prevalent. PA tissue diagnosis is currently accomplished via microscopic examination of histochemically stained tissue sections, and PAs are then subclassified by IHC to determine if they produce a specific hormone. Subclassification based on hormone expression is sometimes predicted by preoperative serum hormone assays, but it is confirmed by pathology to determine both prognosis and the need for subsequent therapy (12). Because the subcellular localization of hormones is not generally important in making subtype classification, we hypothesized that IHC may not be necessary for making this subclassification, and that the relative abundance of any hormone compared to all other possible pituitary hormones in a homogenized tumor tissue sample would accurately predict the tumor subtype.
METHODS
All studies on human tissue were performed with approval of the local University of Washington Institutional Review Board. Tissue protein extraction was performed with the qProteome kit (Qiagen), and analytes [adrenocorticotropic hormone (ACTH), follicle stimulating hormone (FSH), growth hormone (GH),luteinizing hormone (LH), prolactin (PRL), and thyroid-stimulating hormone (TSH)] were assayed with the human pituitary kit HPT-66K (Millipore) on a Luminex 200 instrument (Millipore). Assay characteristics of the HPT-66K kit are reported by the manufacturer (http://www.millipore.com). Total extract protein concentration was measured with a commercial bicinchoninic acid (BCA) assay (Thermo Fisher). All buffer components not included in kits were purchased from Sigma-Aldrich.
We searched the institutional pathology database for all instances where the site of resection was indicated as “pituitary.” We reviewed pathologic diagnoses for these cases, along with relevant clinical information, and retrieved FFPE tissue blocks from all available cases. Per institutional guidelines, to include a tissue block in the study, an attending pathologist verified that three 10-micron sections (the amount of tissue called for in the qProteome kit instructions) could be harvested without exhausting diagnostically relevant tissue from the block. Samples with adequate tissue for the study are summarized in Table 1. To develop an approach to interpreting the data from this analysis, we analyzed 85 samples from 2000–2008 together to generate a training data set from which an analytical classifier was developed. An additional 51 samples were thereafter collected prospectively between 2008 and 2010 as a validation data set by creating an extra, unstained section of each pituitary adenoma case that came through the neuropathology laboratory. The training and validation sample sets were analyzed separately, more than a year apart. No samples from suspected or proven TSH-expressing PAs were included in this study because none of these extremely rare neoplasms were present in the institutional archive.
Table 1.
IHC-determined hormone expression of the pituitary adenoma cases included in this study.
| Number | % | |
|---|---|---|
| Training set | ||
| ACTH | 17 | 20 |
| GH | 9 | 11 |
| LH | 6 | 7 |
| LH plus FSH | 1 | 1 |
| PRL | 7 | 8 |
| Null | 45 | 53 |
| Validation set | ||
| ACTH | 5 | 10 |
| GH | 3 | 6 |
| GH plus PRL | 3 | 6 |
| LH | 4 | 8 |
| LH plus FSH | 3 | 6 |
| PRL | 1 | 2 |
| Null | 32 | 63 |
We performed IHC on 4-micron tissue sections with standard methods, using the following antibody clones and dilutions: ACTH (Dako 02A3, 2000:1), FSH (Dako C10, 1500:1), LH (Dako C93, 1500:1), GH (Sigma GH-C2, 1000:1), TSH (Dako 0042, 2000:1),PRL (Biogenex BGX031A, 50:1), and FSH/LH (Chemicon AB928 polyclonal, 14 000:1). The FSH/LH polyclonal antibody was used as an initial screening antibody, and any positive staining was followed by separate IHC stains for FSH and LH with the respective monoclonal antibodies. Antigen retrieval consisted of15-min microwave pretreatment in 20 mmol/L citrate buffer, pH 6.0, for all antibodies except anti-ACTH, for which no pretreatment was used. A neuropathologist made the diagnosis and reviewed IHC.
