Abstract
Objective
To clarify the role of interleukin-20 (IL-20) in the regulatory mechanism of extracellular matrix expression and to determine the contribution of IL-20 to the phenotype of systemic sclerosis (SSc).
Methods
Protein and messenger RNA (mRNA) levels of collagen, Fli-1, IL-20, and IL-20 receptor (IL-20R) were analyzed using polymerase chain reaction (PCR) array, immunoblotting, immunohistochemical staining, enzyme-linked immunosorbent assay, and real-time PCR.
Results
PCR array revealed that IL-20 decreased gene expression of α2(I) collagen (0.03-fold), Smad3 (0.02-fold), and endoglin (0.05-fold) in cultured normal dermal fibroblasts. Fli-1 protein expression was induced by IL-20 (~2-fold). The inhibition of collagen by IL-20, the induction of Fli-1 by IL-20, and the reduction of Smad3 and endoglin by IL-20 were also observed in SSc fibroblasts. Serum IL-20 levels were reduced only slightly in SSc patients but were significantly decreased in patients with scleroderma spectrum disorders (the prodromal stage of SSc) compared with those in normal subjects (111.3 pg/ml versus 180.4 pg/ml; P < 0.05). On the other hand, IL-20 mRNA expression in SSc skin was decreased compared with that in normal skin (P < 0.05), which may result in the induction of collagen synthesis in SSc dermal fibroblasts. IL-20R was expressed in normal and SSc fibroblasts. Moreover, IL-20 supplementation by injection into the skin reversed skin fibrosis induced by bleomycin in mice (~0.5-fold).
Conclusion
IL-20 reduces basal collagen transcription via Fli-1 induction, while down-regulation of Smad3 and endoglin may cancel the effect of transforming growth factor β in SSc fibroblasts. To confirm the therapeutic value of IL-20 and IL-20R, their function and expression in vivo should be further studied.
Systemic sclerosis (SSc) or scleroderma is one of the rheumatic diseases characterized by tissue fibrosis of the skin and internal organs. In the skin, thickened dermis due to uncontrolled excessive deposition of extracellular matrix (ECM), mainly type I collagen (which consists of α1[I] and α2[I] collagen), is a hallmark of this disease (1,2). The source of the increased ECM is thought to be dermal fibroblasts activated by interactions with endothelial cells, lymphocytes, or macrophages, via various mediators including cytokines and growth factors (3,4). For example, many researchers have suggested that transforming growth factor β1 (TGFβ1) may play a central role in fibroblast activation (5–7). In addition, connective tissue growth factor (7), platelet-derived growth factor (8), insulin-like growth factor, interleukin-1 (IL-1), IL-6, and IL-7 are reported to be involved in the pathogenesis of this disease (9–12). Accordingly, investigating the cytokine network mediating fibroblast activation of SSc is essential to understand the molecular mechanism(s) of this disease.
IL-20 is identified as a member of the IL-10 family (13), which includes IL-10, IL-19, IL-22, IL-24 (melanoma differentiation–associated protein 7), and IL-26 (AK155). Although IL-19, IL-20, and IL-24 have partial homology in their amino acid sequences and share their receptor (14), the main biologic effects of these 3 cytokines seem quite distinct. IL-19 and IL-24 are mainly implicated in immune activity and tumor apoptosis, respectively (15–17). On the other hand, IL-20 is expressed by multiple cell types, including monocytes and skin keratinocytes, and is implicated in the pathogenesis of autoimmune diseases. IL-20 was reportedly elevated in synovial fluid from patients with rheumatoid arthritis (18). Moreover, IL-20 is thought to be correlated with the etiology of lupus nephritis (19,20). In addition, IL-20 expression and IL-20 receptor (IL-20R) complexes are dramatically up-regulated in psoriatic skin lesions (21). However, there have been no reports that validate the function of IL-20 in the regulation of ECM or the pathogenesis of SSc.
In the present study, we investigated the effect of IL-20 on ECM expression in normal fibroblasts. In addition, we compared the expression pattern of IL-20 in the sera and skin between normal subjects and SSc patients, and we demonstrated the involvement of IL-20 signaling in the abnormal ECM regulation seen in SSc.
PATIENTS AND METHODS
Reagents
Recombinant human IL-19, IL-20, and IL-24 and mouse IL-20 were obtained from R&D Systems.
Patients
Serum samples were obtained from 33 patients with SSc (5 males and 28 females, mean age 58.7 years [range 24–85 years]); 13 had diffuse cutaneous SSc (dcSSc), and 20 had limited cutaneous SSc. All patients fulfilled the classification criteria proposed by the American College of Rheumatology (ACR) (22). We also included in the study 10 patients with systemic lupus erythematosus (SLE), 12 patients with dermatomyositis (DM), and 9 patients with scleroderma spectrum disorders who did not fulfill the ACR classification criteria for SSc but who we thought would meet these criteria in the future (23–25) (further information available from the corresponding author). Control serum samples were also obtained from 15 healthy volunteers. Skin biopsy specimens of lesional skin were obtained from SSc patients. Control samples of routinely discarded skin were obtained from healthy human subjects undergoing skin grafts. Institutional review board approval and written informed consent according to the Declaration of Helsinki were obtained before patients and healthy volunteers were entered into this study.
Diagnosis of scleroderma spectrum disorders by the point system
A total score was evaluated as the sum of 5 factors for the diagnosis of scleroderma spectrum disorders (23–25):
Skin sclerosis: swollen fingers = 1 point, sclerodactyly = 3 points, proximal sclerosis = 5 points, diffuse sclerosis = 10 points.
