Abstract
Development and selection of an ideal scaffold is of importance for tissue engineering. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) is a biocompatible bioresorbable copolymer that belongs to the polyhydroxyalkanoate family. Because of its good biocompatibility, PHBHHx has been widely used as a cell scaffold for tissue engineering. This review focuses on the utilization of PHBHHx-based scaffolds in tissue engineering. Advances in the preparation, modification, and application of PHBHHx scaffolds are discussed.
Keywords: PHBHHx, Tissue engineering, Biomaterial
Introduction
Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) is a natural biological polyester material that belongs to the polyhydroxyalkanoate (PHA) family (1,2). Compared with other, well-established PHA family members, poly(3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), PHBHHx has better biodegradability and mechanical properties. PHBHHx has excellent biocompatibility with a variety of cell types, including smooth muscle cells (3), fibroblasts (4), articular cartilage-derived chondrocytes (5,6), osteoblasts (7), and bone marrow cells (8). Additionally, PHBHHx possesses a low degree of crystallinity (2).
Polyhydoxyalkanoates are generally biodegradable and thermoprocessable and thus have been widely used in both conventional medical devices and tissue engineering. Because of its good biocompatibility, PHBHHx is attracting increasing attention as a cell scaffold for tissue engineering (2). Ye et al. (9) demonstrated that a three-dimensional PHB/PHBHHx scaffold seeded with differentiated human adipose-derived stem cells is capable of producing cartilage-like tissue after implantation into the subcutaneous layer of nude mice. PHBHHx scaffolds have been successfully applied in an in vivo tendon repair model, as evidenced by facilitation of tendon movement recovery and complete restoration of load bearing and function in recipient rats (10).
This review describes the two- and three-dimensional properties of PHBHHx and focuses on the improvement of its biological properties via surface modifications. Recent advances in the preparation and application of PHBHHx scaffolds are also discussed.
Scaffolds
Development and selection of a suitable scaffold is a critical aspect of tissue engineering. Scaffolds act not only as a physical support, but also as an artificial extracellular matrix (ECM) that contributes to the attachment, proliferation, and differentiation of seeded cells (11). An ideal scaffold material should meet the following criteria (12,13): 1) nontoxicity of the polymer and its degradation products, 2) excellent biocompatibility with seeded cells, 3) good mechanical properties and flexibility, 4) controlled biodegradability, 5) easily formed as a porous structure, and 6) appropriate thermoplastic properties.
Tissue engineering scaffolds can be roughly divided into natural and synthetic scaffolds. Synthetic scaffolds such as polyglycolic acid and polylactic acid (PLA) have been widely used as medical sutures, stents, and drug delivery carriers (14,15). Natural polymers including collagen, fibrin, chitosan, and PHAs are biocompatible and yield promising results when used as tissue engineering scaffolds (16-18).
Properties of PHBHHx scaffolds
Physical properties
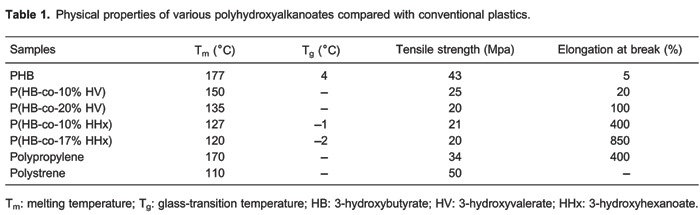
Chen et al. (2) suggest that the changing PHA compositions allow favorable mechanical properties, biocompatibility, and degradation times within desirable time frames under specific physiological conditions (Table 1). From this table, we find that the melting temperature (Tm) and glass-transition temperature (Tg) of PHBHHx are dependent on the content of 3-hydroxyhexanoate (HHx), i.e., the higher the proportion of HHx, the lower the Tm and Tg values. Crystallinity of PHBHHx also decreases with increase in the content of HHx. In the composite system of PHB/PHBHHx, similar findings are observed. Chen et al. (2) also revealed that PHB/PHBHHx blended at a ratio of 1:1 has the maximum surface free energy, a key parameter affecting adhesion, distribution, and differentiation of seeded cells (2,19).