For quantitative analysis, 3 10-micron FFPE tissue sections on glass slides were rehydrated per the qProteome protocol. Tissue was scraped from slides with a razor blade into 1.5-mL microcentrifuge tubes and processed according to the qProteome protocol, which involves heating and agitation in a proprietary buffer containing Tris and sodium dodecyl sulfate. We made dilutions (500:1) of this extract solution in pH 7.4 phosphate buffered saline and analyzed 25 µL of this solution with the Millipore human pituitary kit on the Luminex instrument following the manufacturer’s recommended protocol. The individual performing this quantitative analysis was blinded to the IHC classification of each case.
Because 3 of the hormones present in pituitaries (FSH, LH, TSH) are normally quantified with functional rather than mass units, normalization factors were required to allow hormone-hormone comparisons. The standard practice used in multivariate analysis of scaling each hormone concentration to its population variance was not possible because the distributions of hormone concentrations in adenoma samples were highly irregular, and a typical reference interval study was impossible. Further confounding the issue of determining population reference intervals for these tissue proteins is the well-known macro- and microscopic spatial heterogeneity of pituitary hormone expression in normal pituitaries, such that different portions of pituitary tissue would be expected to be enriched in 1 or more hormones (11).
To address the issue of hormone-to-hormone comparison, each hormone concentration was divided by a factor intended to account for differences in scale between different hormone concentrations in samples. Absent guidance about what hormone concentrations to expect in these samples, we chose to divide measured hormone concentrations by the upper ends of the respective immunoassay linear ranges as stated by the manufacturer (12.5 µg/L for ACTH, 100 mIU/mL for FSH, 200 mIU/mL for LH, 100 µIU/mL for TSH, 50 µg/L for GH, and 100 µg/L for PRL). Scaled hormone concentrations were then expressed as fractions of the total hormone amount by dividing each individual scaled hormone concentration by the sum of all scaled hormone concentrations from each sample. The scaled concentrations were also summed and divided by the total extract protein concentration to arrive at an index of total hormone concentration, expressed as summed scaled concentration per mg of tissue protein. These total hormone indices were expressed and compared as their logarithms.
Confirmatory immunoblot analysis was performed on the FFPE protein extracts using standard methodology, with the same antibodies used for IHC analysis. Results are shown in Supplemental Figure 1.
Statistical analysis, including ROC and principal component analysis (13), was accomplished using the R statistics package (http://www.r-project.org). We compared values with Student’s t-test when normally or log-normally distributed and used χ2 testing to compare the numbers of adenoma subtypes between groups.
RESULTS
Assay Characteristics
Performance characteristics of the commercial immunoassay kit used here are described by the manufacturer (http://www.millipore.com/catalogue/item/HPT-66K#). Comparisons between FFPE and frozen materials were not performed with the commercial immunoassay kit, but additional studies confirming linearity and precision were performed with the tissue homogenates used in this method. Linearity, determined by assaying mixtures of homogenized samples containing different concentrations of hormones to minimize potential matrix effects, is shown in Figure 1 and was acceptable, with r2 values > 0.98. Precision was determined by assaying replicates of multiple samples over multiple days. Within-run and between-run CVs for raw concentrations of all hormones except FSH ranged from 5% to 16% measured on these samples, similar to the manufacturer’s stated performance for other sample types (see Supplemental Table 1). Assay imprecision expressed in terms of fractional hormone concentrations is demonstrated in Supplemental Table 2 and demonstrates that larger imprecision at low absolute hormone concentrations (i.e., for FSH) has no effect on the imprecision of the highest relative hormone concentrations that are used for classification.
Figure. 1.
Assay linearity assessed by performing reciprocal dilutions on duplicate specimens containing high or low concentrations of each analyte. Slope, intercept, and r2 are given in the associated table.
