Abstract
Invasion and dissemination of medulloblastoma (MB) within the central nervous system is the principal factor predicting MB treatment failure and death. Netrin-1 is an axon guidance factor implicated in tumor and vascular biology, including in invasive behaviors. We found that exogenous netrin-1 stimulated invasion of human MB cells and endothelial cells (EC) in contrast to VEGF-A, which promoted invasion of EC but not MB cells. Further, MB cells expressed endogenous netrin-1 along with its receptors, neogenin and UNC5B. Blockades in endogenous netrin-1, neogenin or UNC5B reduced MB invasiveness. Neogenin blockade inhibited netrin-1-induced EC tube formation and recruitment of EC into Matrigel plugs, two hallmarks of angiogenesis. In pediatric MB patients, netrin-1 mRNA levels were increased 1.7-fold in MB tumor specimens compared to control specimens from the same patient. Immunohistochemical analyses showed that netrin-1 was elevated in MB tumors versus cerebellum controls. Notably, urinary levels of netrin-1 were 9-fold higher in MB patients compared to control individuals. Moreover, urinary netrin-1 levels were higher in patients with invasive MB compared to patients with non-invasive MB. Lastly, we noted that urinary netrin-1 levels diminished after MB resection in patients. Our results suggest netrin-1 as a candidate biomarker capable of detecting an invasive, disseminated phenotype in MB patients and predicting their disease status.
Keywords: medulloblastoma, netrin-1, neogenin, urinary biomarker
INTRODUCTION
Medulloblastoma (MB), a malignant embryonal tumor in the cerebellum, is the most common malignant pediatric brain tumor. About 3700 cases of brain embryonal tumors were reported in the United States between 2005 and 2009 (Central Brain Tumor Registry of the United States, CBTRUS) (1). Outcomes have markedly improved, with subgroups of patients achieving near 90% five-year survival rates; however, critical to this success is the ability to achieve a complete or near-complete surgical resection, without tumor invasion or dissemination. For younger children (especially under two years of age) with invasive or disseminated MB, the five-year survival rate can be as low as 32% (2). There is a clear clinical imperative to improve the understanding of the mechanisms underlying invasion and dissemination in MB.
Genomic analysis of MB has identified subgroups that correlate with clinical outcome, better defining tumors prone to invasion and dissemination. MB has been stratified into four subgroups: WNT, Shh (Sonic hedgehog), Group3 and Group4 (3). Shh-MB is the best characterized subgroup. While the Shh group has an overall survival rate that falls in the middle of the four subgroups, a distinct subset of invasive, anaplastic Shh tumors has the worst prognosis of any group, underscoring the impact of invasion on survival. Shh activity is relevant to netrin-1 since neogenin, a netrin receptor, is a Shh target in MB and is required for MB cell cycle progression (4). The expression of another axonal guidance receptor, neuropilin 1 (NRP1), correlates with poor overall survival in MB patients, but receptor blockade inhibits the growth and metastasis of MB (5). Recent studies in our lab have shown that netrin-1 promotes invasiveness and angiogenesis in another brain tumor, glioblastoma, by activating the cysteine protease cathepsin B (CatB) (6).
Netrin-1 is a 60–80kD laminin-like protein, originally demonstrated to serve as an axon guidance molecule during neural development of Caenorhabditis elegans (7). The netrin family consists of three secreted netrins (netrin-1, netrin-3 [also known as netrin-2 chicken-like] and netrin-4). Their activity is mediated by several receptors, including uncoordinated5A-D (UNC5A-D), deleted in colorectal cancer (DCC), its orthologue neogenin and Down syndrome cell adhesion molecule (DSCAM). During brain development, floor plate-secreted netrin-1 diffuses and establishes a gradient to attract growing commissural axons that express netrin receptors to the midline of the central nervous system (7, 8).
Netrin-1 is active outside of the nervous system, contributing to inflammation (9), ischemia in the brain (10) and kidney (11), and tumor progression and angiogenesis (6). Upregulation of netrin-1 mRNA and protein in tumors has been shown in colon cancer (12), neuroblastoma (13) pancreatic cancer (14) and non-small cell lung carcinoma (15). Elevated levels of netrin-1 and UNC5B have been observed in breast cancer patients with distant metastasis (16); however, the functional role of netrin-1 and its receptors in MB are not fully understood.
In this report, we have demonstrated that recombinant netrin-1 stimulates MB cell invasion in four selected human MB cell lines in vitro and that netrin-1 levels are elevated in human MB patient tissues. We analyzed the function of netrin-1 and its receptors, UNC5B and neogenin, in these human MB cell lines. Blockage of endogenous netrin-1 and/or its receptor neogenin by antibody or siRNA results in the suppression of MB cell invasiveness. Netrin-1 is also active in endothelial cells (EC). It stimulates primary mouse brain EC invasion, tube formation in vitro and EC invasion into Matrigel plugs in mice.
These results suggest that netrin-1 might regulate MB invasiveness in the clinic. In fact, pediatric MB tumor specimens demonstrate significant elevation of netrin-1 mRNA and protein relative to normal brain tissue. Urinary analysis is a useful approach to measure soluble protein concentrations non-invasively. We first reported the use of urinary biomarkers, such as MMP-2, MMP-9 and VEGF, in pediatric brain tumors (17). Netrin-1 is a secreted molecule and, thus, might be released into urine. Accordingly, we measured urinary netrin-1 levels to identify MB presence and also response to MB therapy. This unique approach revealed clinical utility, with excellent diagnostic accuracy in differentiating between MB and control patients and with the ability to identify the invasive/metastatic phenotype and effective response to therapeutic interventions. We conclude that the netrin-1/neogenin pathway holds promise as a novel therapeutic target to inhibit MB cell invasiveness and angiogenesis and that measurement of netrin-1 levels demonstrates utility as a non-invasive, diagnostic and prognostic biomarker.
MATERIALS AND METHODS
Cell culture
D425, D458 and D556 human medulloblastoma (MB) cells were supplied by the American Type Culture Collection. D283 cells were kindly provided by Dr. Rakesh Jain (Massachusetts General Hospital, Boston, MA). Cells were cultured in Modified Eagle’s Medium (Gibco) for D283 or DMEM/F12 medium (Gibco) for D425, D458 and D556 and supplemented with 10% FBS. Human umbilical vein endothelial cells (HUVEC) (Lonza) were cultured in EGM2 (Lonza). Mouse brain capillary endothelial cells (EC) were isolated from nude mice as described previously (6). EC were cultured in EGM-2MV (Lonza) supplemented with 10% FBS. The primary cells were used within seven passages.
