One of the challenges in development of molecular models of hypertension as a disease with end-organ failure is the multi-faceted nature of complications that accompany the elevated blood pressure. Co-morbidities can be detected even at early stages of hypertension and in patients with family history of hypertension even before the elevation of the blood pressure 1. Well-documented co-morbidities to hypertension are diabetes, cardiac and large artery remodeling with hypertrophy and fibrosis, atherosclerosis and renal pathogenesis. Hypertension is also accompanied by microvascular rarefaction, immune suppression, and apoptosis, to name just a few such complications. To date no satisfactory mechanism has been proposed to put this manifold of pathophysiological phenomena under one conceptual roof and at the same time provide an explanation for the elevated arterial blood pressure. Oxygen free radical production in hypertensives could be argued to form a bridge between elevated blood pressure and vascular complications. Reactive oxygen species serve as signaling molecules for elevated artery/arteriolar tone by reaction with nitric oxide and they are also involved in cell injury. But no molecular details have emerged in any model of hypertension to provide a conclusive picture for the role of free radicals in some of the more specific cell dysfunctions that accompany hypertension.
Instead an increasing body of evidence implicates an uncontrolled proteolytic process as one of the underlying mechanisms in hypertension, including the family of matrix metalloproteinases (MMPS). MMPs are likely more than just extracellular-matrix-remodeling proteases. Their activity in major complications associated with hypertension, e.g. atherosclerosis and stroke, is well documented 2, 3.
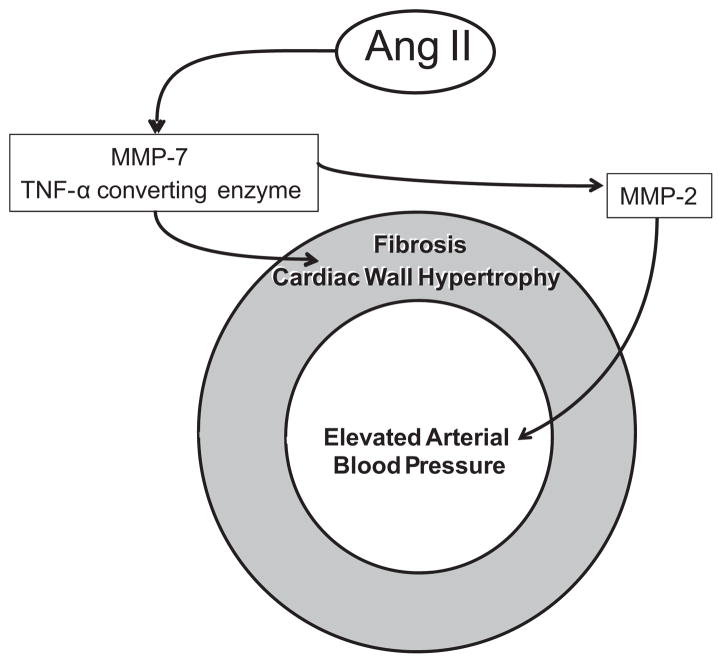
One is justified to ask: Is there a possibility that MMPs are already involved in the early stages of hypertension? Furthermore, is there a possibility that MMPs are actually the mediators that cause hypertension and at the same time may cause co-morbidities? In the article by Dr. Fernandez-Patron and his colleagues 4 evidence is presented in the Angiotensin II (Ang II)–induced hypertensive mouse that MMPs are part of the mechanisms responsible for elevated blood pressure and are also involved in cardiac wall hypertrophy and fibrosis via a previously unrecognized MMP signaling cascade. Following earlier leads suggesting that the TNF-α converting enzyme (TACE) 5 and MMP-7 6 mediate cardiac hypertrophy and fibrosis and that knockdown of TACE serves to partially attenuate MMP-2, the authors demonstrate that TACE and MMP-7 siRNA treatment prevent the MMP-2 activity (Figure 1). The simultaneous knockdown of TACE and MMP-7 also attenuates blood pressure elevation in the Ang-II treated mice as well as the development of cardiac hypertrophy and fibrosis as documented by a variety of cardiac indices and fibrotic markers. In contrast, MMP-2 inhibition by gene knockdown and pharmacological means serves to prevent only a rise in blood pressure but did little to prevent the hypertrophy. This evidence points towards a transcriptional regulation of MMP-2 by MMP-7 and TACE. While AngII-induced cardiovascular disease is signaled via multiple MMPs pathways with unique physiological roles the MMP-2 modulates only the blood pressure in this model of hypertension. MMP-7, which participates in a number of fibrotic processes, is able to modulate the cardiac hypertrophy but needs to activate MMP-2 to modulate blood pressure.