Pulmonary changes: pulmonary fibrosis accompanied by forced vital capacity (FVC) ≥80% predicted = 2 points, pulmonary fibrosis accompanied by FVC <80% predicted = 4 points.
Antinuclear antibodies (ANAs): anti–topoisomerase I antibody = 5 points, anticentromere or anti–U1 RNP antibody = 3 points, antinucleolar antibody = 2 points, other ANAs = 1 point.
Pattern of Raynaud’s phenomenon: biphasic or bilateral = 1 point, biphasic and bilateral = 2 points, triphasic = 3 points.
Nailfold bleeding: 1 or 2 fingers =1 point, ≥3 fingers = 2 points.
A total score of 5–8 is consistent with scleroderma spectrum disorders, and a total score of ≥9 is consistent with SSc.
Cell cultures
Human dermal fibroblasts were obtained by skin biopsies of the affected areas (dorsal forearm) of 7 healthy human subjects and dcSSc patients (26). Institutional review board approval and written informed consent were obtained according to the Declaration of Helsinki. Independently isolated monolayer cultures of fibroblasts obtained from different individuals were maintained at 37°C in 5% CO2 in air. Cells were serum-starved for 24 hours before all experiments.
Cell lysis and immunoblotting
Cultured fibroblasts were washed twice with cold phosphate buffered saline (PBS) and lysed in Denaturing Cell Extraction Buffer (BioSource International). Aliquots of the cell lysates (normalized for protein concentrations) were separated by electrophoresis on 10% sodium dodecyl sulfate–polyacrylamide gels and transferred onto PVDF filters. The PVDF filters were then incubated with antibodies against type I collagen (SouthernBio-tech) or against Ets-1, Fli-1, IL-20R, or β-actin (all from Santa Cruz Biotechnology). The filters were incubated with secondary antibody, and the immunoreactive bands were visualized using an ECL system (Amersham Biosciences).
Immunohistochemistry
Wax-embedded sections (4-μm thickness) were dewaxed in xylene and rehydrated in graded alcohols. For the immunostaining of IL-20 and Smad3, antigens were retrieved by incubation with citrate buffer (pH 6) for 9 minutes with a microwave. Endogenous peroxidase activity was inhibited with a solution of 0.3% H2O2 in methyl alcohol, after which sections were blocked with 5% donkey serum for 20 minutes and then reacted with antibodies to IL-20 (Abcam) or Smad3 (Santa Cruz Biotechnology) at 4°C. After excess antibody was washed out with PBS, samples were incubated with horseradish peroxidase (HRP)–labeled anti-mouse antibody (Nichirei Biosciences) at 37°C.
For immunostaining of IL-20R or Fli-1, antigens were retrieved by incubation with citrate buffer (pH 9) for 9 minutes with a microwave. Antibodies to IL-20R (Abcam) and Fli-1 (Santa Cruz Biotechnology) and HRP-labeled anti-rabbit antibody (Nichirei Biosciences) were used.
The reaction was visualized using a diaminobenzidine substrate system (Dojin). Slides were counterstained with Mayer’s hematoxylin and examined under a light microscope (BX50; Olympus).
RNA isolation, array analysis, and quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted from culture cells with Isogen (Nippon Gene) or from paraffin-embedded sections with an RNeasy FFPE kit (Qiagen). For the array, first-strand complementary DNA (cDNA) was synthesized from RNA using an RT2 First Strand Kit (Qiagen). Complementary DNA was mixed with RT2 SYBR Green/Rox qPCR Master Mix (Qiagen), and the mixture was added to a 96-well Extracellular Matrix and Adhesion Molecules PCR Array (Qiagen). PCR was performed on a Takara Thermal Cycler Dice (TP800) instrument (Takara Bio). The threshold cycle (Ct) for each RNA was extracted using Thermal Cycler Dice Real Time System software, version 2.10B (Takara Bio). The raw Ct was normalized using the values of housekeeping genes. For quantitative real-time PCR, first-strand cDNA was synthesized using a PrimeScript RT reagent Kit (Takara Bio) (27). GAPDH primer was purchased from Qiagen, and other primers were purchased from Takara Bio. Using a Takara Thermal Cycler Dice instrument, DNA was amplified for 40 cycles of denaturation for 5 seconds at 95°C and annealing for 30 seconds at 60°C. The relative expression of each gene of interest and GAPDH was calculated using a standard curve method.
Immunoprecipitation
Phosphorylated Fli-1 was detected as described previously (28). For the detection of acetylated Fli-1, cells were lysed in Pierce IP lysis buffer with the Halt phosphatase inhibitor cocktail (Thermo Scientific Pierce). Cell lysates were precleared with protein G–Sepharose (GE Healthcare) and then were incubated with monoclonal mouse anti–Fli-1 antibody (BD Biosciences) and protein G–Sepharose beads overnight at 4°C. The immunoprecipitated proteins were washed with Pierce buffer. Agarose-bound proteins were extracted by incubation in sample buffer at 95°C. The sample was then assessed by immunoblotting with anti–acetylated lysine antibody (Cell Signaling Technology). The membrane was stripped and reprobed with rabbit polyclonal anti–Fli-1 antibody (Santa Cruz Biotechnology) (29).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed using an EpiQuik ChIP kit (Epigentek) (28). Briefly, after cells were treated with 1% formaldehyde for 10 minutes, the crosslinked chromatin was sonicated. The DNA fragments were immunoprecipitated with IgG isotype control antibody or polyclonal anti–Fli-1 antibody at room temperature. After crosslinking was reversed, the immunoprecipitated chromatin was amplified by PCR of a specific region of the α2(I) collagen genomic locus. The primers used were 5′-CTGGACAGCTCCTGCTTTGAT-3′ (forward) and 5′-C-TTTCAAGGGGAAACTCTGACTC-3′ (reverse). The amplified DNA products were run out on an agarose gel containing ethidium bromide.