Recombinant netrin protein
The netrin-1/pcDNA3.1/V5-His-TOPO plasmid was transfected into 293T cells using FuGENE HD Transfection Reagent (Roche Applied Science) to express His- and V5-tagged netrin-1 protein. Netrin-1 secreted into culture medium was purified on HiTrap HP Chelating columns (GE Healthcare Bio-Sciences Corp.) as previously described by us (6, 18). Recombinant chicken netrin-2 (127-N2) and human netrin-4 (1254-N4) were purchased from R&D.
Invasion assays
Invasion assays were performed in Transwells® (Corning Glass) with an 8.0 µm pore size and coated with BD Matrigel™ Basement Membrane Matrix (BD Biosciences) (0.1 mg/mL) as described by us previously (6). Cells that had migrated through the filters after 16 (for MB cell lines) or 24 (for EC) hours at 37°C were stained with the Diff-Quick cell staining kit (Dade Behring Inc.), and four fields were counted by phase microscopy. For netrin-1 blocking experiments, cells were incubated with 20 µg/mL of neutralizing antibody to netrin-1 (R&D Systems).
Patient population
Tissue and urine were collected as part of an institutional review board-approved protocol at Boston Children’s Hospital (BCH). All of the pediatric patients (age 18 years and under; n=16) presented with previously undiagnosed, untreated tumors and had urine collected before surgery. Tumors were evident on magnetic resonance imaging studies at the time of specimen collection. No pediatric patients had known histories of vascular malformations or recent surgery (within three months of specimen collection). All tumor diagnoses were confirmed with neuropathology. Control patients were healthy, age- and sex-matched. Normal cerebellum tissue for immunohistochemistry was purchased from US Biomax, Inc., Gene Tex, Inc., and Abcam.
Urine collection
Once collected, urine was transported on ice to our laboratory, stored at −20°C, then analyzed with ELISA as previously described by us (17).
Tissue collection
Tissue specimens were obtained from the Division of Neuropathology at BCH. Representative tumor tissue was selected and 5 µm-thick sections were prepared from paraffin-embedded tissue.
Immunohistochemistry
All tissues were processed by our core histopathology laboratory with review as part of clinical care and neuropathology review to confirm diagnosis. Netrin-1 expression was evaluated by immunohistochemical analysis using rabbit polyclonal anti-netrin-1 antibody (Novus Biologicals, NBP1-19822). Formalin-fixed, paraffin-embedded tissue sections were mounted on microscope slides. Following the Closed Loop Assay Development (CLAD) protocol (Ventana Medical Systems), antibody was optimized using the OmniMap DAB anti-Rabbit (HRP) detection kit (Ventana Medical Systems). Standard quality control procedures were undertaken to optimize antigen retrieval, primary antibody dilution, secondary antibody detection and other factors for both “signal and noise.”
Statistical analysis
Statistical analysis was performed by our biostatistician, Dr. David Zurakowski. Age and sex differences were evaluated with the Student’s t-test and Fisher’s exact test. Since the urinary netrin-1 measurements do not closely follow a normal distribution (assessed by the Kolmogorov-Smirnov goodness-of-fit test), medians and interquartile ranges (IQR) were used to summarize the tumor patient data, and controls were compared using the nonparametric Mann-Whitney U-test. Diagnostic accuracy was assessed with receiver operating characteristic (ROC) curve analysis, and the Youden Index was employed to identify cut-off values.
RESULTS
Exogenous netrin-1 stimulates MB cell invasiveness and activates MAPK
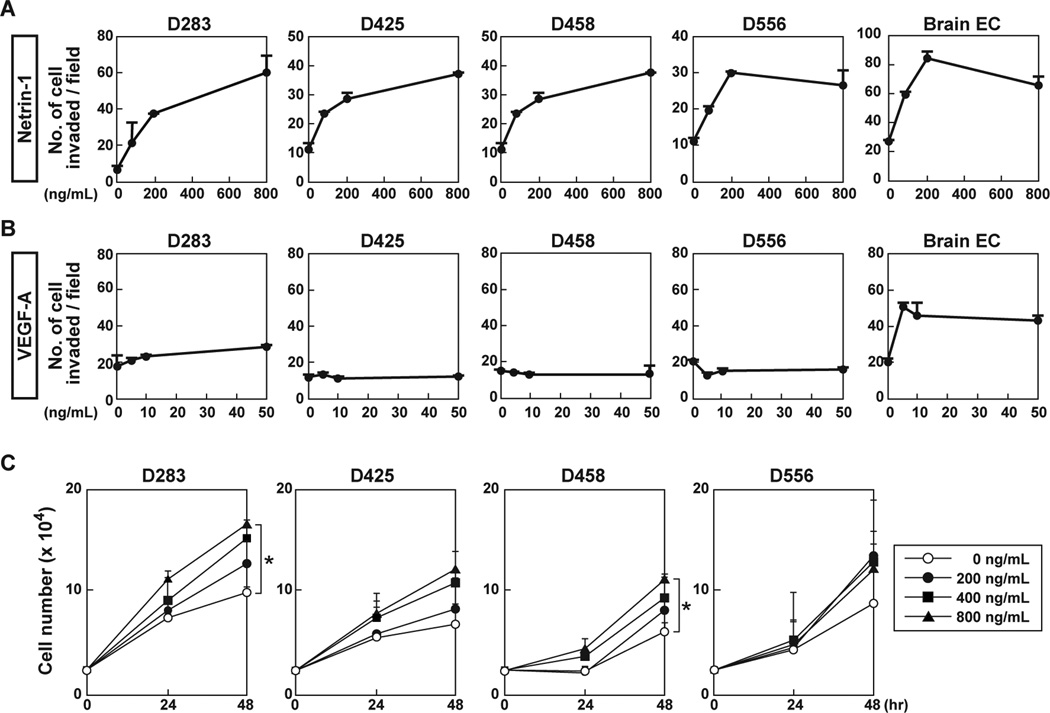
Transwell invasion assays were carried out in four human MB cell lines: D283, D425, D458 and D556. Netrin-1 stimulated their invasiveness in a dose-dependent manner by 16 hours (Fig. 1A). Netrin-1 also stimulated the invasiveness of EC derived from the mouse brain (Fig. 1A). On the other hand, VEGF-A, an angiogenic factor, did not affect these MB cell lines, even at a high concentration (50 ng/mL), yet did stimulate mouse brain EC invasion as a positive control (Fig. 1B). These VEGF-A results could be accounted for by lack of VEGFR2 expression in MB cells (Supplementary Fig. S1A). Thus, netrin-1 might be an advantageous therapeutic target since it stimulates both MB and EC invasiveness, whereas VEGF-A stimulates only EC invasiveness.
