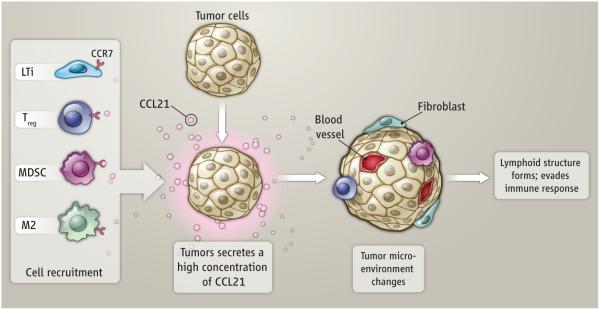
Host immunity can contribute to the elimination of malignant tumors, and cancer growth is often enhanced when immunity is suppressed. Many types of tumors have developed mechanisms to suppress the immune system in order to enhance their survival. Some tumors can escape immune detection by down-regulating their own expression of HLA class I proteins, rendering them invisible to cytotoxic T lymphocytes (1). More commonly, tumors actively suppress immune effector mechanisms by secreting proteins (such as TGF-β or IL-10) that inhibit effector CD8+ T cell responses and promote induction of Treg cells (2). On page _ of this issue, Shields et al. identify a novel mechanism used by melanomas to deceive the immune system (3). Chemokine CCL21-expressing melanomas recruit CCR7+ lymphoid tissue inducer (LTi) cells and reorganize portions of their tumor stroma into lymphoid tissue-like structures. This ingenuous stromal reconstruction directs the recruitment and maintenance of at least two types of suppressor immune cells, CD4+ regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSC), both of which suppress immune recognition and effector functions, thereby promoting immune tolerance and tumor progression.
LTi cells play a crucial role in mammalian development, contributing to the organogenesis of secondary lymphoid tissues, including lymph nodes and Peyer’s patches (4, 5). They express the TNF family members TNF, lymphotoxin (LT)α1β2, and TNF-related activation-induced cytokine (TRANCE) that work together to induce up-regulation of chemokines and adhesion molecules by stromal ‘organizer’ cells during fetal development (6). As the stroma of the lymphoid organ develops, its expression of the adhesion molecules ICAM-1 and VCAM-1 and the chemokines CXCL13 and CCL21 supports stable interactions between LTi and stromal cells and the recruitment of CXCR5+ B-lymphocytes and CCR7+ LTi cells and T-lymphocytes into the developing tissue (7). Ultimately, this localized stromal cell activation triggered by fetal LTi cells results in formation of an organized, compartmentalized lymphoid organ that is prepared as a site for generation of a regulated immune response.
In adult animals, LTi cells are thought to support maintenance of mature spleen architecture (8). Under conditions of persistent inflammation or infection, adult LTi cells can induce reorganization of stromal cells to form ectopic tertiary lymphoid structures (9, 10). This week’s report by Shields et al., that melanomas expressing CCL21 can recruit LTi cells, which reorganize the tumor’s stromal elements and recruit Treg cells, MDSC and other leucocytes, adds malignant transformation to the array of signals that can lead to formation of ectopic lymphoid tissue-like structures. Since other signaling pathways besides CCL21 contribute to secondary and tertiary lymphoid tissue development, it is likely that other tumor types expressing other signaling proteins will be found to induce similar stromal cell reconstruction.
Secondary and tertiary lymphoid tissues are designed to provide an environment favorable for activation of humoral and cellular immunity. Since tumors thrive on escape from immune responses, what is the benefit for the tumor to create surroundings with characteristics of lymphoid tissue? The association of lymphoid structures with tumors underscores the fact that lymphoid tissues act importantly not only to activate but also to down-regulate immune effector pathways. For example, stromal cells in lymph nodes have been shown to present antigen to CD8+ T cells in a way that induces tolerance rather than activation (11). Additionally, lymphoid tissues provide an environment in which naïve T cells, in the presence of TGF-β, can be converted to Treg cells, further favoring suppression of T cell effector functions. By mimicking these functions of normal secondary lymphoid tissues, tumor-induced lymphoid-like stromal structures would promote immune tolerance and suppression.
Do melanoma cells act directly to block or reduce the differentiation of naïve T cells into effector cells? Or, rather, do they act indirectly by recruiting immunoregulatory antigen-presenting cells (APC) into the lymphoid-like structures? Shields et al. observed that B-lymphocytes were not recruited to the site of melanoma cell accumulation. Since B cells play an important role in priming T cells in secondary lymphoid tissues, perhaps their absence reduces priming of T cells within the tumor-induced stroma. Alternatively, TGF-β produced in the tumor may alter local macrophage populations resulting in a switching from classically activated, phagocytic and proinflammatory M1 macrophages to alternatively activated, poorly phagocytic and anti-inflammatory M2 macrophages (also known as tumor-associated macrophages (TAMs)) (12, 13). M2 macrophages secrete extracellular matrix proteins leading to a more robust tissue matrix. It will be of interest to determine whether tumor products regularly alter the type of antigen-presenting cells in the tumor microenvironment in ways that influence the tone of the immune response and promote stromal stability. In particular, understanding how tumor-induced changes in the stroma influence different immune cells will be important for determining ways to modulate these structures. The involvement of LTi cells in the melanoma-induced lymphoid-like structures observed by Shields et al. suggests that LTα1β2 may be an important signal increasing expression of CCL21. This may be significant because generally LTα1β2-dependent structures are plastic. If so, they should be reprogrammable by modulation of LTα1β2 signaling as has been shown for the stromal compartments in the white pulp of the spleen (14). Understanding the extent to which the tumor-induced lymphoid-like structure are plastic will go a long way towards determining if blocking CCL21 and/or LTi function can disrupt the tolerogenic tumor-induced structures, releasing host immunity to aid in elimination of the malignant cells.
Figure 1. Formation of Lymphoid Stroma.
CCL21-expressing tumor cells recruit CCR7+ LTi cells, Treg cells, and MDSCs. LTi cells direct the reorganization of local stroma into lymphoid-like structures that support maintenance of Treg cells and MDSCs that lead to immune tolerance of the tumor. TAMs secrete ECM proteins that promote stromal stability.
References
- 1.Meissner M, et al. Clin Cancer Res. 2005 Apr 1;11:2552. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Nat Med. 2004 Sep;10:900. doi: 10.1038/nm0904-900. [DOI] [PubMed] [Google Scholar]
- 3.Shields JD, Kourtis IC, Tomei AA, Swartz MA. Science. 2010;328 doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 4.Mebius RE, Rennert P, Weissman IL. Immunity. 1997 Oct;7:493. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, et al. Int Immunol. 1999 May;11:643. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 6.Kim D, et al. J Exp Med. 2000 Nov 20;192:1467. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansel KM, et al. Nature. 2000 Jul 20;406:309. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 8.Withers DR, et al. Eur J Immunol. 2007 Nov;37:3240. doi: 10.1002/eji.200737541. [DOI] [PubMed] [Google Scholar]
- 9.Aloisi F, Pujol-Borrell R. Nat Rev Immunol. 2006 Mar;6:205. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 10.Cupedo T, Jansen W, Kraal G, Mebius RE. Immunity. 2004 Nov;21:655. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, et al. Nat Immunol. 2007 Feb;8:181. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Trends Immunol. 2002 Nov;23:549. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 13.Sica A, Allavena P, Mantovani A. Cancer Lett. 2008 Aug 28;267:204. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Zindl CL, et al. Immunity. 2009 Mar 20;30:408. doi: 10.1016/j.immuni.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]