Abstract
In this issue of Molecular Cell, Gelato et al (2014) identify the signaling molecule phosphatidylinositol-5-phosphate (PI5P) as an allosteric regulator that determines the mode of chromatin binding for the DNA methylation maintenance factor Uhrf1. This work links nuclear lipids to chromatin signaling in the maintenance of DNA methylation and epigenetic regulation.
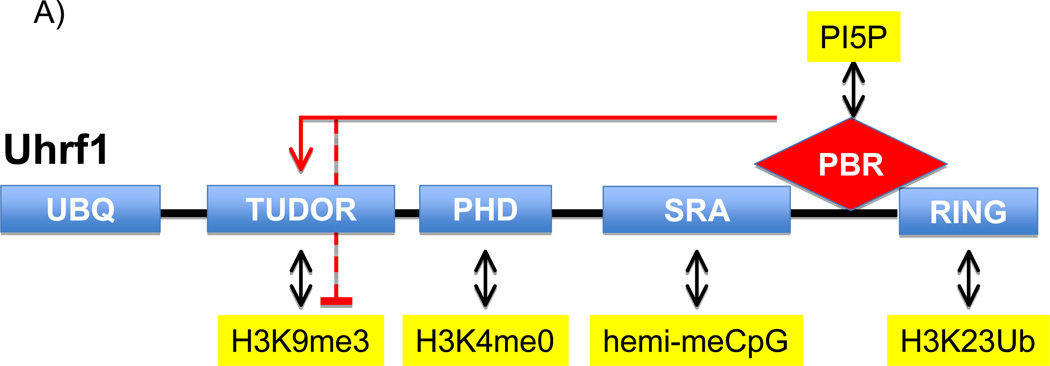
The genomic pattern of methylation at the 5-position of cytosine within the sequence CpG is key for epigenetic inheritance and is maintained through cell division (Rose and Klose, 2014). DNMT1 (DNA methyltransferase 1), which is most active on hemi-methylated DNA (the state of newly replicated methylated DNA), is tasked with copying the methylation pattern from the parental stand to the daughter strand during semiconservative replication. A critical step in maintenance of DNA methylation is the proper targeting of DNMT1 to replication forks moving through regions of the genome that contain methylated DNA. A major player in this targeting step is Uhrf1 (Ubiquitin-like with PHD and Ring Finger domains 1), a protein that is required for efficient maintenance methylation (Bostick et al., 2007; Sharif et al., 2007). Uhrf1 contains multiple distinct domains, including an E3-ligase RING domain, a SRA (SET and RING finger associated) domain, PHD (Plant Homeodomain) finger, and a TTD (Tandem Tudor domain) (Figure 1A) that work in concert during DNMT1 targeting. For example, the RING domain of Uhrf1 acts as an E3 ubiquitin ligase to mediate ubiquitylation of histone H3 at lysine 23 (H23KUb), which in turn serves as a docking site for DNMT1 at chromatin (Nishiyama et al., 2013). The SRA domain recognizes hemi-methylated DNA and therefore increases the probability that Uhrf1 generated H3K23Ub is placed at regions that require DNMT1 activity. Notably, the PHD finger and TTD of Uhrf1 both function as histone “reader” modules; domains that bind to histones in a modification-sensitive manner. The PHD finger recognizes the very N-terminus of H3 and this interaction is disrupted by methylation at lysine 4 (Lallous et al., 2011). Whereas, the TTD binds preferentially to H3 tails that are methylated at lysine 9 (H3K9me) (Karagianni et al., 2008) and cooperates with the SRA domain to stabilize Uhrf1 at chromatin enriched for both H3K9me3 and methylated DNA (Liu et al., 2013). In addition to the two domains potentially recognizing H3 independent of one another, an elegant study demonstrated that the PHD finger and TTD can also form a single functional unit that recognizes with higher affinity a distinct H3 tail signature comprising no methylation at lysine 4 and di- or tri-methylation at lysine 9 (H3K4m0K9me2/3) (Rothbart et al., 2013). However, prior to Gelato et al, a molecular mechanism for how these different histone-binding activities present on Uhrf1 are coordinated was not well understood. Strikingly, Gelato et al now report that a signaling lipid in the nucleus, phosphatidylinositol-5-phosphate (PI5P), binds to Uhrf1 and regulates its mode of association with chromatin (Figure 1B).