Figure 1. Exogenous netrin-1 induces MB cell invasiveness.
A, Four MB cell lines and brain capillary EC were cultured in Matrigel-coated Transwell chambers with indicated netrin-1 concentrations at 16 hours. Invading cells on the membrane were counted in four different fields. B, VEGF-A was analyzed on the same MB cell lines. C, For proliferation, MB cells (2.5 × 104) were cultured in serum reduced medium (0.5% FBS) in the absence or presence of netrin-1. Cell number was counted at 24 and 48 hours. Data represent the mean ± SD (n=3), * p < 0.05.
Netrin-1 stimulated Erk phosphorylation in D458 cells and brain EC in a time-course dependent manner (Supplementary Fig. S1B), consistent with our previous results that netrin-1 induced Erk phosphorylation in glioblastoma cells (6). VEGF-A did not stimulate Erk phosphorylation in MB cells, but did in brain EC (Supplementary Fig. S1C).
MB cell proliferation was analyzed in response to netrin-1 treatment (Fig. 1C). Although invasiveness in response to netrin-1 occurred at 16 hours, exogenous netrin-1 had no effect on MB cell proliferation even at 24 hours, with only minimal increases in proliferation in some MB cell lines at 48 hours. In addition, 200 ng/mL of netrin-1 used in the invasion assays was insufficient to induce MB cell proliferation at any time point (Fig. 1C). Thus it appears, in MB cells, that invasion can be independent of proliferation.
Inhibition of endogenous netrin-1 decreases MB cell invasion
Endogenous netrin-1 secreted into conditioned medium was measured by ELISA (Supplementary Fig. S1D). The netrin-1 protein levels in the four MB cell lines ranged from 20 pg/mL to 110 pg/mL. Antibody specificity was addressed by testing the ELISA with positive and negative controls. We had previously prepared U87MG glioblastoma cells overexpressing netrin-1 (6). As predicted, ELISA detected abundant netrin-1 in conditioned media of netrin-1 transfectants (250 pg/mL), but none at all in the conditioned media of parental U87MG cells (Supplementary Fig. S1D). To further confirm the reliability of the ELISA, all samples were re-tested by an independent laboratory member on different dates with new kits. There was high concordance of netrin-1 levels between samples, supporting the reproducibility of the assay.
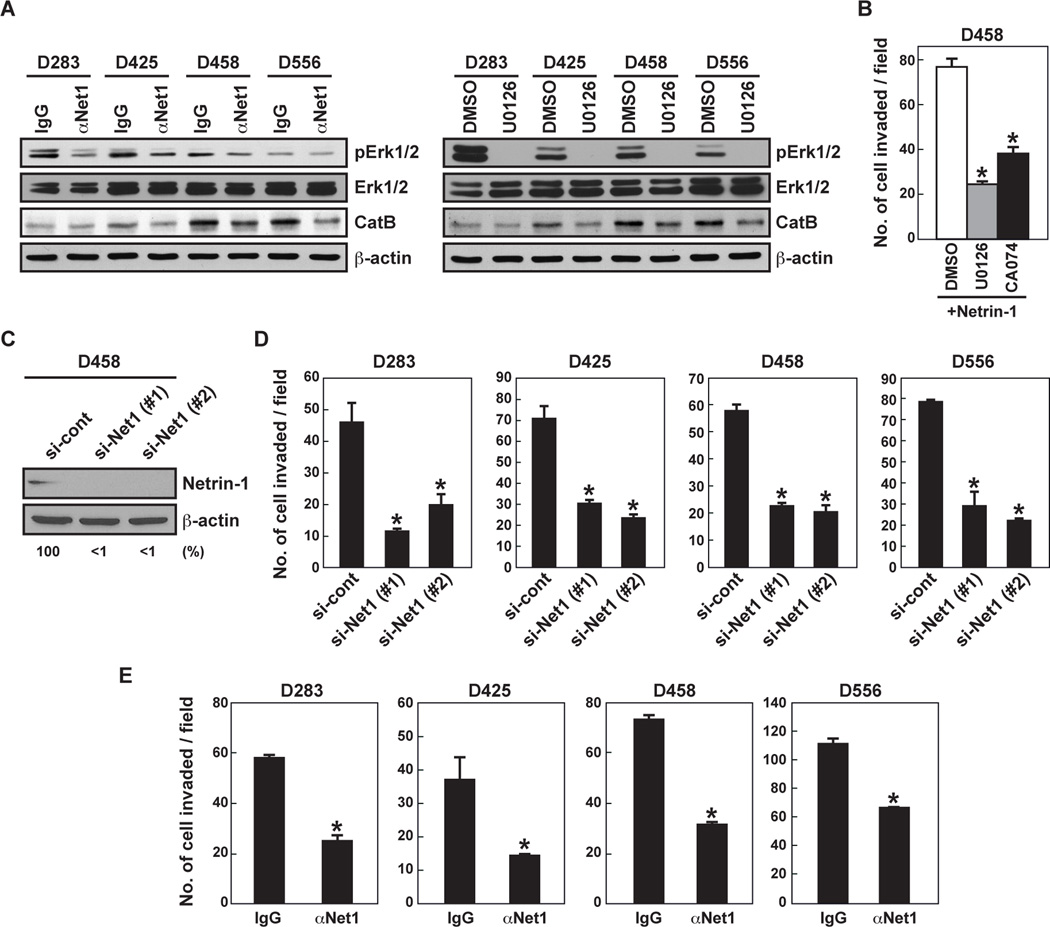
Netrin-1 stimulates glioblastoma cell invasion by activating CatB, a cysteine protease, via the MAPK pathway (6). Netrin-1 neutralizing antibody suppressed Erk phosphorylation in four MB cell lines, as did U0126 treatment (Fig. 2A). CatB protein expression was reduced in three of the MB cell lines but not in D283 (Fig. 2A). MEK inhibitor U0126 and CatB inhibitor CA074 inhibited netrin-1-induced D458 cell invasion by 71% and 53%, respectively (Fig. 2B). When cells were treated with two different siRNA constructs, both reduced netrin-1 protein expression by >90% (Fig. 2C) and MB cell invasion by 56–74% (Fig. 2D). Netrin-1 neutralizing antibody also inhibited MB invasion (D293 by 55%, D425 by 62%, D458 by 56% and D556 by 39%) compared to cells treated with control IgG (Fig. 2E). These inhibition results suggest that netrin-1 secreted by MB cells stimulates MB cell invasiveness by activating CatB via the MAPK pathway.