Toxicity of PHBHHx and its degradation products
PHBHHx is a copolyester of 3-hydroxybutyrate (HB) and HHx. PHB is not only an inert storage polymer found in the cytoplasm of many bacteria, but also a universal, interactive, solvating biopolymer involved in important physiological functions (20). Martin et al. (21) reported that, as the degradation products of PHBHHx, neither HB nor HHx are toxic, and even provide some therapeutic or nutritional benefits. It has been documented that HB facilitates neuronal survival and differentiation.
In vitro and in vivo PHBHHx biodegradation
Chen and Tong (22) evaluated, for the first time, the biodegradability of PHBHHx films vs PHB and Ecoflex and examined the effect of HHx content on PHBHHx degradation. They reported that, after 18 days of incubation, 40% P(HB-co-12%-HHx) and 20% PHB were degraded, while Ecoflex only lost 5% of its weight. Compared with PHB, P(HB-co-5%-HHx), and P(HB-co-20%-HHx), P(HB-co-12%-HHx) was degraded at a faster rate. Scanning electron microscopy analysis revealed that P(HB-co-12%-HHx) films had the most porous surface after degradation. These findings indicate that surface morphology plays an important role in the degradation of PHBHHx. P(HB-co-12%-HHx), with a low crystallinity and rough surface, had the highest degradation rate.
In vivo studies of PHBHHx and a PHBHHx/polyethyl-ene glycol (PEG) blend implanted in rabbits for 6 months found that PHBHHx decreased in weight faster than PHB but slower than PLA (3,19). The degradation of PHBHHx was greatly improved by blending with PEG, but such blends led to a strong tissue response. Therefore, PHBHHx is a good biodegradable material and may be suitable for in vivo applications.
Biocompatibility properties
It has been reported that mouse fibroblast L929 cells plated on PHB/PHBHHx films grew better than those plated on PHB and PLA films, suggesting the PHB/PHBHHx blend is highly biocompatible (23). Similarly, Zhao et al. (24) demonstrated that chondrocyte cells grown on PHBHHx/PHB scaffolds possessed the physiological functions needed for effective cartilage tissue regeneration. However, the biocompatibility of PHAs is inadequate because of their hydrophobic properties (23). These findings suggest that improvement of the biocompatibility of PHBHHx is pivotal for its applications in tissue engineering. The methods commonly used to improve the biocompatibility of materials are discussed in detail in the third section of this review (Surface modification of PHBHHx for improved biocompatibility).
PHA family members for tissue engineering applications
PHAs
PHAs are a family of biodegradable polyesters synthesized by bacteria as intracellular carbon and energy storage compounds (2). More than 150 kinds of monomeric PHAs have been reported. Although PHAs have the potential to be used as tissue engineering biomaterials, only a few of them, such as PHB, PHBV, and PHBHHx, have been produced industrially (25). PHB is one of the most thoroughly researched members of the PHA family and has good biocompatibility for adrenocortical cells, osteoblasts, epithelial cells, fibroblasts, endothelium cells, and isolated hepatocytes (26). However, it is highly brittle and degrades slowly, and both of these properties have limited its applications (27). Another PHA family member, PHBV, can be produced as fibers that have been shown to promote reepithelialization (28). After surface modification, PHB and PHBV are able to promote osseointegration (29). However, long-term exposure to PHB and PHBV may induce an inflammatory response.
Brief introduction of PHBHHx
The new PHA family member, PHBHHx, is considered a very promising tissue engineering material with adjustable mechanical properties and good biocompatibility and biodegradability (3). Experimental studies have indicated that PHBHHx has high biocompatibility for a wide range of cell types such as smooth muscle cells, fibroblasts, chondrocytes, osteoblasts, and bone marrow cells (5,30-33). The PHBHHx films were created using solvent-casting, electrospinning, and compression molding methods, and the behavior of human mesenchymal stem cells (hMSCs) grown on these films has been investigated (34). hMSCs cultured on highly porous electrospun PHBHHx films have better cell viability than those cultured on compression-molded films.
PHBHHx particles have been used as drug delivery systems in targeted anticancer therapy (35-37). PHBHHx combined with folic acid and loaded with etoposide (a cell cycle-specific anticancer drug) allows improvement of the targeted delivery of drugs to tumors. In another application, PHBHHx nanoparticles loaded with an insulin-phospholipid complex have been used in a long-acting release formulation of insulin. However, practical applications of PHBHHx have been limited because of some disadvantages such as high hydrophobicity and low crystallization rate. To overcome these limitations, many attempts have been made to modify the physicochemical properties of PHBHHx and to develop new formulations.