Figure 1.
Schematic of Matrix Metalloproteinases (MMPs) differential signaling in the arterial wall of Angiotensin II (AngII)–induced hypertension and cardiac wall hypertrophy.
What molecules could serve as MMP-2 substrates? Besides cleavage of big endothelin or calcitonin gene-related peptide, shown by the Fernandez-Patron group, adrenomedullin, a vasodilator and inhibitor of myocardial fibrosis expressed in vascular smooth muscle and in endothelium, may serve as a substrate for MMM-2 that generates a series of peptides, which have in part vasoconstrictor activity. Thus MMP-2 may have a dual role and a direct impact on the vascular tone in arteries and arterioles 7. To what degree this MMP-2 mediated hypertension is unique to the Ang-II hypertensive model remains to be determined.
In the spontaneously hypertensive rat, a model with multiple elevated MMP levels, we proposed an alternative hypertensive mechanism by MMP-mediated cleavage of the β2-adrenergic receptor 8. In the presence of receptor cleavage, the normal vasodilatory stimulus provided by this receptor upon agonist binding is suppressed causing a lack of vasodilatory input to the arterial/arteriolar tone and consequently an arterial blood pressure elevation. An interesting feature of this mechanism is that proteolytic receptor cleavage may affect others, e.g. the insulin receptor thereby causing insulin resistance (i.e. type II diabetes) or the vascular endothelial growth factor receptor causing endothelial apoptosis and capillary rarefaction.
The role of the MMPs in the development of hypertension and its co-morbidities is likely to be a fruitful area of exploration. There may be a possibility to uncover other MMP-specific signaling cascades for co-morbidities in hypertension. Diverse leads exist in a rich literature on MMPs. For example, MMP14 (MT-1 MMP) has been implicated in cell migration through the extracellular matrix via its ability to break down the extracellular matrix proteins 9 and at the same time in fibroblast proliferation during cardiac fibrosis 10.
Possible future management of MMPs will require a nuanced approach since the MMPs also play a major role in tissue repair. Interventions against MMPs will depend not only on the activity of specific members of the MMP family and their endogenous inhibitors, but also on the organ specific patterns in which the MMPs are synthesized and activated. Understanding eventually the genetic and/or environmental cause of the MMP activation may be one of the major requirements for optimal management of hypertension and its complications.
Acknowledgments
Sources of Funding: Supported by NHLBI grant HL 10881
This is a commentary on article Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, Kassiri Z, Fernandez-Patron C. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2011;57(1):123-30.
Footnotes
Conflicts: NONE.
References
- 1.Lacy F, O'Connor DT, Schmid-Schönbein GW. Plasma hydrogen peroxide production in hypertensives and normotensive subjects at genetic risk of hypertension. J Hypertens. 1998;16:291–303. doi: 10.1097/00004872-199816030-00006. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez JA, Orbe J, Martinez de Lizarrondo S, Calvayrac O, Rodriguez C, Martinez-Gonzalez J, Paramo JA. Metalloproteinases and atherothrombosis: MMP-10 mediates vascular remodeling promoted by inflammatory stimuli. Front Biosci. 2008;13:2916–2921. doi: 10.2741/2896. [DOI] [PubMed] [Google Scholar]
- 3.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, Kassiri Z, Fernandez-Patron C. MMP-2 mediates angiotensin ii-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.110.159525. in press. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, Kassiri Z, Fernandez-Patron C. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension. 2009;54:575–582. doi: 10.1161/HYPERTENSIONAHA.108.127670. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Chow FL, Oka T, Hao L, Lopez-Campistrous A, Kelly S, Cooper S, Odenbach J, Finegan BA, Schulz R, Kassiri Z, Lopaschuk GD, Fernandez-Patron C. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119:2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 7.Martinez A, Oh HR, Unsworth EJ, Bregonzio C, Saavedra JM, Stetler-Stevenson WG, Cuttitta F. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J. 2004;383:413–418. doi: 10.1042/BJ20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues SF, Tran ED, Fortes ZB, Schmid-Schönbein GW. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H25–35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh Y. MT1-MMP: a key regulator of cell migration in tissue. IUBMB Life. 2006;58:589–596. doi: 10.1080/15216540600962818. [DOI] [PubMed] [Google Scholar]
- 10.Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]