Figure 2. Inhibition of netrin-1 blocks MB cell invasion and Erk phosphorylation.
A, MB cells were treated with netrin-1 neutralizing antibody (20 µg/mL, for 120 minutes) or U0126 (10 µM, for 30 minutes) prior to cell lysate collection. Cell lysates were analyzed by western blot. B, D458 cells were incubated with MEK inhibitor (U0126, 10 µM) or CatB inhibitor (CA074, 10 µM) and cell invasiveness was assessed with Matrigel-coated Transwells. C, D458 cells were transfected with control or netrin-1-specific siRNA (#1 and #2) (20 nM). After 24 hours, the silencing effect of netrin-1 siRNA on netrin-1 protein levels was analyzed by western blot. The intensity of netrin-1 bands was normalized to their respective β-actin controls. Numbers below gel lanes represent the fold-change in intensity relative to controls. D, MB cells were transfected by netrin-1 siRNA and invasion assay was performed. Data represent the mean ± SD (n=3), * p < 0.05. E, MB cells were incubated with control IgG or netrin-1 neutralizing antibody (20 µg/mL). Cell invasiveness was assessed via Matrigel-coated Transwells. Data represent the mean ± SD (n=3), * p < 0.05.
Netrin-1 increases MB invasiveness through UNC5B and neogenin
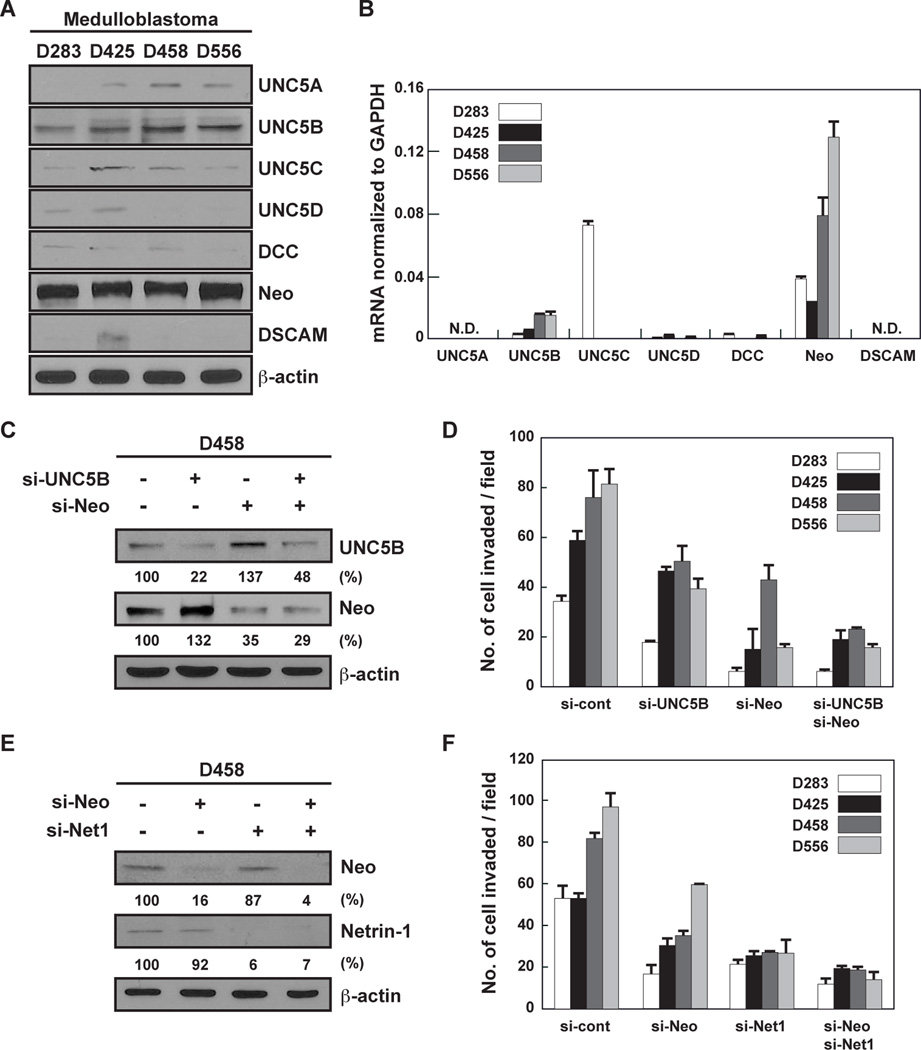
Netrin-1 has multiple receptors (7). Of these receptors, neogenin and UNC5B expression levels appeared to be the highest in all four MB cell lines (Fig. 3A,B). UNC5B and neogenin siRNA reduced protein expression by about 80% or 65%, respectively, in D458 cells (Fig. 3C). Silencing of UNC5B and neogenin blocked MB cell invasion by 38% and 70%, respectively (Fig. 3D). There was no additional effect on MB cell invasion using a combination of UNC5B and neogenin siRNA (Fig. 3D). Since MB cell lines express netrin-1 and neogenin, we knocked down both proteins. Neogenin and netrin-1 siRNA reduced protein expression by about 85% or 95%, respectively, in D458 cells (Fig. 3E). The combination of neogenin and netrin-1 siRNA strongly suppressed MB cell invasion (76%) compared to neogenin (52%) and netrin-1 (63%) knockdown alone (Fig. 3F). These results indicate that the netrin-1/neogenin loop could be a target to repress the invasive activity of MB cells.
Figure 3. MB cell invasiveness is mediated by neogenin and UNC5B.
A,B, The netrin-1 receptor protein (A) and mRNA (B) expression levels were analyzed by western blot and qRT-PCR, respectively. N.D. stands for “not detected.” C,D, D458 cells were transfected with control, UNC5B or neogenin siRNA. Receptor expression was measured by western blot, and MB cell invasiveness was assessed in Matrigel-coated Transwells. E,F, D458 cells were transfected with control, neogenin or netrin-1 siRNA. Receptor expression was measured by western blot, and MB cell invasiveness was assessed in Matrigel-coated Transwells. Data represent the mean ± SD (n=3), * p < 0.05.