Surface modification of PHBHHx for improved biocompatibility
Surface treatment
Surface modification is an effective way to improve the hydrophilic properties of PHAs. Different strategies, including ultraviolet treatment, plasma treatment, grafting techniques, and surface hydrolysis (38-40), have been applied to increase the hydrophilicity of polymer materials. These treatments can be used not only to increase the hydrophilicity of the surface material, but also to introduce biologically active molecules to polymer surfaces, thus modifying the biocompatibility of materials. Alkali and lipase treatments are very effective to improve the wetting and adhesion properties of polymer surfaces (32,41). It has been demonstrated that NaOH treatment enhances the attachment of cells to PHBHHx films (42). However, these surface modification techniques do have some disadvantages. For example, grafting makes the surface of the material very unstable because of the very weak interaction between the bulk polymer and the bioactive molecules. Direct ultraviolet radiation may change the mechanical properties of polymer films and make them very brittle. Plasma treatment may only temporarily enhance the hydrophilicity of PHAs.
Blending with other materials
The blending of materials with different properties is a commonly used approach to offset individual defects. Mei et al. (43) evaluated the biocompatibility of PHBHHx modified by silk fibroin (SF). They found that the water contact angle on the PHBHHx/SF was smaller than that on the pure PHBHHx scaffold, and the number of cells attached to the surface of PHBHHx/SF films was larger than on the control films, indicating an improvement in hydrophilicity and biocompatibility.
Additionally, PHA family members may be blended with each other. Scaffolds made from PHBHHx and PHB at a ratio of 1:1 have a higher surface free energy and are more effective for chondrocyte adhesion than scaffolds made of PHBHHx alone (19). In another study, adipose-derived mesenchymal stem cells inoculated on PHB/PHBHHx scaffolds and implanted into animals were found to generate cartilage-like tissues 14 days after implantation (2).
Biomodification with amphiphilic proteins
Recently, biomodification of polymer surfaces has emerged as a novel approach to improve their wetting and adhesion properties. Several amphiphilic proteins have been identified to be located on the surface of in vivo PHA granules including PhaC synthase, poly-beta-hydroxybutyrate depolymerase (PhaZ), and granule-associated protein (PhaP) (44). Previous studies showed that PhaP fusion proteins are able to bind to natural PHA granules, allowing for the development of low-cost approaches for protein purification, specific drug delivery, and cell sorting. Arginine-glycine-aspartate (RGD) tripeptide is normally involved in cell binding to a number of ECM glycoproteins by interaction with integrins (45). The PhaP-RGD fusion protein, when used to coat PHBHHx, has been shown to enhance cell adhesion, spreading, migration, and proliferation (31). Similarly, You et al. (5) showed that PhaP-RGD-coated PHBHHx scaffolds facilitate chondrogenic differentiation of human bone marrow mesenchymal stem cells inoculated onto them.
Scaffold fabrication
There are currently several methods available for fabrication of PHBHHx scaffolds, including solution casting, electrospinning, phase separation, solvent casting, fiber bonding and particulate leaching, solid freeform fabrication, and particle sintering (46,47).
Solvent evaporation method
Solvent evaporation is a simple method and the most commonly used to fabricate PHBHHx films (34). In detail, a PHBHHx solution is prepared by dissolving purified PHBHHx in chloroform, which is then evenly distributed into glass dishes. The chloroform is allowed to evaporate at room temperature, resulting in the formation of PHBHHx films on the Petri dishes. Vacuum drying is generally applied to eliminate the residual solvent, because of its cytotoxicity.
Phase separation and freeze-drying
Freeze-drying is commonly used to prepare three-dimensional scaffolds (33). The principle involved is that the polymer solution, emulsion, or hydrogel is frozen to induce phase separation, and the solvent is then removed by vacuum freeze-drying to form a porous scaffold. The pore morphology, mechanical properties, biocompatibility, and degradability of the scaffold are affected by the concentration of the polymer solution and the phase separation temperature, the quenching temperature, and the types of polymer and solvent.
