Abstract
During infection of the lung epithelium, Mycobacterium tuberculosis must infect and survive within macrophages long enough to be transported into deeper lung tissues. Cambier et al. (2013) show that pathogenic mycobacteria use the coordinated action of two cell wall glycolipids to regulate macrophage recruitment to initial infection sites.
The cell wall of Mycobacterium tuberculosis and its close relative M. marinum contains phthiocerol dimycocerosate (PDIM) and the structurally related phenolic glycolipids (PGL). However, the functions of these lipids have remained largely unclear, leading to much fascination and frustration over several decades. Researchers reported in 1974 that clinical M. tuberculosis isolates lacking PDIM were attenuated in the guinea pig model of infection, and 25 years later, two groups independently presented genetic proof for this association (Stanley and Cox, 2013). Because spontaneous loss of M. tuberculosis PDIM occurs frequently during in vitro passaging, many mycobacterial geneticists regard PDIM meta-stability as an impetus for genetic complementation of in vivo phenotypes. The structurally related PGL also has a perplexing history: laboratory strains and many clinical isolates are naturally deficient in PGL production (Reed et al., 2004), and the contribution of the glycolipid to M. tuberculosis virulence has been controversial.
The roles of PDIM and PGL during infection have also flummoxed the field because of the inherent difficulties associated with their analysis. Lipids, for example, are not directly genetically encoded and therefore are not amenable to traditional tagging methods. Moreover, cell wall lipids may have multiple, overlapping functions (Passemar et al., 2013). As a consequence of both technical and biological challenges, the literature of mycobacterial lipids is replete with controversy, and the mechanisms by which these molecules contribute to pathogenesis remain unclear.
Proposals for PDIM and PGL functions are numerous but generally fall into three categories (Passemar et al., 2013). First, the glycolipids may have structural roles, both within the bacterial envelope and once inserted into host membranes. Second, they may act as barriers to or repositories for toxic molecules. Third, they may directly interact with the immune system. These hypotheses are not mutually exclusive, and indeed Cambier et al. (2013) now demonstrate that mycobacterial glycolipids play both structural and immunomodulatory roles during the earliest stage of infection.
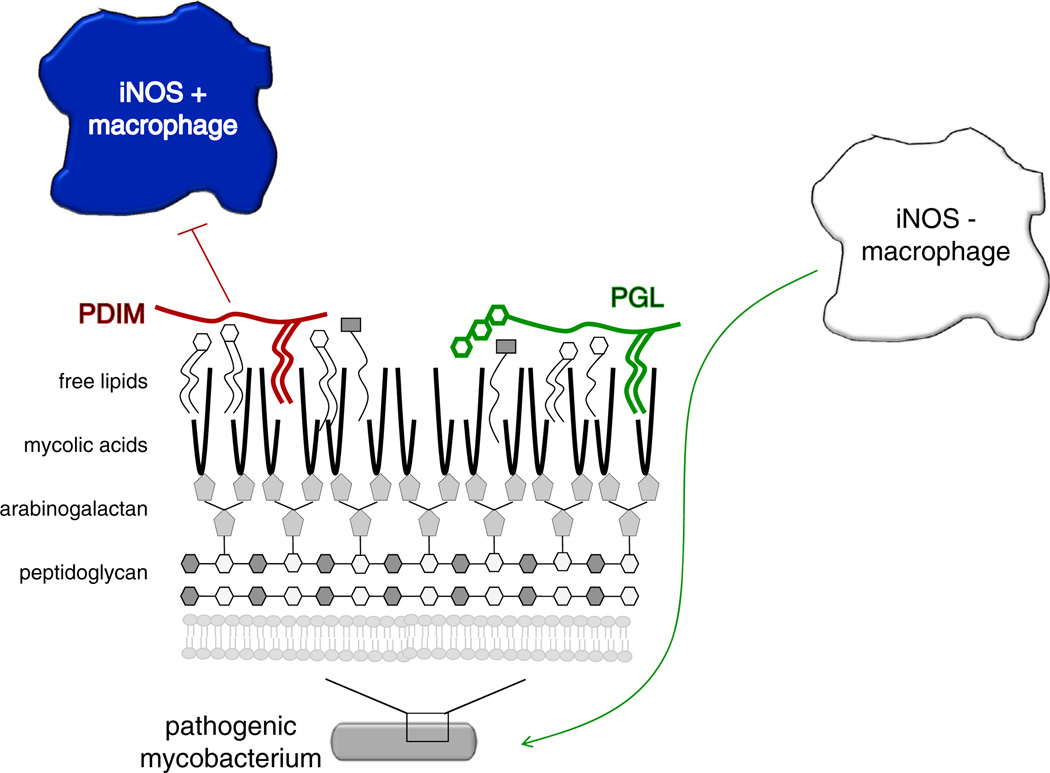
Cambier et al. (2013) began their study by noting that Toll-like receptor (TLR)-mediated immunity is important for protection against M. tuberculosis in vitro but dispensable in both human and animal studies. Given that the mycobacterial cell wall contains a plethora of pathogen-associated molecular patterns (PAMPs), any of which should theoretically induce TLR signaling, they hypothesized that countermeasures that shield these PAMPs from innate immune detection might also be present. To test this idea, Cambier et al. (2013) employed the M. marinum-zebrafish model of infection. M. marinum is a close genetic relative of M. tuberculosis and the causative agent of ectotherm tuberculosis. By manipulating both host