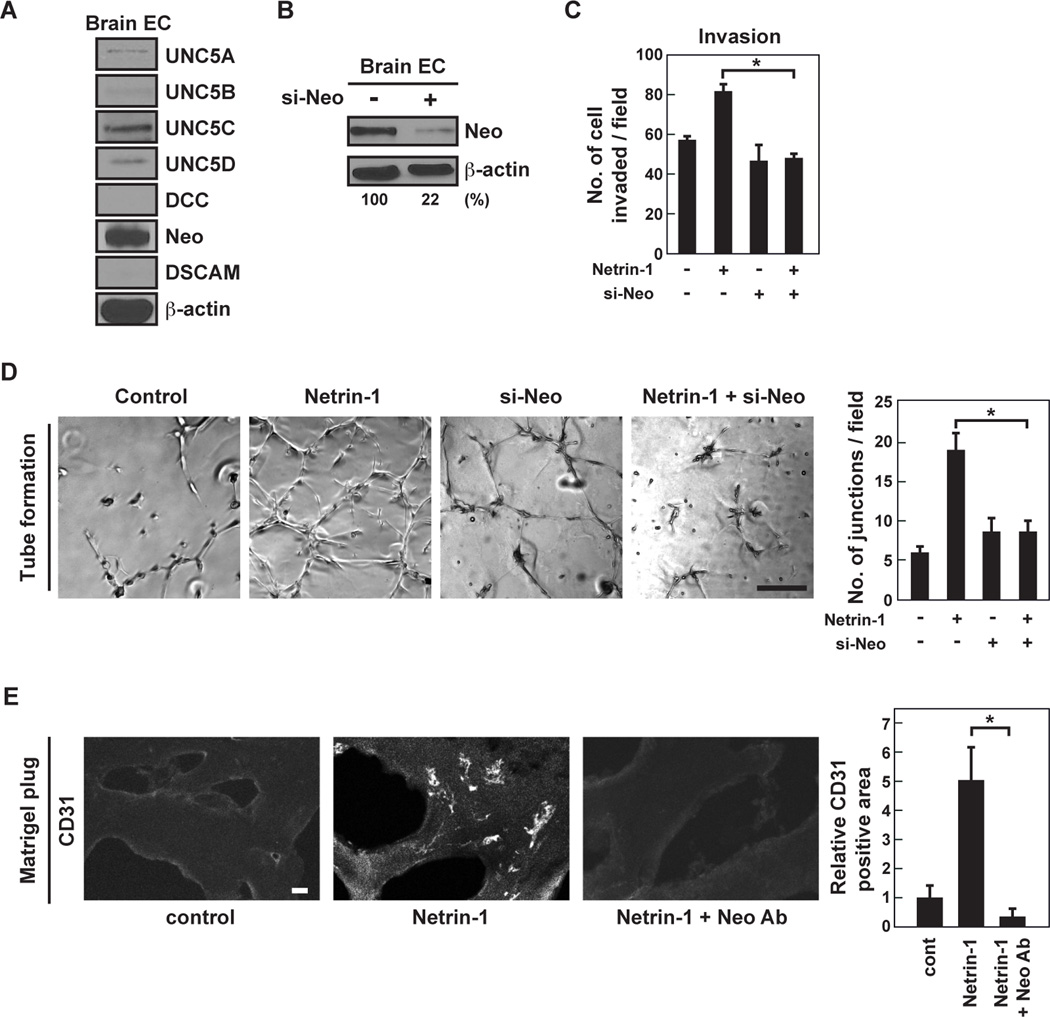
Netrin-1 mediates EC activity through neogenin
Netrin-1 induced mouse brain capillary EC invasion (Fig. 1A) as well as EC sprouting (Supplementary Fig. S2). Neogenin expression was highest among the netrin-1 receptors in brain EC (Fig. 4A). Neogenin siRNA reduced protein by 80% in brain EC (Fig. 4B). Knockdown of neogenin in EC significantly decreased netrin-1-induced EC invasion by 40% (Fig. 4C) and tube formation by 50% (Fig. 4D). Furthermore, netrin-1 induced CD31-positive EC infiltration into Matrigel, which was abrogated by neogenin neutralizing antibody by 90% (Fig. 4E). It appears that netrin-1 has pro-angiogenic properties.
Figure 4. Neogenin regulates netrin-1-mediated EC activation.
A, Netrin-1 receptor protein expression levels were analyzed by western blot. B, Brain EC were transfected with control or neogenin siRNA. Cell lysates were analyzed by western blot. C, Mouse primary EC were transfected with control or neogenin siRNA. EC invasiveness was assessed in Matrigel-coated Transwells in the presence or absence of netrin-1 (200 ng/mL). D, Mouse primary EC were transfected with control or neogenin siRNA, and were seeded on Matrigel-coated well plates in the presence or absence of netrin-1 (200 ng/mL). The number of tube junctions/field was measured by ImageJ software. The scale bar indicates 100 µm. E, Matrigel plugs, either left untreated or mixed with netrin-1 (16 µg/mL) in the presence or absence of neogenin-blocking antibody (300 µg/mL), were implanted into CD1 mice. Matrigel plugs were removed and frozen sections were stained with anti-CD31 antibody. CD31 positive cells were measured using ImageJ software. The scale bar indicates 10 µm. Data represent the mean ± SD (n=3), * p < 0.05.