Wang et al. (48) fabricated PHBHHx scaffolds by thermally induced phase separation. In this process, polymers are dissolved in 1,4-dioxane under vigorous agitation for 60 min at 65°C and the mixture is then kept frozen at -80°C for 2 h. After removal of the solvent via freeze-drying, porous scaffolds are obtained, with a pore size of approximately 60-100 μm in diameter and a porosity of 93±1.4%. Such porous three-dimensional scaffolds provide additional space for seeded cells to grow.
Electrospinning techniques
Electrospinning techniques have been used to create fibrous scaffolds that mimic the natural ECM for study of in vivo-like phenotypic shape and gene expression (49). Interestingly, the orientation of electrospun fibers is able to provide contact guidance for the attached cells, and has an impact on their morphology and even their differentiation capacity. It has been reported that aligned electrospun fibers promote Schwann cell maturation, neurite outgrowth, vascular endothelial cell growth, and myotube formation (6). The regular alignment of fibers leads to enhanced osteogenic responses in MSCs compared with the fibers having a randomly networked architecture.
The application of electrospinning techniques in tissue engineering has been reviewed (50). Three-dimensional fibrous and nanofibrous PHBHHx-based scaffolds that have been successfully produced by electrospinning show an osteoinductive potential. However, it is difficult to control the porosity of scaffolds produced using this technique.
Particle leaching
Highly porous scaffolds can be fabricated by particle leaching using sodium chloride (NaCl) particles as a porogen (51). Sun et al. (51) demonstrated that the porosity of scaffolds is almost independent of the porogen size, but instead is dependent on the amount of porogen. The maximum porosity of scaffolds can reach about 85%, when the weight ratio of porogen to polymers is 9:1. The fabrication process began with dissolving 2 g PHBHHx powder in 20 mL chloroform in a 50-mL conical flask and then 0.04 g NaCl crystals was suspended in the PHBHHx-chloroform solution and mixed by shaking. Tube construction was carried out using a 2.5-mm diameter stainless steel mandrill, which was dipped into the homogenized PHBHHx/NaCl solution for 1 s, followed by solvent evaporation in a flow hood for 2 min. This process was repeated five times, after which the mandrill was fixed vertically for 60 min to allow the solvent to fully evaporate and the polymer to become rigid. The polymer tube was carefully removed by hand and immersed in deionized H2O overnight to introduce porosity through NaCl crystal dissolution.
Applications of PHBHHx-based biopolyesters
In recent years, PHBHHx-based biopolyesters have been developed for various potential medical applications, especially as tissue engineering and drug delivery materials.
PHBHHx as a material for bone and cartilage tissue engineering
Cells for bone and cartilage regeneration have been evaluated for their proliferative and differentiative responses when inoculated into PHBHHx-based scaffolds (32,52). It has been reported that PHBHHx scaffolds increase both chondrocyte proliferation and protein secretion in rabbit articular cartilage cells seeded onto them (53). Chondrocyte proliferation and synthesis of glycosaminoglycans (GAGs) and type II collagen are generally required for successful cartilage regeneration.
Compared to those seeded on PLA and PHB, rabbit bone marrow cells inoculated on three-dimensional PHBHHx scaffolds show more differentiation into osteoblast-like cells, with a round cell shape, high alkaline phosphatase activity, strong calcium deposition, and fibrillar collagen synthesis. This finding suggests that PHBHHx is a suitable biomaterial for promotion of osteoblast attachment, proliferation, and differentiation.
Chondrocytes isolated from rabbit articular cartilage were inoculated onto numerous PHBHHx/PHB porous scaffolds (54). Total collagen content in all scaffolds containing PHBHHx increased with the duration of incubation. Moreover, the use of PHB/PHBHHx at a ratio of 1:2 in scaffolds resulted in a significant increase in the production of GAG, a major composition of ECM, after 7 days of culture, compared with scaffolds having different component ratios. Therefore, the proportion of PHBHHx in the PHB/PHBHHx composite system is of importance for the promotion of ECM synthesis by articular cartilage chondrocytes. The inoculated cells grew better on the scaffolds consisting of PHBHHx/PHB at the ratios of 2:1 and 1:2 than on those having a 1:1 ratio.