Plasmid construction
A construct consisting of the full-length human α2(I) collagen gene fragment linked to the chloramphenicol acetyltransferase (CAT) reporter gene and a series of 5′-deletions of the construct were generated as previously described (30).
Transient transfection
For CAT assay, fibroblasts were transfected with promoter constructs employing Lipofectamine 2000 (Invitrogen), as described previously (30). In order to correct minor variations in transfection efficiency, pSV-β-galactosidase vector (Promega) was included in all transfections.
For reverse transfection of small interfering RNAs (siRNAs), siRNAs were mixed with Lipofectamine RNAiMAX (Invitrogen) and then added when the cells were plated, followed by incubation at 37°C in 5% CO2. Small interfering RNA against Fli-1 and control siRNA were purchased from Dharmacon.
CAT assay
After 48 hours of incubation after the transfection of constructs with CAT reporter, cells were harvested. CAT activity in cell lysates was assayed colorimetrically using a CAT ELISA kit (Roche) (31).
Immunofluorescence microscopy
Fibroblasts were grown in 4-well LAB TEK chambers (Nunc) to subconfluence as described above. Immunofluorescence was performed using antibody to IL-20R as primary antibody and fluorescein isothiocyanate–conjugated donkey anti-goat IgG (Santa Cruz Biotechnology) as secondary antibody. A Zeiss fluorescence microscope was used (6) to visualize fluorescence.
Measurement of serum IL-20 concentrations
Serum IL-20 levels were measured with a specific enzyme-linked immunosorbent assay (ELISA) kit (Human IL-20 Immunoassay; R&D Systems) (27,32). Briefly, antibody to IL-20 was precoated onto microtiter wells. Aliquots of serum were added to each well, followed by peroxidase-conjugated antibodies to IL-20. Color was developed with H2O2 and tetramethylbenzidine peroxidase, and absorbance at 450 nm was measured. Wavelength correction was performed by absorbance at 540 nm. The concentration of IL-20 in each sample was determined by interpolation from a standard curve.
Intradermal treatment with bleomycin
Bleomycin (Nippon Kayaku) was dissolved in PBS at a concentration of 1 mg/ml and sterilized by filtration. Bleomycin (300 μg) or PBS was injected intradermally into the shaved backs of 6-week-old BALB/c mice 6 times per week for 1 month, as described previously (33,34). Injection was performed using a 27-gauge needle. The back skin was removed on the day after the final injection. The skin samples were then fixed in 10% formalin solution and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E). Dermal thickness was evaluated by 2 investigators (HK, WN) in a blinded manner who measured the distance between the epidermal–dermal junction and the dermal–fat junction in H&E-stained sections under 100× magnification.
Collagen assay
The amount of collagen in the paraffin-embedded skin sections was quantified using a Semi-Quantitative Collagen Assay Kit (Chondrex). After being dewaxed in xylene and rehydrated in graded alcohols, 20-μm thick sections were immersed in staining solution at room temperature for 30 minutes. The staining solution was removed and a bleaching solution was added to measure the absorbance at 540 nm and 605 nm in an ND-1000 spectrophotometer (NanoDrop Technologies). The collagen concentration was calculated based on the following formula: collagen (μg/section) = (optical density [OD] at 540 nm – [OD605 × 0.291])/37.8 × 1,000 (35).
Statistical analysis
Data presented as bar graphs are the mean ± SEM of at least 3 independent experiments. The statistical analysis was carried out using the Mann-Whitney U test for the comparison of medians and Fisher’s exact probability test for the analysis of frequency. P values less than 0.05 were considered significant.
RESULTS
Effect of IL-20 on expression of ECM-related genes in cultured normal dermal fibroblasts
As an initial experiment, to determine the effect of IL-20 on ECM expression, we performed a PCR array of 84 ECM-related genes using RNA obtained from 3 dermal fibroblasts either unstimulated or stimulated with IL-20 for 12 hours. When a 16-fold difference by the ΔΔCt method was considered meaningful, 3 of the 84 genes were up-regulated and 13 genes were down-regulated in IL-20–treated fibroblasts in comparison with untreated cells (Table 1). Among them, human α2(I) collagen gene expression was decreased 0.03-fold by IL-20. Consistent with the array result, quantitative real-time PCR using specific primers for α1(I) or α2(I) collagen and an increased number of samples (n = 7) showed that collagen messenger RNA (mRNA) expression was significantly reduced by IL-20 (Figure 1A). Furthermore, immunoblotting revealed that the protein synthesis of type I collagen was also decreased in a dose-dependent manner by treatment with IL-20, and the decrease was statistically significant (P < 0.05) (Figure 1B).
Table 1.