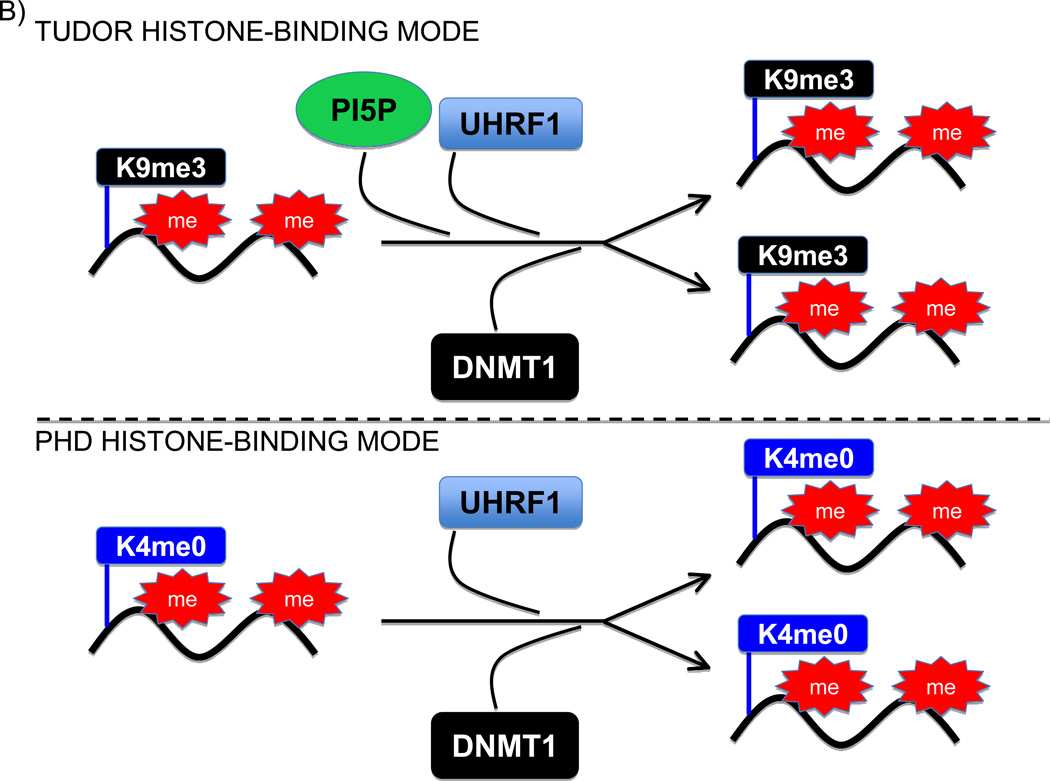
Figure 1.
Regulation of the Uhfr1 histone-binding mode by PI5P. (A) Schematic illustrating the key domains of Uhfr1 and their main molecular activities. The red lines connecting the PBR with the tudor domain indicate that engagement of the PBR with the tudor domain inhibits the tudor-H3K9me3 interaction, which is relieved upon PI5P binding to the PBR. (B) Model for the two PI5P-regulated histone-binding modes for Uhrf1 during replication and maintenance of DNA methylation. Top panel: PI5P helps stabilize Uhrf1 and then DNMT1 at genomic regions containing H3K9me3 and undergoing replication. Bottom Panel: In the absence of PI5P, Uhrf1 and DNTM1 are stabilized at genomic regions undergoing replication that contain H3K4me0.
In experiments designed to characterize the binding specificities of Uhrf1’s two histone-binding modules within the context of the full-length protein, the authors discovered a role for a small cellular co-factor present in nuclear extracts. In the absence of the co-factor, the PHD finger of Uhrf1 is the dominant activity (binding to unmodified H3 tail). Addition of nuclear extract uncoupled the pairing of the TTD and PHD finger and switched Uhrf1 specificity to a TTD-dependent H3K9me3-binding mode. Interestingly, a polybasic region (PBR) present in the carboxy terminus of Uhrf1 was identified as a cis-acting element that bound the TTD and blocked the interaction between the TTD and H3K9me3. Further, the authors found that the activity contained within nuclear extract that switched Uhrf1 to the H3K9me3-binding mode required an intact PBR, suggesting that a co-factor within extracts was binding to the PBR and preventing this region from inhibiting the TTD. Utilizing a candidate-based approach the authors identified PI5P as aco-factor that directly binds to the PBR and allosterically modulates Uhrf1 activity.