Training Set Hormone Quantification
Relative scaled pituitary hormone concentrations from FFPE adenoma samples are shown in Supplemental Table 3, which gives details on both training set and validation set quantifications. ROC analysis of the assay’s ability to distinguish between adenomas with positive IHC classifications is shown in Figure 2. For the samples in the training data set with positive IHC classifications, the single hormone with the highest proportional scaled concentration from the quantitative assay correctly predicted the IHC classification in 88% of cases (Figure 3). It is important to note that discrepant cases were not evenly distributed across adenoma subtypes. Rather, IHC-quantitative assay discrepancies were all either IHC-defined ACTH-expressing adenomas (4 of 17 incorrectly identified) or an LH-expressing adenoma (1 of 6 incorrectly identified), and all other samples were concordant (9 of 9 expressing GH, 1 of 1 expressing FSH, and 7 of 7 expressing PRL). All discrepant cases were classified as nonfunctioning clinically.
Figure 2.
ROC Analysis comparing the ability of the multiplex pituitary hormone quantification to predict IHC diagnoses. Solid line, training set; dashed line, validation set. AUC values are given for each analyte, with the first number the AUC for the training set, and the second number the AUC for the validation set. In each panel, samples with IHC-positive diagnoses for each hormone are counted as true positives, and all other samples (IHC-positive for another hormone or IHC-null) are counted as true negatives.
Figure 3.
Performance of the quantitative assay on pituitary adenoma samples with different IHC diagnoses. Each column represents samples with the same IHC diagnosis, and the colored segments of the bar correspond to the number of samples that contained the highest fractional concentration of the indicated hormone.
The most frequent IHC classification in the training set was “null,” meaning that IHC failed to identify any specific hormone expression in 53% of adenomas in the training cases. This fraction of null adenomas is larger than the 25%–30% fraction reported in the literature (14), a discrepancy perhaps accounted for by the fact that null adenomas are more likely to be referred for surgical resection, and hence to be included in this sample set, than adenomas like prolactinomas that are amenable to medical treatment. The total hormone concentration measured with this quantitative assay in IHC-positive samples was higher than that of IHC-null adenomas [log(total hormone concentration) = 1.0 (1.0) vs −1.6 (0.9), P = 0.002]. Often in these IHC-null cases, single scaled hormone concentrations made up more than half of the total hormone expression: 7 of 45 expressed > 50% ACTH, 9 of 45 GH, 10 of 45 LH, and 2 of 45 PRL. These relative frequencies of hormone overexpression were not significantly different from the distributions of overexpression in IHC-positive samples by χ2 analysis (P = 0.2).
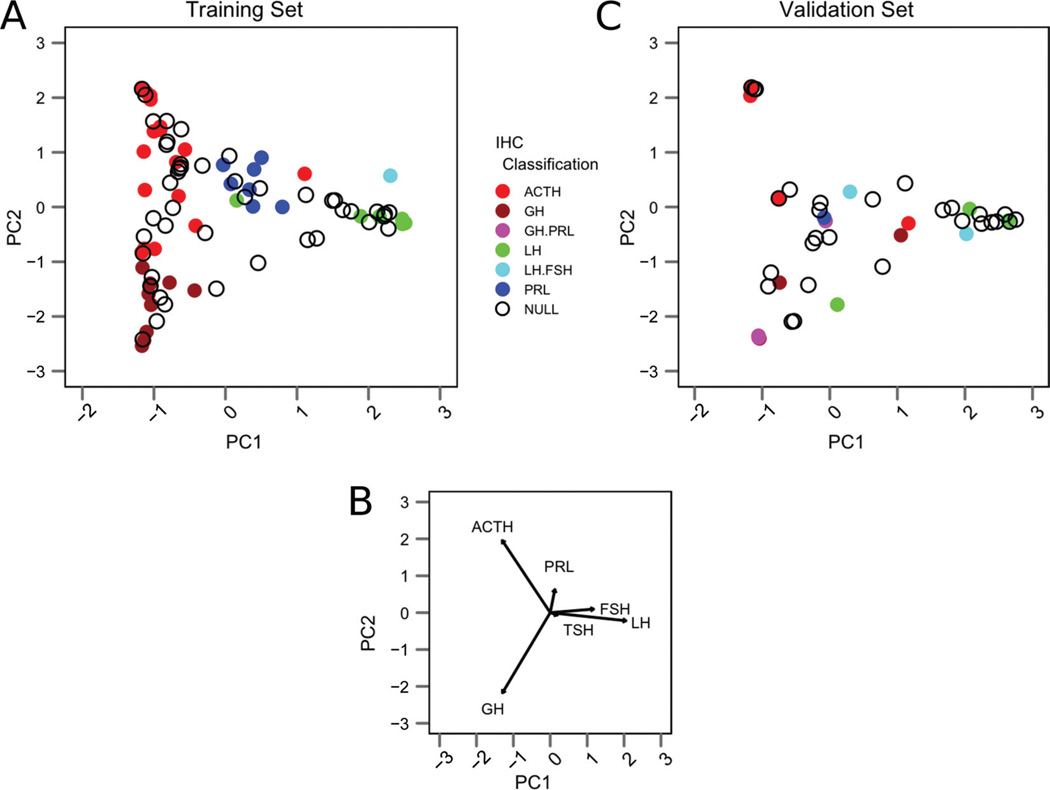