Nerve and brain tissue repair
PHAs have shown good potential as medical implant biomaterials. Neural stem cells (NSCs) grown on/in PHA scaffolds may be useful for repairing central nervous system (CNS) injury (46). Among the PHA family members, PHBHHx appears to have the strongest potential to promote NSC differentiation into neurons. It has been suggested that PHBHHx nanofiber scaffolds that promote NSC growth and differentiation could be developed for treating CNS defects.
Porous nerve conduits with either uniform or nonuniform wall porosity were prepared using a particle leaching method. The conduits were used to bridge a 10-mm defect in the sciatic nerve of rats (55). PHBHHx nerve conduits prepared in this study showed satisfactory mechanical strength and biodegradability. Histological assessment together with transmission electron microscopy showed that the performance of conduits with nonuniform wall porosity was similar to that of nerve autografts in promoting functional recovery, but better than the conduits with uniform wall porosity. Thus, PHBHHx-based conduits may represent a promising alternative for peripheral nerve damage repair.
Soft tissue repair and smooth muscle engineering
Considering that its strength and elastic properties can be adjusted by changing its monomer contents, PHBHHx could be tailored to meet the requirements for regenerating both bone and soft tissues. PHBHHx, with its excellent mechanical and thermal properties, has been used as a scaffold for tissue-engineered cardiovascular products (19). Qu et al. (56) prepared and modified PHBHHx by ammonia-plasma treatment and fibronectin coating and then inoculated human umbilical vein endothelial cells (HUVECs) and rabbit aorta smooth muscle cells on the surface of the modified PHBHHx scaffold (PFn-PHBHHx). They found that HUVECs proliferated more on the PFn-PHBHHx than the PHBHHx surface and formed a confluent monolayer 3 days after seeding. This finding suggests that PFn-PHBHHx may be a good material as a luminal surface of vascular grafts.
Due to good elasticity and strength, PHBHHx may be a promising candidate material for the generation of artificial esophagus. Indeed, some studies using PHBHHx as artificial esophagus in dogs indicated that PHBHHx can stimulate regeneration of removed esophagus tissue (2).
PHBHHx as biodegradable drug carriers
PHBHHx particles have been used for drug delivery because of their biodegradability and nontoxic, nonimmunogenic degradation products. For example, PHBHHx nanoparticles and micron particles were used as drug delivery carriers for the controlled release of 5-fluorouracil (57). In combination with folic acid, PHBHHx nanoparticles have been used to deliver etoposide, a cell cycle specific anticancer drug, to tumors (58). Insulin phospholipid complex loaded biodegradable PHBHHx nanoparticles are effective in the delivery of long-acting insulin (58).
Conclusions
PHBHHx offers great practical potential as a tissue engineering scaffold material. Numerous production techniques are available to modify or improve the physicochemical and biocompatible properties of PHBHHx. With novel composite material generation coupled with technical innovations, PHBHHx-based scaffolds may be of clinical benefit in regenerating a variety of tissues.
Acknowledgments
Research supported by the Natural Science Foundation of China (#81000416), the Fundamental Research Funds for the Central Universities (2011) and the Funds for the Second Hospital of Xi'an Jiaotong University (2011, 2012, 2013).
Footnotes
First published online May 30, 2014.