Netrin-1 is elevated in human MB patients
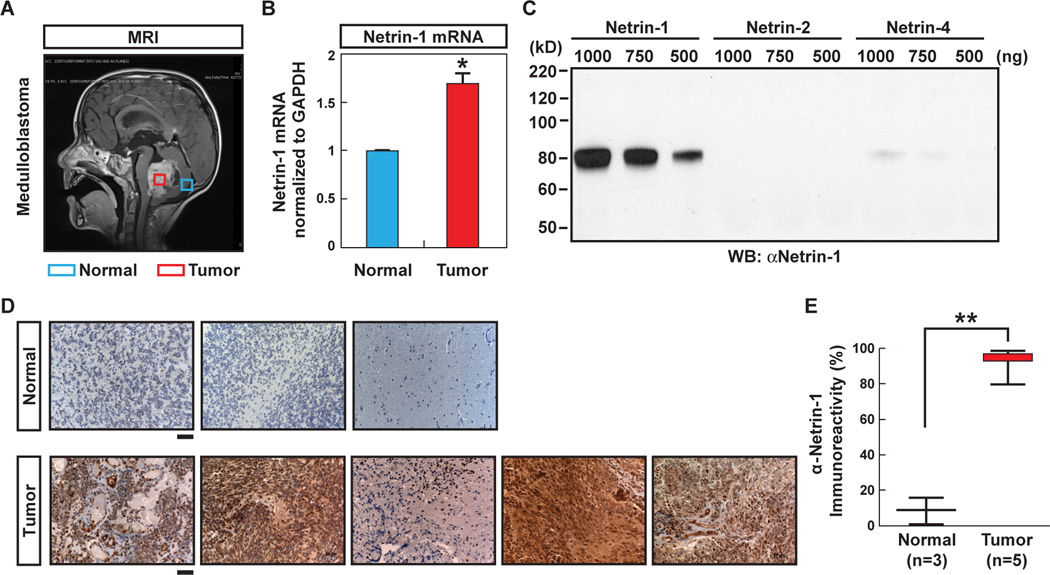
Since netrin-1 might be aberrantly expressed in human MB tissue, we compared netrin-1 mRNA expression levels using pediatric tissue samples. RNA was extracted from the MB tumor of one patient, as indicated by the red box, and normal adjacent cerebellum was obtained from the same patient, as indicated by the blue box (Fig. 5A). qPCR showed that netrin-1 mRNA expression in the MB tumor was 1.7 times higher than in the normal cerebellum tissue (n=1) (Fig. 5B). Furthermore, netrin-1 expression in tumor samples from MB patients (n=5) and normal cerebellum (n=3) were analyzed by immunohistochemistry (IHC). Antibody specificity in IHC was tested by western blot. The antibody detected netrin-1 but not chicken netrin-2 (similar to human netrin-3) or human netrin-4 proteins (Fig. 5C). While IHC staining showed minimal netrin-1 expression in normal cerebellum (n=3), netrin-1 was expressed in the majority of MB cells (n=5) (Fig. 5D). Quantification revealed a marked increase of netrin-1 expression in MB tissue (93% in MB vs. 13% in control, p < 0.0001) (Fig. 5E).
Figure 5. Netrin-1 is elevated in MB.
A, Sagittal T1 MRI with contrast of patient with MB. Boxes indicate area of tissue sampling, with red marking the tumor and blue marking the area of normal cerebellum sampled (as part of planned surgical approach). B, The mRNA levels of netrin-1 expression in normal cerebellum and tumor - both taken from the same patient - were compared. * p < 0.001. C, The specificity of anti-netrin-1 antibody for IHC was tested by western blot. D, Pathologically confirmed specimens of MB, resected as part of routine pediatric clinical care, were prepared as paraffin sections and subjected to IHC with staining for netrin-1. Five representative patient tumors were analyzed, with three controls of non-tumor cerebellum for comparison. The scale bar indicates 50 µm. E, Quantification of the percentage of cells demonstrating the presence of immunoreactivity for netrin-1 (positive staining). Data are shown in box plot format (median, 25–75%), ** p < 0.0001.
Measurement of urinary netrin-1 levels demonstrates clinical utility as a biomarker
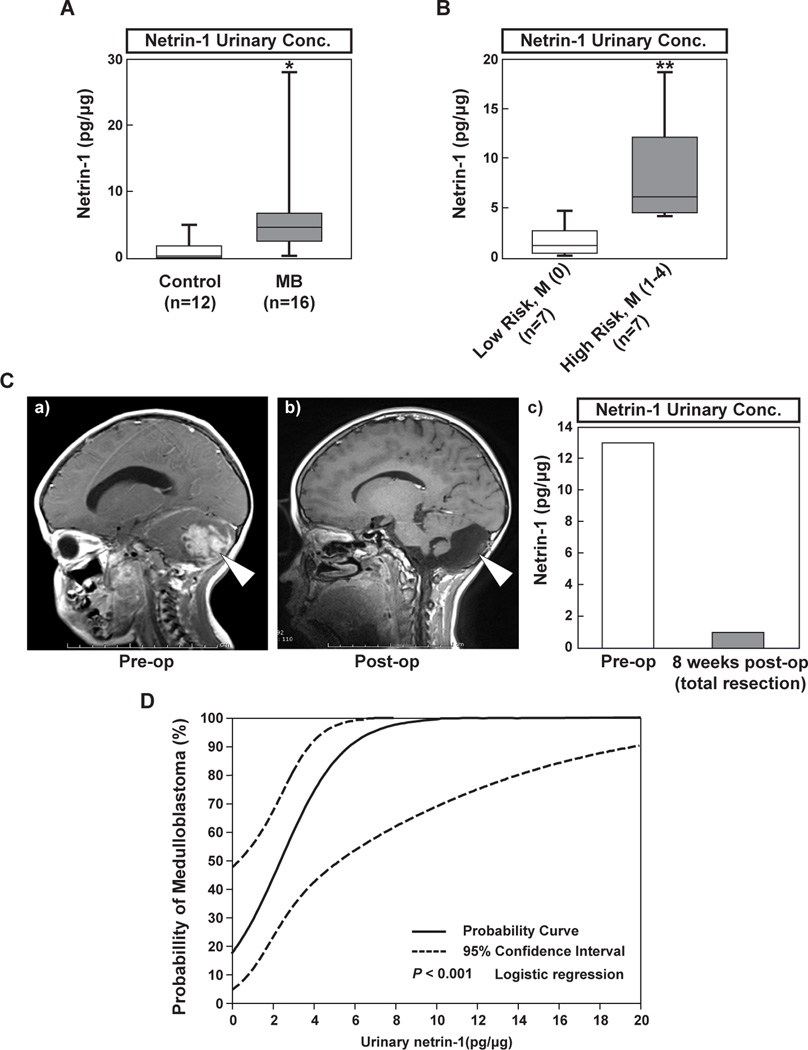
Recent studies from our laboratory demonstrated that urinary biomarkers such as MMP-2, MMP-9 and VEGF predicted brain tumor presence or response to therapy (17). Given that netrin-1 is a secreted protein, we hypothesized that we might detect netrin-1 from patient urinary samples. There were no significant age or sex differences between the 16 MB patients and 12 controls (age, p=0.48 and gender, p=0.92). Median (IQR) urinary netrin-1 levels were 0.5 pg/µg for controls and 4.8 pg/µg for MB patients (p < 0.001), suggesting that urinary netrin-1 levels are elevated in patients with MB (Fig. 6A).
Figure 6. The potential utility of netrin-1 as a urinary biomarker.