To better visualize the multidimensional results obtained with this analysis, we performed principal component analysis (13) of the hormone concentrations measured in the training set samples. Principal component analysis is a mathematical method that facilitates visualization of the prominent sources of variation in a data set by allowing one to project the results of a multivariate analysis in fewer (ideally 2) dimensions. A plot of the first 2 principal components derived from this analysis is shown in Figure 4A, and the eigenvectors associated with this analysis (Figure 4B) show the directions in which the various adenoma subtypes cluster. This analysis demonstrates that hormone-expressing samples with the same IHC classifications tend to cluster together because their respective hormone concentrations are similar. Additionally, many samples with IHC-null classifications tend to cluster in principal component space near IHC-positive cases, indicating they share similar quantitative hormone concentration patterns.
Figure 4.
(A) The first 2 principal components taken from principal component analysis of the training set data. IHC-positive samples are color-coded, according to the legend, and IHC-null samples are shown as open circles. (B) Biplot showing the eigenvectors associated with each hormone indicates the direction in which IHC-positive samples cluster. (C) Relative hormone concentration data from the validation set projected into the principal component space defined by the training set.
Validation Set Hormone Quantification
We collected 51 pituitary adenomas prospectively for validation. Notably, the distribution of IHC classifications among the validation samples differed quite substantially from the training samples (see Table 1), and included several samples with IHC-demonstrated coexpression of 2 hormones (either FSH plus LH or GH plus PRL).
ROC analysis of the validation samples is superimposed in Figure 2, demonstrating that the multiplex immunoassay strategy is quite effective at differentiating IHC-positive diagnoses from each other and from IHC-null adenomas. For samples in the validation set with positive IHC diagnoses, the single hormone with the highest proportional scaled concentration from the quantitative assay correctly predicted the IHC-defined hormone (or 1 of the 2 IHC-defined hormones) in 79% of cases (Figure 3). Discrepant cases included 1 of 5 IHC-defined ACTH-expressing adenomas, 1 of 3 IHC-defined GH-expressing adenomas, 1 of 4 IHC-defined LH-expressing adenomas, and 1 of 3 IHC-defined LH/FSH-coexpressing adenomas. All discrepant cases were classified as nonfunctioning clinically.
As in the training set, the most frequent IHC classification in the validation set was “null,” occurring in 63% of cases. Again, the total hormone concentration from each sample was higher, on average, in the IHC-positive samples than in IHC-null adenomas [log(total hormone concentration) = −0.7 (1.2) vs −1.3 (0.9), P = 0.02]. Among these IHC-null samples, 3 of 32 expressed > 50% ACTH, 5 of 32 GH, 1 of 32 FSH, 7 of 32 LH, and 3 of 32 PRL. The relative distribution of predominant quantitative hormone expression between the IHC-null cases in the training and validation sets was not statistically significant by χ2 analysis (P = 0.7).