and bacterium, Cambier et al. (2013) demonstrated that PDIM inhibits TLR signaling via the common adaptor protein MyD88, which in turn prevents the recruitment of microbicidal macrophages to the earliest sites of infection. They further demonstrate that the permissive macrophages associated with early wild-type M. marinum infection are recruited via a host chemokine receptor 2 (CCR2) pathway that is dependent on the presence of PGL. Together, these data suggest that the PDIM and PGL glycolipids of virulent mycobacteria help orchestrate the composition of the first responding immune sentinels (Figure 1).
Figure 1. Mycobacterial Cell Wall Lipids Modulate Macrophage Composition at the Earliest Sites of Infection.
PDIM suppresses the recruitment of microbicidal, iNOS-positive macrophages by inhibiting TLR signaling, while PGL stimulates the recruitment of more permissive, iNOS-negative macrophages via the CCR2 pathway.
In contrast to the macrophages recruited to the initial sites of wild-type M. marinum infection, the majority of macrophages recruited to PDIM-deficient M. marinum expressed inducible nitric oxide synthase (iNOS), an enzyme required for production of microbicidal, reactive nitrogen intermediates (RNS). Cambier et al. (2013) were able to rescue the growth of the mutant strain by the addition of iNOS inhibitors, confirming that RNS were responsible for bacterial attenuation in the absence of PDIM. Although many studies have found that M. tuberculosis lacking PDIM are attenuated in vivo, they have differed in their assessment of when the defect occurs and what components of host immunity are involved, particularly the relative contributions of interferon gamma (IFNγ) cytokine signaling and RNS (Kirksey et al., 2011; Murry et al., 2009; Rousseau et al., 2004). Because Cambier et al. (2013) were able to transfer the MyD88, RNS-dependent attenuation phenotype to PDIM-positive bacteria by infecting in the presence of PDIM-negative bacteria, their experiments support a model in which pathogenic mycobacteria use the glycolipid to evade TLR detection and subsequent RNS produced by microbicidal macrophages at the earliest stages of infection. Notably, these data do not rule out additional roles for the mycobacterial glycolipids in vivo, particularly within macrophages or during later stages of infection (Stanley and Cox, 2013).
Because they were able to attenuate wild-type M. marinum infection by coinjecting crushed M. marinum, Cambier et al. (2013) concluded that PDIM likely functions in immune evasion by physically masking cell wall PAMPs. Their findings emphasize the importance of investigating innate immune signaling in the context of an intact pathogen and with the full repertoire of immune cells present in an intact host. However, many questions remain regarding the molecular mechanisms by which the glycolipids function. For example, does PDIM directly block host access to underlying PAMPs, or does it prevent release of the molecules? What are the host components that bind PGL in the CCR2 pathway or PAMPs in PDIM-deficient strains? And finally, when and where are glycolipids and cell wall PAMPs presented to the host immune system? Answering these questions may require the development of new tools with which to probe cell wall components in vivo.