Expression profiles of extracellular matrix–related genes in the presence or absence of IL-20 in the PCR array*
| Gene symbol | Gene name | Fold change |
|---|---|---|
| Genes up-regulated by IL-20 | ||
| THBS2 | Thrombospondin 2 | 113.77 |
| TGIF1 | TGFB-induced factor homeobox 1 | 57.28 |
| MMP9 | Matrix metallopeptidase 9 | 33.13 |
| Genes down-regulated by IL-20 | ||
| MMP3 | Matrix metallopeptidase 3 | 0.02 |
| SERPINA1 | Serpin peptidase inhibitor, clade A, member 1 | 0.02 |
| SMAD3 | SMAD family member 3 | 0.02 |
| DCN | Decorin | 0.03 |
| PLAU | Plasminogen activator, urokinase | 0.03 |
| LOX | Lysyl oxidase | 0.03 |
| COL1A2 | Collagen, type I, α2 | 0.03 |
| ENG | Endoglin | 0.05 |
| CAV1 | Caveolin 1, caveolae protein, 22 kd | 0.05 |
| IL5 | Interleukin 5 | 0.06 |
| FASLG | Fas ligand | 0.06 |
| IL10 | Interleukin 10 | 0.06 |
| IL1A | Interleukin 1, α | 0.06 |
A mixture of equal amounts of mRNAs from 3 normal fibroblasts was prepared in the presence or absence of interleukin-20 (IL-20), and the mRNA expression profile was evaluated using a polymerase chain reaction (PCR) array. Fold change was calculated as 1/2(raw Ct of each mRNA – Ct of housekeeping genes). Genes up- or down-regulated >16-fold by IL-20 stimulation are shown.
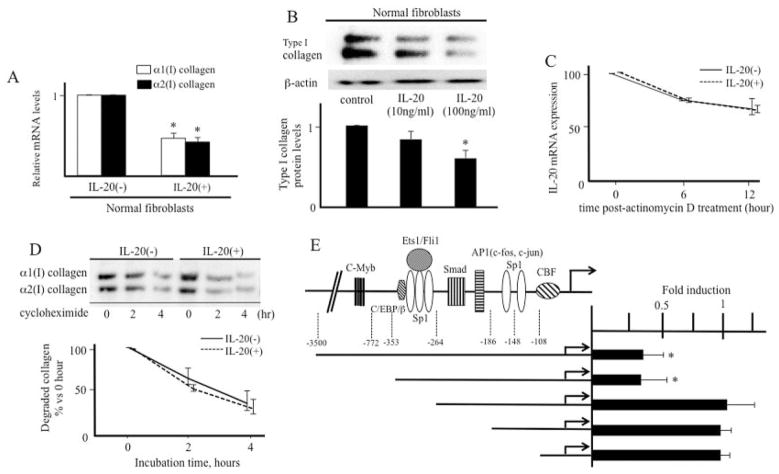
Figure 1.
Effect of interleukin-20 (IL-20) on collagen expression. A, Normal fibroblasts were treated with IL-20 (100 ng/ml) for 12 hours. Levels of collagen mRNA were determined by real-time polymerase chain reaction (PCR) (n = 7 samples). ⅞* = P < 0.05 versus untreated fibroblasts (set to 1.0). B, Normal fibroblasts were treated with IL-20 (100 ng/ml) for 24 hours. Cell lysates were subjected to immunoblotting. The protein levels of type I collagen quantitated by scanning densitometry and corrected for the levels of β-actin in the same samples are shown relative to those in untreated fibroblasts (set to 1.0) (n = 3 samples). * = P < 0.05 versus untreated fibroblasts. C, Fibroblasts were incubated in the presence or absence of IL-20 for 12 hours before the addition of 2.5 μg/ml actinomycin D for 6 or 12 hours. IL-20 mRNA expression was analyzed by real-time PCR. D, Fibroblasts were incubated in the presence or absence of IL-20 for 24 hours before the addition of cycloheximide (10 μg/ml). Cells were harvested at the indicated time points after cycloheximide was administered, and immunoblotting was performed. The protein levels of type I collagen quantitated by scanning densitometry and corrected for the levels of β-actin in the same samples are shown relative to those in untreated fibroblasts (set to 1.0) (n = 3 samples). E, The indicated α2(I) collagen promoter deletion constructs were transfected into normal fibroblasts in the absence or presence of IL-20 (100 ng/ml) for 24 hours (n = 3 samples). The bar graph represents fold stimulation of chloramphenicol acetyltransferase activities stimulated with IL-20 relative to those not stimulated with IL-20 (set to 1.0). * = P < 0.05 versus cells not stimulated with IL-20. Values are the mean ± SEM. c/EBPβ = CCAAT/enhancer binding protein β; AP-1 = activator protein 1; CBF = CCAAT-binding transcription factor.
To determine whether the down-regulation of collagen by IL-20 takes place at the transcriptional level or translational level, stability of collagen mRNA was examined. Because the steady-state level of mRNA is controlled by the level of gene transcription and/or the stability of mRNA, de novo mRNA synthesis was blocked by the RNA synthesis inhibitor actinomycin D in normal fibroblasts in the presence or absence of IL-20. As shown in Figure 1C, after actinomycin D treatment, there was no significant difference in the decrease rate of α2(I) collagen mRNA between cells with and those without IL-20. Similarly, when de novo protein synthesis and the expression of proteolytic enzymes were blocked with cycloheximide, IL-20 stimulation had little effect on the half-lives of collagen protein (Figure 1D). Taken together, these results indicate that the stability of collagen mRNA and protein was not altered by IL-20, and proteolytic enzymes were less likely to be involved in the effect of IL-20 on collagen expression. Thus, collagen synthesis is thought to be decreased by IL-20 at the transcriptional level.