References
- 1.Wang YW, Yang F, Wu Q, Cheng YC, Yu PH, Chen J, et al. Effect of composition of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on growth of fibroblast and osteoblast. Biomaterials. 2005;26:755–761. doi: 10.1016/j.biomaterials.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Chen GQ, Wu Q, Wang YW, Zheng Z. Application of microbial polyesters-polyhydroxyalkanoates as tissue engineering materials. Key Engineering Materials. 2005;288-289:437–440. doi: 10.4028/www.scientific.net/KEM.288-289.437. [DOI] [Google Scholar]
- 3.Qu XH, Wu Q, Zhang KY, Chen GQ. In vivo studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) based polymers: biodegradation and tissue reactions. Biomaterials. 2006;27:3540–3548. doi: 10.1016/j.biomaterials.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Yang HX, Sun M, Zhang Y, Zhou P. Degradable PHBHHx modified by the silk fibroin for the applications of cardiovascular tissue engineering. Materials Science. 2011 doi: 10.5402/2011/389872. [DOI] [Google Scholar]
- 5.You M, Peng G, Li J, Ma P, Wang Z, Shu W, et al. Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials. 2011;32:2305–2313. doi: 10.1016/j.biomaterials.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Gao R, Wang PP, Jian J, Jiang XL, Yan C, et al. The differential effects of aligned electrospun PHBHHx fibers on adipogenic and osteogenic potential of MSCs through the regulation of PPARgamma signaling. Biomaterials. 2012;33:485–493. doi: 10.1016/j.biomaterials.2011.09.089. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Jiang XL, Peng SW, Guo XY, Shang GG, Chen JC, et al. Induced apoptosis of osteoblasts proliferating on polyhydroxyalkanoates. Biomaterials. 2013;34:3737–3746. doi: 10.1016/j.biomaterials.2013.01.088. [DOI] [PubMed] [Google Scholar]
- 8.Yu BY, Chen PY, Sun YM, Lee YT, Young TH. Response of human mesenchymal stem cells (hMSCs) to the topographic variation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) films. J Biomater Sci Polym Ed. 2012;23:1–26. doi: 10.1163/092050610X541386. [DOI] [PubMed] [Google Scholar]
- 9.Ye C, Hu P, Ma MX, Xiang Y, Liu RG, Shang XW. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials. 2009;30:4401–4406. doi: 10.1016/j.biomaterials.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Webb WR, Dale TP, Lomas AJ, Zeng G, Wimpenny I, El Haj AJ, et al. The application of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds for tendon repair in the rat model. Biomaterials. 2013;34:6683–6694. doi: 10.1016/j.biomaterials.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Bryant SJ, Anseth KS. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64:70–79. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 12.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/S0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 13.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 14.Zhong T, Deng C, Gao Y, Chen M, Zuo B. Studies of in situ-forming hydrogels by blending PLA-PEG-PLA copolymer with silk fibroin solution. J Biomed Mater Res A. 2012;100:1983–1989. doi: 10.1002/jbm.a.33307. [DOI] [PubMed] [Google Scholar]
- 15.Jeong CG, Hollister SJ. A comparison of the influence of material on in vitro cartilage tissue engineering with PCL, PGS, and POC 3D scaffold architecture seeded with chondrocytes. Biomaterials. 2010;31:4304–4312. doi: 10.1016/j.biomaterials.2010.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, et al. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30:2544–2551. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Wang CC, Yang KC, Lin KH, Liu HC, Lin FH. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials. 2011;32:7118–7126. doi: 10.1016/j.biomaterials.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011;32:8927–8937. doi: 10.1016/j.biomaterials.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z, Bei FF, Tian HL, Chen GQ. Effects of crystallization of polyhydroxyalkanoate blend on surface physicochemical properties and interactions with rabbit articular cartilage chondrocytes. Biomaterials. 2005;26:3537–3548. doi: 10.1016/j.biomaterials.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Pereira TF, Silva MAC, Oliveira MF, Maia IA, Silva JVL, Costa MF, et al. Effect of process parameters on the properties of selective laser sintered Poly(3-hydroxybutyrate) scaffolds for bone tissue engineering. Virtual Phys Prototyp. 2012;7:275–285. doi: 10.1080/17452759.2012.738551. [DOI] [Google Scholar]