To better visualize these multidimensional results, we projected the relative hormone concentrations from the validation set onto the principal component space of the training set (Figure 4C). In this analysis, the IHC-positive validation samples tended to cluster in areas similar to those of the training set samples, and the IHC-null samples again tended to cluster near IHC-positive samples.
Discrepancies Between IHC And Quantitative Analysis
IHC-ACTH adenomas
All of the IHC-defined ACTH-expressing adenomas in both training and validation sets that did not predominantly express ACTH on quantitative tissue analysis were clinically silent, with no patient having increased serum ACTH or signs and symptoms of Cushing disease.
IHC-GH adenomas
A single IHC-defined GH-expressing adenoma in the validation set (V8), from a patient with a clinically defined nonfunctioning adenoma, did not demonstrate excess GH on quantitative tissue analysis. Of note, in this case, the quantitative GH result of 38% was very close to that of the predominant hormone (PRL at 39%), and IHC identified rare positive PRL-expressing cells.
IHC-PRL adenomas
All of the IHC-defined PRL-expressing adenomas were found to express PRL predominantly on quantitative tissue analysis. In cases with IHC-defined GH and PRL coexpression, GH and PRL were the 2 most abundant hormones found on quantitative tissue analysis.
IHC-LH and/or FSH adenomas
Of the IHC-defined LH-expressing and LH/FSH-coexpressing adenomas that were not found to express 1 or both hormones predominantly on quantitative tissue analysis, all but 1 was clinically silent. This IHC-defined LH/FSH coexpressing adenoma (V17) from the validation set was found to overexpress PRL on quantitative tissue analysis and was associated with a clinical presentation of galactorrhea. The clinical explanation for this finding was the so-called “stalk effect,” which occurs when compression of the pituitary stalk leads to a decrease in the normal dopaminergic inhibition of pituitary PRL release (11). The 1 IHC-defined LH-expressing adenoma without increased LH concentration on quantitative tissue analysis in the validation set (V14) was instead found to have increased GH and was found to be extensively hemorrhagic and necrotic, in keeping with a clinical presentation of pituitary apoplexy.
IHC-null adenomas
The majority of cases in both training and validation sets were classified by IHC as null, meaning that no hormone was identified. Not all IHC-null classifications were made in cases with clinically silent adenomas, however, suggesting that some IHC classifications were false negatives. For example, 2 of the 9 IHC-null adenomas found to express high GH in the training set by quantitative tissue analysis were clinically diagnosed with acromegaly (T44, T45), and 2 more were found to have increased GH on preoperative serum testing (T42, T66). Additionally, 1 IHC-null adenoma (V19) was derived from a clinical case of Cushing disease and found on quantitative tissue analysis to have predominant GH (30.8%) and ACTH (30.5%) expression; another IHC-null adenoma (T63) was noted to have rare ACTH-positive cells by IHC (interpreted as “negative” by IHC in consideration of the clinicopathologic presentation), but found to have prominent ACTH overexpression on quantitative tissue analysis. Another adenoma called IHC-null on the basis of the clinicopathologic presentation (T49) was noted to have rare-positive IHC staining for LH and was found to have predominant LH expression on quantitative tissue analysis. Finally, 1 case of a clinically silent adenoma (T40) was noted to have diffuse, weak ACTH staining by IHC that was interpreted as nonspecific (with a final diagnosis rendered as IHC-null), but was found on quantitative tissue analysis to contain predominantly ACTH.
Only a single (IHC-null) adenoma case studied was found to express a high relative concentration of TSH (T52). In this case, there was no clinical indication of TSH overexpression, and in fact the patient suffered from clinical hypothyroidism and hypogonadism before resection.