Next, to identify the element of the human α2(I) collagen promoter that responds to IL-20 stimulation, we compared activities of serial 5′-deletions of the promoter linked to the CAT reporter gene in the presence or absence of IL-20 in normal fibroblasts (Figure 1E). The full-length −3500 to +58 bp construct and the shorter construct with deletion end points at −353 to +58 bp showed a similar fold decrease of promoter activities by IL-20 relative to the values in untreated fibroblasts (~0.3-fold). The −264 to +58 bp construct and shorter constructs did not respond to IL-20 stimulation, indicating that the element of the α2(I) collagen promoter gene that responds to IL-20 is located between −353 and −264 bp.
This region contains the binding sites for Sp1, Ets family transcription factors (e.g., Ets-1 and Fli-1), and CCAAT/enhancer binding protein β (c/EBPβ) (Figure 1E). According to the results of the PCR array, the expression of c/EBPβ and Sp1 was not affected by IL-20 (not shown). Real-time PCR showed that IL-20 induced the expression of Fli-1 but not that of Ets-1 (Figure 2A). In addition, the protein expression of Fli-1 was also increased by IL-20 (Figure 2B). Fli-1 is thought to share binding sites with Ets-1, and it inhibits α2(I) collagen promoter activity by competing with Ets-1 using an over-expression system in vitro (36,37), indicating that the balance of Ets-1 and Fli-1 controls collagen expression (36,38). We found that the ratio of Ets-1:Fli-1 protein expression was significantly decreased by IL-20. Several lines of evidence revealed that posttranslation modification, such as phosphorylation and acetylation, tightly regulates the protein stability and transcriptional activity of Fli-1 (29). Although both phosphorylation and acetylation of Fli-1 were not changed by IL-20 (Figure 2C), the cytokine increased the association of Fli-1 with the collagen promoter in chromatin immunoprecipitates (Figure 2D). These data indicate that IL-20 increased FIi-1 protein through the induction of Fli-1 mRNA levels directly without the alterations of phosphorylation and acetylation.
Figure 2.
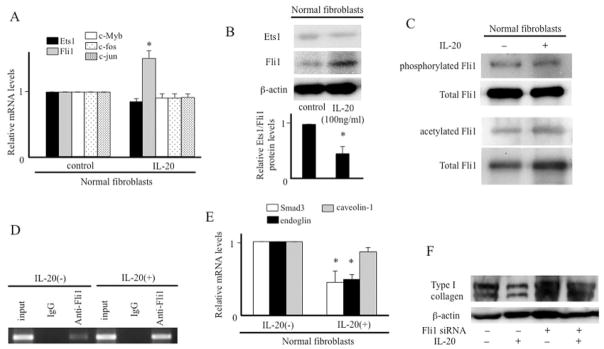
Effect of interleukin-20 (IL-20) on Fli-1 expression. A, Normal fibroblasts were incubated in the presence or absence of IL-20 for 12 hours. Messenger RNA levels were determined by real-time polymerase chain reaction (PCR) (n = 7 samples). * = P < 0.05 versus untreated fibroblasts (set to 1.0). B, Normal fibroblasts were treated with IL-20 for 24 hours. Immunoblotting was performed using antibody to Ets-1 or Fli-1. Protein levels of Ets-1 and Fli-1 were quantitated as described in Figure 1B, and the ratio of Ets-1:Fli-1 protein expression is shown. * = P < 0.05 versus untreated fibroblasts (set to 1.0) (n = 3 samples). C, Normal fibroblasts were treated with IL-20 (100 ng/ml) for 24 hours. Levels of phosphorylated and acetylated Fli-1 were determined as described in Patients and Methods. D, Normal fibroblasts were incubated in the presence or absence of IL-20 for 12 hours. Cellular DNA was sheared, and chromatin (input DNA) was immunoprecipitated with anti–Fli-1 antibody or IgG isotype control antibody. The presence of α2(I) collagen promoter fragments in the precipitates was detected using PCR followed by agarose gel electrophoresis. E, Levels of Smad3, endoglin, and caveolin 1 mRNA were determined as described in A. * = P < 0.05 versus untreated fibroblasts (set to 1.0) (n = 7 samples). F, Normal fibroblasts were treated with IL-20 (100 ng/ml) for 12 hours in the presence or absence of Fli-1 small interfering RNA (siRNA). The expression of type I collagen was determined by immunoblotting. Values are the mean ± SEM.
We also examined the mRNA expression of other human α2(I) collagen promoter–related molecules by real-time PCR. The expression of c-Myb, c-Fos, and c-Jun was not affected by IL-20 (Figure 2A). Caveolin 1 was down-regulated by IL-20 in the array (Table 1), but the decrease became insignificant in real-time PCR with increasing the number of samples (n = 7) (Figure 2E). Smad3 and endoglin, mediators of TGFβ signaling, were significantly decreased by IL-20 (Figure 2E), consistent with the array results. However, the decrease of Smad3 or endoglin could not explain the reduction of α2(I) collagen expression by IL-20 in normal fibroblasts, because their inhibition was shown to have no effect on basal collagen transcription (39,40). Therefore, our results suggest that IL-20 reduces collagen transcription mainly via Fli-1 expression. Consistent with our hypothesis, the inhibitory effect of IL-20 on collagen was blocked by the transfection of Fli-1 siRNA (Figure 2F).