- 21.Martin DP, Peoples OP, Williams SE, Zhong LH. Nutritional and therapeutic uses of 3-hydroxyalkanoate oligomers. 1999 US Patent Appl 359086. [Google Scholar]
- 22.Chen W, Tong YW. PHBV microspheres as neural tissue engineering scaffold support neuronal cell growth and axon-dendrite polarization. Acta Biomater. 2012;8:540–548. doi: 10.1016/j.actbio.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Zhao K, Chen GQ. Effect of surface treatment on the biocompatibility of microbial polyhydroxyalkanoates. Biomaterials. 2002;23:1391–1397. doi: 10.1016/S0142-9612(01)00260-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhao K, Deng Y, Chun CJ, Chen GQ. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials. 2003;24:1041–1045. doi: 10.1016/S0142-9612(02)00426-X. [DOI] [PubMed] [Google Scholar]
- 25.Weng YX, Wang XL, Wang YZ. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polymer Testing. 2011;30:372–380. doi: 10.1016/j.polymertesting.2011.02.001. [DOI] [Google Scholar]
- 26.Shishatskaya EI, Volova TG. A comparative investigation of biodegradable polyhydroxyalkanoate films as matrices for in vitro cell cultures. J Mater Sci Mater Med. 2004;15:915–923. doi: 10.1023/B:JMSM.0000036280.98763.c1. [DOI] [PubMed] [Google Scholar]
- 27.Unverdorben M, Spielberger A, Schywalsky M, Labahn D, Hartwig S, Schneider M, et al. A polyhydroxybutyrate biodegradable stent: preliminary experience in the rabbit. Cardiovasc Intervent Radiol. 2002;25:127–132. doi: 10.1007/s00270-001-0118-3. [DOI] [PubMed] [Google Scholar]
- 28.Tezcaner A, Bugra K, Hasirci V. Retinal pigment epithelium cell culture on surface modified poly(hydroxybutyrate-co-hydroxyvalerate) thin films. Biomaterials. 2003;24:4573–4583. doi: 10.1016/S0142-9612(03)00302-8. [DOI] [PubMed] [Google Scholar]
- 29.Kose GT, Ber S, Korkusuz F, Hasirci V. Poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) based tissue engineering matrices. J Mater Sci Mater Med. 2003;14:121–126. doi: 10.1023/A:1022063628099. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Peng SW, Wang YY, Zheng SB, Wang Y, Chen GQ. The use of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds for tarsal repair in eyelid reconstruction in the rat. Biomaterials. 2010;31:7512–7518. doi: 10.1016/j.biomaterials.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Dong Y, Li P, Chen CB, Wang ZH, Ma P, Chen GQ. The improvement of fibroblast growth on hydrophobic biopolyesters by coating with polyhydroxyalkanoate granule binding protein PhaP fused with cell adhesion motif RGD. Biomaterials. 2010;31:8921–8930. doi: 10.1016/j.biomaterials.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Wang YW, Wu Q, Chen GQ. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials. 2004;25:669–675. doi: 10.1016/S0142-9612(03)00561-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Wang ZH, Shen CY, You ML, Xiao JF, Chen GQ. Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials. 2010;31:1691–1698. doi: 10.1016/j.biomaterials.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 34.Yu BY, Chen PY, Sun YT, Lee YT, Young TH. The behaviors of human mesenchymal stem cells on the poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) membranes. Desalination. 2008;234:204–211. doi: 10.1016/j.desal.2007.09.087. [DOI] [Google Scholar]
- 35.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 36.Khang G, Kim SW, Cho JC, Rhee JM, Yoon SC, Lee HB. Preparation and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) microspheres for the sustained release of 5-fluorouracil. Biomed Mater Eng. 2001;11:89–103. [PubMed] [Google Scholar]
- 37.Peng Q, Sun X, Gong T, Wu CY, Zhang T, Tan J, et al. Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater. 2013;9:5063–5069. doi: 10.1016/j.actbio.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 38.Shangguan YY, Wang YW, Wu Q, Chen GQ. The mechanical properties and in vitro biodegradation and biocompatibility of UV-treated poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Biomaterials. 2006;27:2349–2357. doi: 10.1016/j.biomaterials.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Qu XH, Wu Q, Liang J, Zou B, Chen GQ. Effect of 3-hydroxyhexanoate content in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on in vitro growth and differentiation of smooth muscle cells. Biomaterials. 2006;27:2944–2950. doi: 10.1016/j.biomaterials.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Li XT, Sun J, Chen S, Chen GQ. In vitro investigation of maleated poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for its biocompatibility to mouse fibroblast L929 and human microvascular endothelial cells. J Biomed Mater Res A. 2008;87:832–842. doi: 10.1002/jbm.a.31890. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Yun H, Gong Y, Zhao N, Zhang X. Effects of surface modification of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) on physicochemical properties and on interactions with MC3T3-E1 cells. J Biomed Mater Res A. 2005;75:985–998. doi: 10.1002/jbm.a.30504. [DOI] [PubMed] [Google Scholar]