A, Urinary netrin-1 levels were quantified by ELISA and compared between children with MB (n=16) and age- and gender-matched healthy controls (n=12). Data are shown in box plot format (median, 25–75%), * p < 0.001. B, When MB patients are classified by non-invasive (n=7) and invasive (n=7) phenotypes, invasive tumors are associated with higher levels of netrin-1 (see Table 1). Data are shown in box plot format (median, 25–75%), ** p = 0.002. C, Pre- and post-operative urinary netrin-1 levels from one patient, with corresponding MRI at times of urine collection (pre-op and 8 weeks post-op). D, Logistic regression analysis is employed to produce a clinically relevant predictive graph, enabling assessment of the risk of tumor presence based on the patients’ urinary levels of netrin-1.
We compared urinary netrin-1 levels between invasive phenotypes (local recurrence and/or dissemination) and non-invasive, non-disseminated tumors. As described in Table 1, the phenotype of the invasive tumor was defined as the presence of either a high-risk patient (age <3 years, subtotal resection with >1.5 cm residual tumor, M+ with leptomeningeal seeding or location outside of posterior fossa) or an M+ positive patient as defined by a Modified Chang Staging (M1-M4, but not M0) (2, 19–22). Patients with invasive tumors (High Risk: 8.3 pg/µg, n=7) had urinary netrin-1 levels approximately five times greater than patients with non-invasive tumors (Low Risk: 1.6 pg/µg, n=7, p=0.002) (Fig. 6B). In a single patient case (shown as proof-of-principle), urinary netrin-1 levels were elevated at presentation (Fig. 6C a) at 13 pg/µg, and then markedly decreased at 8 weeks post-operatively to 0.8 pg/µg (Fig. 6C c), correlated with an MRI showing no evident residual tumor (Fig. 6C b). Taken together, urinary netrin-1 levels correlate with invasive/disseminated phenotype and drop following surgical resection.
Table 1.
Urinary netrin-1 levels in MB patients
| Low Risk, Chang M (0) | High Risk, Chang M (1–4) |
|---|---|
| 2 (6F) | 4.5 (11F) |
| 0.1 (7F) | 4 (6F) |
| 2.4 (10M) | 18.8 (7M) |
| 4.5 (12M) | 5.2 (14M) |
| 1.3 (8M) | 6.2 (1F) |
| 0.5 (11F) | 12 (9F) |
| 0.5 (6F) | 7.2 (18M) |
The phenotype of the invasive tumor was defined as the presence of either a high-risk patient (age <3 years, subtotal resection with >1.5 cm residual tumor, M+ with leptomeningeal seeding or location outside of posterior fossa) or an M+ positive patient as defined by a Modified Chang Staging (M1–M4, but not M0). In our series, all patients were high-risk (including 3 of 7 with residual >1.5 cm, indicative of invasive disease) and all had metastasis (>M0). Values represent the netrin-1 level (pg/µg) and age (in years) and sex in the parentheses average of age in low risk: 8.6 years, 5F; in high risk: 9.1 years, 4F).
Urinary netrin-1 levels as predictive biomarkers of MB
Receiver operating characteristic (ROC) curve analysis indicated that urinary netrin-1 provided excellent diagnostic accuracy as a predictive biomarker in differentiating between tumor and control groups (area under the curve [AUC]: 0.875, p<0.001). The Youden Index revealed that the optimal cut-off value is >2.3 pg/µg, which translates into 81.3% sensitivity. Multivariable logistic regression indicated that the odds of MB were estimated to be over 12 times greater for patients with urinary netrin-1 levels over 2.3 pg/µg (odds ratio: 12.6, 95%; CI: 2.1–47.5, p=0.002) (Fig. 6D).
DISCUSSION
We analyzed four different human MB cell lines (D238, D425, D458 and D556) for reproducibility. Exogenous addition of netrin-1 increased MB cell invasion in a dose-dependent manner. All four MB cell lines secreted netrin-1. Blocking endogenous netrin-1 by neutralizing antibody and siRNA inhibited MB invasiveness by 70%; however, administration of the angiogenic factor VEGF-A did not stimulate MB invasiveness, due to lack of VEGFR2. Thus, unlike VEGF-A, netrin-1 has a dual role in promoting MB invasiveness and EC activity.
Previous work in glioblastoma cell lines revealed that netrin-1 administration results in receptor-mediated regulation of intracellular phosphorylation of kinases. This subsequently regulates the expression of CatB, a protease, and that blockade of CatB results in the abrogation of netrin-1 mediated invasion (6). Netrin-1 administered to MB cells promotes the phosphorylation of Erk1/2, and this increase of p-Erk1/2 correlates with increased invasion. Blockade of Erk1/2 phosphorylation with a pharmacologic inhibitor (U0126) resulted in loss of netrin-1-induced invasion. In a similar fashion, pharmacologic blockade of CatB resulted in loss of invasion. Taken together, these data reveal that netrin-1 induces the phosphorylation of Erk1/2, which is followed by CatB synthesis and release, promoting MB invasion.
Seven netrin receptors have been reported (7). Of these receptors, UNC5B and neogenin appear to be the most predominant in all four MB cell lines. As with netrin-1, inhibiting neogenin and UNC5B protein expression by respective siRNAs suppressed MB cell invasion in vitro, revealing that netrin-1 exerts its invasive effects by interacting specifically with these receptors. When paired, there was a slight synergy between the two receptors. These results demonstrate a novel role for the neogenin and UNC5B receptors in MB invasion, with the identification of key mechanism checkpoints.
Netrin-1 increased MB invasiveness at 16 hours. On the other hand, netrin-1 did not facilitate MB cell proliferation at 24 hours, but did slightly increase proliferation in some MB cell lines by 48 hours. Moreover, 200 ng/mL of netrin-1 induced MB invasion, whereas it was necessary for at least 800 ng/mL to induce MB proliferation. These results demonstrate marked temporal distinctions between invasion and proliferation activities by netrin-1; however, we cannot exclude that netrin-1 also stimulates proliferation and is multifunctional to some degree.
Netrin-1 has pro-angiogenic properties. It stimulated EC invasion, tube formation, sprouting from EC spheroids in vitro and the recruitment of invasive ECs into Matrigel plugs in mice. Blocking neogenin with a neutralizing antibody completely blocked EC infiltration into Matrigel in vivo. Netrin-1 stimulates angiogenesis in other systems, such as murine ischemic hindlimb model and the corneal micropocket assay (23, 24). On the other hand, netrin-1 inhibits angiogenesis through UNC5B in mice or in zebrafish models (25, 26). This disparity could be due to the presence of bifunctional receptors, with some mediating repulsion (UNC5A-D) and others, attraction (DCC, neogenin and DSCAM).