As described above, IL-19, IL-20, and IL-24 share a receptor (14), although their functions are different. We investigated whether IL-19 and IL-24 also regulate collagen expression in vitro. Immunoblotting revealed that protein synthesis of type I collagen was not significantly affected by treatment with IL-19 or IL-24 in comparison with untreated cells (further information available from the corresponding author). When the ratio of Ets-1:Fli-1 was also examined by immunoblotting, we did not find significant change (further information available from the corresponding author). Therefore, among IL-20R–related cytokines, the reduction of collagen expression via Fli-1 up-regulation is likely to be specific to IL-20.
Expression levels of IL-20 in sera and involved skin of patients with SSc
Next, we measured the serum levels of IL-20 in various rheumatic diseases by ELISA. As shown in Figure 3A, the mean serum IL-20 levels in patients with SSc, SLE, and DM were slightly but not significantly decreased compared with those in normal subjects. On the other hand, patients with scleroderma spectrum disorders who did not fulfill the ACR classification criteria for SSc but who we thought would develop SSc in the future (23–25) had IL-20 levels significantly lower than those in normal subjects.
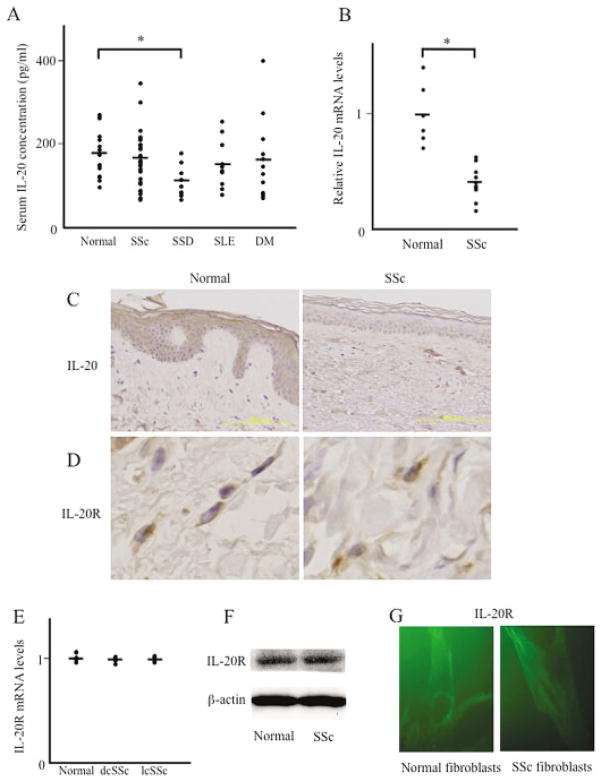
Figure 3.
Interleukin-20 (IL-20) levels in sera and skin tissues from patients with rheumatic diseases. A, Shown are serum IL-20 levels as determined by enzyme-linked immunosorbent assay. Serum samples were obtained from 33 patients with systemic sclerosis (SSc), 9 patients with scleroderma spectrum disorders (SSD), 10 patients with systemic lupus erythematosus (SLE), 12 patients with dermatomyositis (DM), and 15 normal subjects. Symbols represent individual samples; bars show the mean. * = P < 0.05. B, Total RNA was extracted from skin tissues obtained from 12 SSc patients and 6 normal subjects. IL-20 mRNA expression was analyzed by real-time polymerase chain reaction (PCR). Symbols represent individual samples; bars show the mean. * = P < 0.05. C, Paraffin-embedded sections of normal and SSc-involved skin were subjected to immunohistochemical analysis for IL-20. Original magnification × 200 (n = 3 samples). D, Paraffin-embedded sections of normal and SSc-involved skin were subjected to immunohistochemical analysis for IL-20 receptor (IL-20R). Original magnification × 400 (n = 3 samples). E, Total RNA was extracted, and IL-20R mRNA expression was analyzed by real-time PCR as described in B. Symbols represent individual samples; bars show the mean. F, Cell lysates were obtained from cultured normal and SSc dermal fibroblasts and subjected to immunoblotting with antibody to IL-20R. Results are representative of 5 normal and 5 SSc fibroblasts. G, Expression of IL-20R in cultured normal dermal fibroblasts and SSc fibroblasts was visualized by immunofluorescence microscopy. Original magnification × 1,000. dcSSc = diffuse cutaneous SSc; lcSSc = limited cutaneous SSc.
We determined the association of serum IL-20 levels with clinical features and laboratory findings in SSc patients (further information available from the corresponding author). Patients with decreased IL-20 levels (below the average of normal subjects) had a significantly higher prevalence of esophageal involvement than did those with normal IL-20 levels (52.9% versus 12.5%; P < 0.05). The modified Rodnan skin thickness score (41) was also increased, although not significantly, in those with decreased IL-20 levels. In contrast, as determined by real-time PCR, IL-20 mRNA expression in involved skin of SSc patients was significantly decreased compared with that in skin of normal subjects (Figure 3B). Immunohistochemical staining using paraffin-embedded skin sections also showed that IL-20 protein was detected strongly in the epidermis of normal skin but hardly detected in SSc atrophic epidermis (Figure 3C). Thus, serum IL-20 levels may be specifically decreased in the prodromal stage of SSc, while IL-20 expression in involved skin of SSc patients may be constitutively decreased.