The interactions between the host milieu and bacterial cell wall are likely to be bidirectional and to change over the course of infection. For example, in vivo PDIM abundance may depend on expression of the biosynthetic enzymes, which decreases upon extended macrophage infection (Rohde et al., 2012), shifts in metabolic flux, which occur during host lipid catabolism (Jain et al., 2007), and insertion of the molecule into host membranes (Astarie-Dequeker et al., 2009). Thus, there may be variable amounts of PDIM on the mycobacterial cell surface at different time points after infection. One corollary of this hypothesis is that there may also be differential exposure of M. tuberculosis PAMPs, which in turn could shape the evolving relationship between host and pathogen over the course of an infection.
M. tuberculosis preferentially initiates human infection in the distal lung, a surface that is largely devoid of the commensal and environmental microbes found in the upper respiratory tract. It may be that the TLR signaling stimulated by the PAMPs from these organisms overrides PDIM- and PGL-mediated immune evasion. In support of this hypothesis, Cambier et al. (2013) found that coinfection of M. marinum with Staphylococcus aureus or Pseudomonas aeruginosa, common oropharyngeal colonizers of the human lung, attenuated wild-type M. marinum in a MyD88-dependent fashion. Thus, TLR signaling by commensal flora may protect against M. tuberculosis infection and force the bacterium to infect via the less accessible lower respiratory tract.
Finally, this study highlights the many advantages of the M. marinum-zebrafish infection model. First, Cambier et al. (2013) were able to discriminate between relative contributions of different immune components at both the level of cell type and chemokine signaling. The optical transparency of the zebrafish embryo, moreover, allowed real-time, quantitative imaging of pathogenic mycobacteria infecting an intact host at the single-cell level. Although bulk population measurements of colony-forming units (cfu) in various organs have yielded important insights into host-pathogen interactions, they offer static portraits of bacterial burden with limited insight into the kinetics of infection. As demonstrated by Cambier et al. (2013), single-cell tracking of both zebrafish host and mycobacterial pathogen can facilitate complex, dynamic models of tuberculosis.
REFERENCES
- Astarie-Dequeker C, Le Guyader L, Malaga W, Seaphanh F-K, Chalut C, Lopez A, Guilhot C. PLoS Pathog. 2009;5:e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier CJ, Takaki KK, Larson RP, Hernandez RE, Tobin DM, Urdahl KB, Cosma CL, Ramakrishnan L. [Published online December 15, 2013];Nature. 2013 doi: 10.1038/nature12799. http://dx.doi.org/10.1038/nature12799. [DOI] [PMC free article] [PubMed]
- Jain M, Petzold CJ, Schelle MW, Leavell MD, Mougous JD, Bertozzi CR, Leary JA, Cox JS. Proc. Natl. Acad. Sci. USA. 2007;104:5133–5138. doi: 10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirksey MA, Tischler AD, Siméone R, Hisert KB, Uplekar S, Guilhot C, McKinney JD. Infect. Immun. 2011;79:2829–2838. doi: 10.1128/IAI.00097-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry JP, Pandey AK, Sassetti CM, Rubin EJ. J. Infect. Dis. 2009;200:774–782. doi: 10.1086/605128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passemar C, Arbués A, Malaga W, Mercier I, Moreau F, Lepourry L, Neyrolles O, Guilhot C, Astarie-Dequeker C. [Published online September 13, 2013];Cell. Microbiol. 2013 doi: 10.1111/cmi.12214. http://dx.doi.org/10.1111/cmi.12214. [DOI] [PubMed]
- Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE., 3rd Nature. 2004;431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- Rohde KH, Veiga DFT, Caldwell S, Balázsi G, Russell DG. PLoS Pathog. 2012;8:e1002769. doi: 10.1371/journal.ppat.1002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau C, Winter N, Pivert E, Bordat Y, Neyrolles O, Avé P, Huerre M, Gicquel B, Jackson M. Cell. Microbiol. 2004;6:277–287. doi: 10.1046/j.1462-5822.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Cox JS. Host–Pathogen Interactions During Mycobacterium tuberculosis infections. In: Pieters J, McKinney JD, editors. Current Topics in Microbiology and Immunology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. [DOI] [PubMed] [Google Scholar]