DISCUSSION
In this report, we describe a novel approach to tissue proteomic analysis via a multiplex immunoassay system that is already used for quantitative assays in many clinical laboratories. Measuring the concentrations of proteins in homogenized FFPE tissue samples with bead-based sandwich immunoassays could therefore be preferable to other proposed clinical proteomic strategies such as reversed-phase protein microarrays, because the throughput and cost of Luminex assays have already been shown to be compatible with clinical laboratory workflow and resources.
To assess the performance of our analysis, the highest fractional hormone concentrations were compared with adenoma classifications rendered by IHC, which was regarded as a gold standard. However, IHC results were sometimes at odds with patient clinical presentations when quantitative tissue analysis was not. For example, more than half of all cases in this study were classified as IHC-null, yet many of these appeared to express 1 hormone preferentially on quantitative tissue analysis. ROC analysis including these IHC-null cases as true negatives, shown in Figure 2, is therefore confounded because some IHC-null cases were likely not truly negative. A second ROC analysis omitting the IHC-null samples from the true negatives is shown in Supplemental Figure 2, and the higher AUCs found on this analysis may be a better estimate of the method’s true performance. That the total hormone concentrations measured in IHC-positive adenomas were on average 4-fold greater than in IHC-null adenomas further indicates that IHC may be insufficiently sensitive to detect all cases of clinically meaningful hormone expression.
Particular attention was focused on the discrepancies between IHC classification and the quantitative assay. These discrepancies could be due to numerous factors, including preanalytical errors (too little or too much tissue fixation, inappropriate antigen retrieval), analytical errors (suboptimal IHC technique, insufficiently sensitive or specific antibodies, inappropriate antibody titers, operator error by the interpreting pathologist, inappropriate sampling of non-adenoma tissue in the quantitative method, poor performance of the quantitative classification scheme), and non-analytical factors (incorrect or incomplete clinical data in the medical record, ectopic production of hormones). That the quantitative assay was more often able to detect a clearly predominant hormone in adenoma tissue samples is likely due to preanalytical specimen preparation. Tissue pretreatment for IHC used 15 min of heating in a mild citrate buffer intended to unmask epitopes while leaving tissue morphology intact, whereas the lengthy protein extraction conditions used in this assay (20 min at 100 °C and 2 h at 80 °C with 750-rpm agitation in an SDS buffer) are probably more efficient in extracting a greater quantity of immunoreactive proteins. A head-to-head comparison of these protein extraction conditions to test this hypothesis is not possible, unfortunately, because the detection strategies for each method are incompatible with the protein extraction conditions used for the other assay.
For the reasons outlined above, it is unclear how to interpret the classification discrepancies between IHC and the quantitative approach. That 4 cases of clinically GH-expressing adenomas were classified as null by IHC but GH-dominant on quantitative tissue analysis, however, likely indicates that quantitative analysis may outperform IHC in clinical sensitivity in at least a subset of clinical situations. Use of this assay in clinical practice, therefore, might require a rethinking of the optimal approach to classification of pituitary adenomas. For example, IHC-classified PRL-expressing adenomas are sometimes treated with dopamine agonists, but it will require a prospective clinical study to know whether a patient with an IHC-null adenoma but increased PRL on the quantitative tissue assay would benefit from such therapy. Additionally, it will require more study to determine whether this technique can be applied to TSH-expressing pituitary adenomas, which are so rare that no known cases existed in our tissue archive.
The technique described here is relatively simple compared to other proteomic analyses, and the instrumentation used is much less expensive than what is required for mass spectrometry–based proteomics. The sample requirement (several histologic sections), processing time (less than 1 day per run), and sample throughput (up to dozens of samples in a run) are similar to what is required for IHC in a standard clinical laboratory, but many or all steps of this assay could also be automated with existing tissue disruption, liquid handling, and immunoassay platforms. Reagent costs are also significantly lower for this assay compared with IHC because of multiplexing (reagents currently cost approximately $20 per sample for extracting proteins and quantitating hormones), but since there are many other costs associated with clinical assays, including professional fees, a direct comparison of cost vs IHC depends on many factors that vary between institutions. From a quality perspective, because daily quantitative quality control and quantitative inter-laboratory comparisons are possible with this method, this assay could allow more rigorous monitoring of assay performance and increased reliability of hormone classification for pituitary adenomas compared with routine clinical IHC.