Although there has been no previous report indicating that IL-20R is expressed in dermal fibroblasts, immunostaining revealed that IL-20R protein was expressed to a similar extent in fibroblast-like spindle-shaped cells of normal and SSc skin in vivo (Figure 3D). This result was consistent with similar IL-20R expression levels in normal and SSc skin by real-time PCR in vivo (Figure 3E) or by immunoblotting (Figure 3F) and immunofluorescence (Figure 3G) in vitro using cultured fibroblasts. Thus, dermal fibroblasts may express IL-20R, and its expression levels are likely to be similar in normal and SSc fibroblasts. Taken together, these results indicate that IL-20 may have an inhibitory effect on collagen expression, and down-regulation of IL-20 levels in SSc skin in vivo contributes to increased collagen accumulation and tissue fibrosis.
Effect of IL-20 on type I collagen expression in TGFβ-stimulated normal fibroblasts and SSc fibroblasts
We next investigated the effect of IL-20 on type I collagen expression in TGFβ-treated normal fibroblasts. Exogenous IL-20 decreased basal collagen expression, and TGFβ could not induce collagen expression in the presence of IL-20 (Figure 4A), suggesting that IL-20 both suppresses the effect of TGFβ and reduces basal collagen expression. As expected, the protein expression of Fli-1 was increased by IL-20 in the presence of TGFβ (Figure 4B), while the mRNA expression levels of Smad3 and endoglin were decreased significantly (Figure 4C). We assume that IL-20 reduces basal collagen transcription via Fli-1 induction, while the down-regulation of Smad3 and endoglin may cancel the effect of TGFβ. The inhibition of collagen by IL-20 (Figure 4D), the induction of Fli-1 by IL-20 (Figure 4E), and the reduction of Smad3 and endoglin by IL-20 (Figure 4F) were also observed in SSc fibroblasts.
Figure 4.
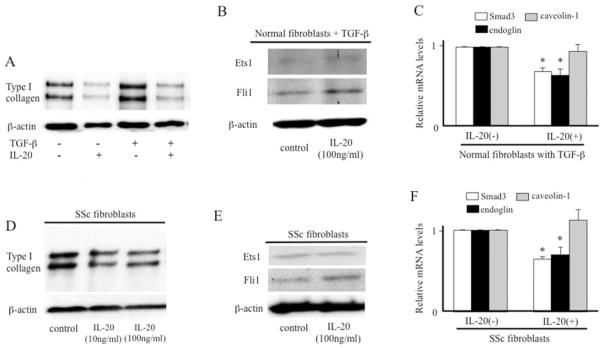
Effect of IL-20 on collagen expression in transforming growth factor β (TGFβ)–stimulated normal fibroblasts or SSc fibroblasts. A, Normal fibroblasts were treated with IL-20 (100 ng/ml) and/or TGFβ (2 ng/ml) for 24 hours. The expression of type I collagen was evaluated by immunoblotting. B, Normal fibroblasts were treated with IL-20 in the presence of TGFβ (2 ng/ml) for 24 hours. Immunoblotting was performed using antibody to Ets-1 or Fli-1. C, Normal fibroblasts were treated with IL-20 (100 ng/ml) for 12 hours in the presence of TGFβ (2 ng/ml). Real-time PCR was performed to evaluate levels of Smad3, endoglin, and caveolin 1 mRNA (n = 7 samples). * = P < 0.05 versus untreated fibroblasts (set to 1.0). D, SSc fibroblasts were treated with IL-20 for 24 hours. Immunoblotting was performed using antibody to type I collagen. E, SSc fibroblasts were treated with IL-20 for 24 hours. Immunoblotting was performed using antibody to Ets-1 or Fli-1. F, SSc fibroblasts were treated with IL-20 (100 ng/ml) for 12 hours. Real-time PCR was performed to evaluate levels of Smad3, endoglin, and caveolin 1 mRNA (n = 7 samples). * = P < 0.05 versus untreated fibroblasts (set to 1.0). Values are the mean ± SEM. See Figure 3 for other definitions.
Effect of IL-20 on bleomycin-induced skin fibrosis in mice
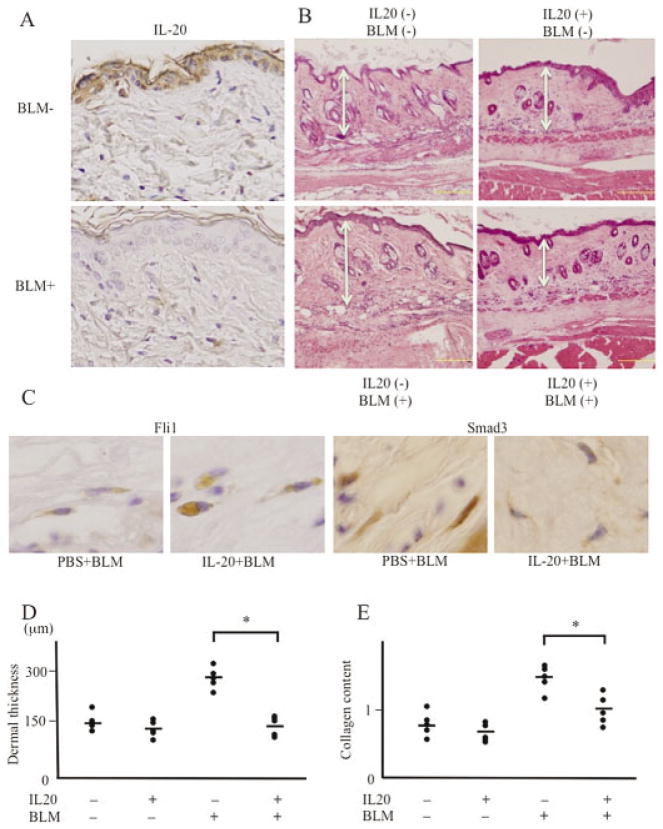
Skin fibrosis induced by bleomycin injection in mice is used as a murine model of SSc (33). The epidermis of control mouse skin expressed IL-20, while IL-20 expression was decreased in bleomycin-treated mouse epidermis (Figure 5A), as seen in SSc skin. The expression of IL-20 in inflammatory cells or endothelial cells was slight and similar between control and bleomycin-treated mouse skin. Accordingly, we tried to determine whether IL-20 supplementation could reverse skin fibrosis in SSc.