The seven different PIP species, including PI5P are generated by a number of PIP kinases and phosphatases (Shah et al., 2013). The various PIPspecies mediate signaling by modulating the subcellular localization and/or activity of distinct PIP-binding proteins. The authors used a series of classic PIP-binding assays to demonstrate the preferential binding of Uhrf1 to PI5P versus the other PIPs, including the other mono-phosphorylated species PI3P and PI4P. Moreover, association of Uhrf1 with heterochromatin was decreased in cells depleted of endogenous PI5P. The specificity of Uhrf1 for PI5P is intriguing because levels of this phospholipid have been shown to undergo dynamic fluctuations within the nucleus in response to a variety of stimuli and to regulate chromatin functions through modulation of PHD finger proteins like ING2 (Shah et al., 2013). For instance, nuclear PI5P levels increase by more than an order of magnitude during S phase (Shah et al., 2013). Thus, PI5P has been proposed to be a critical regulator of nuclear signaling events.
The work presented by Gelato et al uncovers an exciting new mode of action for PI5P in regulating nuclear functions and raises a number of intriguing models and questions (Figure 1). One could envision that the increase in PI5P during S phase serves as a signal to reinforce DNMT1 targeting to H3K9me3-rich genomic regions that are undergoing duplication (Figure 1B). In this regard, the specific and high fidelity targeting of DNMT1 to H3K9me3-chromatin by Uhrf1 would be mediated by multiple concerted molecular events: binding of the SRA to hemi-methylated DNA, binding of the PBR to PI5P, binding of the TTD to H3K9me3, and then placement of H23KUb to create a favorable platform for DNMT1 at DNA methyl-rich replication forks. Focal or global down-regulation of nuclear PI5P levels could switch DNMT1 from being targeted to regions marked by H3K9me3 to localization at genomic regions in which H3K4 is unmethylated (Figure 1B). Taken together, PI5P-mediated regulation of Uhrf1 conformation provides an additional signaling mechanism to ensure the faithful inheritance of genome-wide DNA methylation patterns during replication.
There are many other chromatin-regulatory proteins that like Uhrf1 contain multiple reader modules. It is a reasonable to postulate that these other proteins have PIP-binding PBRs and that PI5P or different PIP species – by the mechanism revealed in Gelato et al – may influence diverse nuclear pathways beyond the Uhrf1-DNMT1 pathway elucidated here. In addition, future work focused on identifying nuclear PIP-binding proteins (for example by utilizing proteomic strategies) should provide insight into how PIP signaling dynamics integrate with other nuclear signaling systems to govern fundamental nuclear processes. Finally, going forward, a fundamental question in the field will be to understand the physical state that PIPs exist in the nucleus. Are they stabilized in protein-lipid structures or is there a yet to be discovered lipidbased structure within the nucleoplasm? Even though many questions remain, Gelato et al have addressed a longstanding question that is posed in the famous Robert Frost poem “The Road Not Taken”, which ends with these words:
“Two roads diverged in a wood, and I— I took the one less traveled by, And that has made all the difference.”
For Uhrf1, PI5P has made that choice, and that road is paved with H3K9me3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- Gelato KA, Tauber M, Ong MS, Winter S, Hiragami-Hamada K, Sindlinger J, Lemak A, Bultsma Y, Houliston S, Schwarzer D, et al. Mol. Cell. This issue. 2014 doi: 10.1016/j.molcel.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Karagianni P, Amazit L, Qin J, Wong J. Molecular and cellular biology. 2008;28:705–717. doi: 10.1128/MCB.01598-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallous N, Legrand P, McEwen AG, Ramon-Maiques S, Samama JP, Birck C. PloS one. 2011;6:e27599. doi: 10.1371/journal.pone.0027599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gao Q, Li P, Zhao Q, Zhang J, Li J, Koseki H, Wong J .Nature communications. 2013;4:1563. doi: 10.1038/ncomms2562. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yamaguchi L, Sharif J, Johmura Y, Kawamura T, Nakanishi K, Shimamura S, Arita K, Kodama T, Ishikawa F, et al. Nature. 2013;502:249–253. doi: 10.1038/nature12488. [DOI] [PubMed] [Google Scholar]
- Rose NR, Klose RJ. Biochimicaetbiophysicaacta. 2014 (volume and page – or DOI?) [Google Scholar]
- Rothbart SB, Dickson BM, Ong MS, Krajewski K, Houliston S, Kireev DB, Arrowsmith CH, Strahl BD. Genes & development. 2013;27:1288–1298. doi: 10.1101/gad.220467.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZH, Jones DR, Sommer L, Foulger R, Bultsma Y, D'Santos C, Divecha N. The FEBS journal. 2013;280:6295–6310. doi: 10.1111/febs.12543. [DOI] [PubMed] [Google Scholar]
- Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, Shinga J, Mizutani-Koseki Y, Toyoda T, Okamura K, et al. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]