Supplementary Material
ABBREVIATIONS
- IHC
immunohistochemistry
- FFPE
formalin-fixed, paraffin-embedded
- PA
pituitary adenoma
- ACTH
adrenocorticotropic hormone
- FSH
follicle-stimulating hormone
- GH
growth hormone
- LH
luteinizing hormone
- PRL
prolactin
- TSH
thyroid-stimulating hormone
Footnotes
AUTHOR CONTRIBUTIONS: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
AUTHORS’ DISCLOSURES OR POTENTIAL CONFLICTS OF INTEREST: No authors declared any potential conflicts of interest.
REFERENCES
- 1.Oien K. Pathologic evaluation of unknown primary cancer. Semin Oncol. 2009;36:8–37. doi: 10.1053/j.seminoncol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Carlson J, Fletcher C. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cell lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509–514. doi: 10.1111/j.1365-2559.2007.02794.x. [DOI] [PubMed] [Google Scholar]
- 3.Gown A. Unmasking the mysteries of antigen or epitope retrieval and formalin fixation. Am J Clin Pathol. 2004;121:172–174. doi: 10.1309/9G5F-Y3U3-QB4R-15DR. [DOI] [PubMed] [Google Scholar]
- 4.Hede K. Breast cancer testing scandal shines spotlight on black box of clinical laboratory testing. J Natl Cancer Inst. 2008;100:836–844. doi: 10.1093/jnci/djn200. [DOI] [PubMed] [Google Scholar]
- 5.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 6.Gross DS, Rothfeld JM. Quantitative immunocytochemistry of hypothalamic and pituitary hormones: validation of an automated, computerized image analysis system. J Histochem Cytochem. 1985;33:11–20. doi: 10.1177/33.1.2578140. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe S. Estrogen and progesterone receptor determinations in breast cancer: technology, biology and clinical significance. Acta Oncol. 1988;27:1–19. doi: 10.3109/02841868809090312. [DOI] [PubMed] [Google Scholar]
- 8.Schneiderhan-Marra N, Sauer G, Kazmaier C, Hsu HY, Koretz K, Deissler H, Joos TO. Multiplexed immunoassays for the analysis of breast cancer biopsies. Anal Bioanal Chem. 2010;397:3329–3338. doi: 10.1007/s00216-010-3873-7. [DOI] [PubMed] [Google Scholar]
- 9.Janik D, Lindau-Shepard B, Comeau A, Pass K. A multiplex immunoassay using the Guthrie specimen to detect T-cell deficiencies including severe combined immunodeficiency disease. Clin Chem. 2010;56:1460–1465. doi: 10.1373/clinchem.2010.144329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird G, Montine T. Multiplex immunoassay analysis of cytokines in idiopathic inflammatory myopathy. Arch Pathol Lab Med. 2008;132:232–238. doi: 10.5858/2008-132-232-MIAOCI. [DOI] [PubMed] [Google Scholar]
- 11.Asa S. Practical pituitary pathology: what does the pathologist need to know? Arch Pathol Lab Med. 2008;132:1231–1240. doi: 10.5858/2008-132-1231-PPPWDT. [DOI] [PubMed] [Google Scholar]
- 12.Vance M. Pituitary adenoma: a clinician’s perspective. Endocr Pract. 2008;14:757–763. doi: 10.4158/EP.14.6.757. [DOI] [PubMed] [Google Scholar]
- 13.Jolliffe IT. Principal component analysis. 2nd ed. New York: Springer; 2002. p. 478. [Google Scholar]
- 14.Carey WD, editor. Cleveland Clinic. Current clinical medicine. 2nd ed. Philadelphia: Saunders/Elsevier; 2010. p. 1354. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.