Clinical MB tumor samples have significantly increased netrin-1 mRNA levels (1.7-fold). Protein levels of netrin-1 in MB are elevated by 5–10-fold compared to normal cerebellum. Netrin-1 is a secreted protein; thus, urine could be a source of netrin-1 as a non-invasive biomarker, suitable for ELISA, which can be used to predict MB tumor status. Urine collection is a cost-efficient method that carries no risk. We obtained urine samples from children with pathology-proven MB. Quantification of urinary netrin-1 revealed significant elevations (9-fold) in samples from children with MB (4.8 pg/µg) as compared to healthy matched controls (0.5 pg/µg). Patient tumors that manifested an invasive phenotype had increased levels of urinary netrin-1 (High Risk: 8.3 pg/µg; Low Risk: 1.6 pg/µg), linking netrin-1 expression to tumor invasion and dissemination. Importantly, tumor resection resulted in a strong drop in urinary netrin-1 levels (pre-op: 13 pg/µg; post-op: 0.8 pg/µg), implicating the MB tumor as the source of netrin-1 in the urine. In addition to the patient in Fig. 6C, a second patient demonstrated similar results when tested two years after surgery (pre-op: 2.6 pg/µg; post-op: 0.5 pg/µg), consistent with clinical cure. A notable weakness of this study is the small sample size. Although the data achieves statistical significance, it is obvious that there is variability in netrin-1 urinary levels. One objective of subsequent studies would be to expand this proof-of-principle series of experiments to include a larger sample size, including a greater number of controls.
In summary, our data implicates netrin-1 as a potent inducer of MB invasiveness, acting via neogenin and UNC5B. These in vitro results are compatible with clinical studies of MB patients showing significant elevations of netrin-1 in tumor samples. Together, these clinical studies suggest a novel application of netrin-1 as a non-invasive biomarker, with statistically significant correlations between netrin-1 levels and tumor status that could distinguish invasive and non-invasive MB and predict whether an MB tumor is responding to therapy.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Melissa Anderson and Kristin Johnson for preparation of the manuscript and Dr. Tadanori Mammoto and Elisabeth Jiang for experimental support.
Grant support: Research reported in this publication was supported by the National Institutes of Health/National Cancer Institute number CA37392, The American Brain Tumor Association (ABTA) and the Fellows Brain Tumor Research Fund. H.N. is supported by the Strategic Young Researcher Overseas Visiting Program for Accelerating Brain Circulation (No. S2207 to Dr. Shigeki Higashiyama, Ehime University) and Postdoctoral Fellowships for Research Abroad from Japan Society for the Promotion of Science, Japan.
Footnotes
There are no conflicts to disclose.
REFERENCES
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milla LA, Arros A, Espinoza N, Remke M, Kool M, Taylor MD, et al. Neogenin1 is a Sonic Hedgehog target in medulloblastoma and is necessary for cell cycle progression. Int J Cancer. 2013 doi: 10.1002/ijc.28330. [DOI] [PubMed] [Google Scholar]
- 5.Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152:1065–1076. doi: 10.1016/j.cell.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu A, Nakayama H, Wang P, Konig C, Akino T, Sandlund J, et al. Netrin-1 promotes glioblastoma cell invasiveness and angiogenesis by multiple pathways including activation of RhoA, cathepsin B, and cAMP-response element-binding protein. J Biol Chem. 2013;288:2210–2222. doi: 10.1074/jbc.M112.397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 8.Mehlen P, Delloye-Bourgeois C, Chedotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2011;11:188–197. doi: 10.1038/nrc3005. [DOI] [PubMed] [Google Scholar]
- 9.Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185:3750–3758. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchiya A, Hayashi T, Deguchi K, Sehara Y, Yamashita T, Zhang H, et al. Expression of netrin-1 and its receptors DCC and neogenin in rat brain after ischemia. Brain Res. 2007;1159:1–7. doi: 10.1016/j.brainres.2006.12.096. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Reeves WB, Pays L, Mehlen P, Ramesh G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am J Pathol. 2009;175:1010–1018. doi: 10.2353/ajpath.2009.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 13.Delloye-Bourgeois C, Fitamant J, Paradisi A, Cappellen D, Douc-Rasy S, Raquin MA, et al. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J Exp Med. 2009;206:833–847. doi: 10.1084/jem.20082299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumartin L, Quemener C, Laklai H, Herbert J, Bicknell R, Bousquet C, et al. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138:1595–1606. 606 e1–606 e8. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 15.Delloye-Bourgeois C, Brambilla E, Coissieux MM, Guenebeaud C, Pedeux R, Firlej V, et al. Interference with netrin-1 and tumor cell death in non-small cell lung cancer. J Natl Cancer Inst. 2009;101:237–247. doi: 10.1093/jnci/djn491. [DOI] [PubMed] [Google Scholar]
- 16.Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY, et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci U S A. 2008;105:4850–4855. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith ER, Zurakowski D, Saad A, Scott RM, Moses MA. Urinary biomarkers predict brain tumor presence and response to therapy. Clin Cancer Res. 2008;14:2378–2386. doi: 10.1158/1078-0432.CCR-07-1253. [DOI] [PubMed] [Google Scholar]
- 18.Bielenberg DR, Shimizu A, Klagsbrun M. Semaphorin-induced cytoskeletal collapse and repulsion of endothelial cells. Methods Enzymol. 2008;443:299–314. doi: 10.1016/S0076-6879(08)02015-6. [DOI] [PubMed] [Google Scholar]
- 19.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 20.Harisiadis L, Chang CH. Medulloblastoma in children: a correlation between staging and results of treatment. Int J Radiat Oncol Biol Phys. 1977;2:833–841. doi: 10.1016/0360-3016(77)90181-x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21:1581–1591. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 22.Yao MS, Mehta MP, Boyett JM, Li H, Donahue B, Rorke LB, et al. The effect of M-stage on patterns of failure in posterior fossa primitive neuroectodermal tumors treated on CCG-921: a phase III study in a high-risk patient population. Int J Radiat Oncol Biol Phys. 1997;38:469–476. doi: 10.1016/s0360-3016(97)00010-2. [DOI] [PubMed] [Google Scholar]
- 23.Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson BD, Ii M, Park KW, Suli A, Sorensen LK, Larrieu-Lahargue F, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larrivee B, Freitas C, Trombe M, Lv X, Delafarge B, Yuan L, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.