- 42.Lu XY, Wang LL, Yang ZQ, Lu HX. Strategies of polyhydroxyalkanoates modification for the medical application in neural regeneration/nerve tissue engineering. Adv Biosci Biotechnol. 2013;4:731–740. doi: 10.4236/abb.2013.46097. [DOI] [Google Scholar]
- 43.Mei N, Zhou P, Pan LF, Chen G, Wu CG, Chen X, et al. Biocompatibility of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) modified by silk fibroin. J Mater Sci Mater Med. 2006;17:749–758. doi: 10.1007/s10856-006-9686-8. [DOI] [PubMed] [Google Scholar]
- 44.Potter M, Steinbuchel A. Poly(3-hydroxybutyrate) granule-associated proteins: impacts on poly(3-hydroxybutyrate) synthesis and degradation. Biomacromolecules. 2005;6:552–560. doi: 10.1021/bm049401n. [DOI] [PubMed] [Google Scholar]
- 45.Lee DE, Hong YD, Choi KH, Lee SY, Park PH, Choi SJ. Preparation and evaluation of 99mTc-labeled cyclic arginine-glycine-aspartate (RGD) peptide for integrin targeting. Appl Radiat Isot. 2010;68:1896–1902. doi: 10.1016/j.apradiso.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Xu XY, Li XT, Peng SW, Xiao JF, Liu C, Fang G, et al. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials. 2010;31:3967–3975. doi: 10.1016/j.biomaterials.2010.01.132. [DOI] [PubMed] [Google Scholar]
- 47.Lomas AJ, Webb WR, Han J, Chen GQ, Sun X, Zhang Z, et al. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate)/collagen hybrid scaffolds for tissue engineering applications. Tissue Eng Part C Methods. 2013;19:577–585. doi: 10.1089/ten.tec.2012.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YW, Wu Q, Chen GQ. Reduced mouse fibroblast cell growth by increased hydrophilicity of microbial polyhydroxyalkanoates via hyaluronan coating. Biomaterials. 2003;24:4621–4629. doi: 10.1016/S0142-9612(03)00356-9. [DOI] [PubMed] [Google Scholar]
- 49.Collins MN, Birkinshaw C. Hyaluronic acid based scaffolds for tissue engineering - a review. Carbohydr Polym. 2013;92:1262–1279. doi: 10.1016/j.carbpol.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 50.Li XT, Zhang Y, Chen GQ. Nanofibrous polyhydroxyalkanoate matrices as cell growth supporting materials. Biomaterials. 2008;29:3720–3728. doi: 10.1016/j.biomaterials.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Sun M, Zhou P, Pan LF, Liu S, Yang HX. Enhanced cell affinity of the silk fibroin-modified PHBHHx material. J Mater Sci Mater Med. 2009;20:1743–1751. doi: 10.1007/s10856-009-3739-8. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Bian YZ, Wu Q, Chen GQ. Evaluation of three-dimensional scaffolds prepared from poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for growth of allogeneic chondrocytes for cartilage repair in rabbits. Biomaterials. 2008;29:2858–2868. doi: 10.1016/j.biomaterials.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 53.Deng Y, Zhao K, Zhang XF, Hu P, Chen GQ. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials. 2002;23:4049–4056. doi: 10.1016/S0142-9612(02)00136-9. [DOI] [PubMed] [Google Scholar]
- 54.Zhao K, Deng Y, Chen G-Q. Effects of surface morphology on the biocompatibility of polyhydroxyalkanoates. Biochem Eng J. 2014;16:115–123. [Google Scholar]
- 55.Bian YZ, Wang Y, Aibaidoula G, Chen GQ, Wu Q. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials. 2009;30:217–225. doi: 10.1016/j.biomaterials.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 56.Qu XH, Wu Q, Liang J, Qu X, Wang SG, Chen GQ. Enhanced vascular-related cellular affinity on surface modified copolyesters of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx) Biomaterials. 2005;26:6991–7001. doi: 10.1016/j.biomaterials.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 57.Lu XY, Zhang Y, Wang L. Preparation and in vitro drug-release behavior of 5-fluorouracil-loaded poly(hydroxybutyrate-co-hydroxyhexanoate) nanoparticles and microparticles. J Appl Polym Sci. 2010;116:2944–2950. [Google Scholar]
- 58.Peng Q, Zhang ZR, Gong T, Chen GQ, Sun X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials. 2012;33:1583–1588. doi: 10.1016/j.biomaterials.2011.10.072. [DOI] [PubMed] [Google Scholar]