Figure 5.
Effect of interleukin-20 (IL-20) on bleomycin (BLM)–induced skin fibrosis in vivo. A, Paraffin-embedded sections of skin were subjected to immunohistochemical analysis for IL-20. Top, Phosphate buffered saline (PBS)–treated wild-type mouse. Bottom, Bleomycin-treated mouse. Results shown are representative of 5 samples. Original magnification × 200. B, PBS- or bleomycin-treated mouse skin was injected with control PBS (left) or IL-20 (right) and stained with hematoxylin and eosin. Double-headed arrows indicate thickness of the dermis. Results shown are representative of 5 samples. Bars = 0.1 mm. C, Paraffin-embedded sections of bleomycin-treated mouse skin injected with control PBS or IL-20 were subjected to immunohistochemical analysis for Fli-1 or Smad3. Results shown are representative of 5 samples. Original magnification × 400. D, Dermal thickness was measured in PBS- or bleomycin-treated mouse skin injected with control PBS or IL-20 (n = 5 samples per group). Symbols represent individual samples; bars show the mean. * = P < 0.05. E, The amount of collagen content in skin sections was quantified using an assay kit (n = 5 samples per group). Symbols represent individual samples; bars show the mean. * = P < 0.05.
Bleomycin (300 μg) or control PBS was locally injected into the back of BALB/c mice 6 times per week for 1 month, and at the same time PBS or IL-20 (3.5 μg) was injected once per week (4 times per month) (further information available from the corresponding author). In the absence of bleomycin injection, IL-20 reduced dermal thickness slightly, but the difference was not significant. Bleomycin treatment without IL-20 induced dermal fibrosis with an increased number of thickened collagen bundles (Figure 5B). However, IL-20 reduced the extent of bleomycin-induced skin thickening (Figure 5B). In immunohistochemical staining using paraffin-embedded mouse skin sections, the expression of Fli-1 in fibroblast-like spindle cells was increased by IL-20, while the expression of Smad3 was decreased (Figure 5C), consistent with in vitro results. We found that reversal of bleomycin-induced dermal thickening by IL-20 was statistically significant (Figure 5D). In addition, the increased amount of collagen induced by bleomycin in skin tissue was also significantly decreased by IL-20 (Figure 5E). Taken together, these results indicate that IL-20 supplementation could attenuate bleomycin-induced skin fibrosis.
DISCUSSION
In the skin, IL-20 is known to induce keratinocyte proliferation, but the effect on dermal fibroblasts has not been investigated. Our study revealed that α2(I) collagen mRNA expression was reduced by IL-20 at the transcriptional level, and that the decrease was mediated by Fli-1 induction in normal dermal fibroblasts. We did not determine the regulatory mechanism of the type I collagen α1 chain in the present study, but there may be similar mechanisms in IL-20–mediated α1(I) collagen down-regulation, because Fli-1 also controls α1(I) collagen expression (33).
There was a significant difference in serum IL-20 levels between patients with scleroderma spectrum disorders and normal subjects. As described above, scleroderma spectrum disorder is a prodromal stage of SSc, and a similar condition was also reported as “limited SSc” by LeRoy and Medsger (42). Because fibrotic change in SSc is usually irreversible, new strategies are needed to diagnose patients as early as possible and to follow them up carefully. For that purpose, the concept of scleroderma spectrum disorder is helpful. Serum IL-20 levels may be useful for differentiating patients with scleroderma spectrum disorders from normal subjects.
Generally, patients with scleroderma spectrum disorders lack a fibrotic response but show prominent clinical symptoms associated with vasculopathy (further information available from the corresponding author). Given that there have been a couple of articles regarding the role of IL-20 in angiogenesis (43,44), decreased serum IL-20 levels in patients with scleroderma spectrum disorders suggests that IL-20 is potentially linked to the pathogenesis of vasculopathy leading to the development of fibrosis. However, as the limitation of this study, scleroderma spectrum disorders potentially include undifferentiated connective tissue diseases developing into collagen diseases other than SSc. Therefore, it is basically impossible to say that all patients with scleroderma spectrum disorders included in this study have an established clinical entity that will develop into SSc. Longitudinal studies of serum IL-20 levels in patients with SSc developed from scleroderma spectrum disorders are needed in the future.
We found that IL-20 expression was decreased in SSc epidermis. We have a hypothetical model of IL-20–mediated tissue fibrosis in SSc (further information available from the corresponding author). Reduction of IL-20 expression in SSc skin may further down-regulation of Fli-1, with subsequent collagen overexpression in SSc fibroblasts; Fli-1 was reported to be constitutively decreased in SSc fibroblasts, probably due to TGFβ signaling (45), and reduction of IL-20 expression may enhance Fli-1 down-regulation. The addition of ectopic IL-20 reduces collagen expression effectively, via Fli-1 recovery and suppression of Smad3 and endoglin. In addition, the epidermis of SSc skin is known to become atrophic, and this may be because of reduced keratinocyte proliferation resulting from decreased IL-20. On the other hand, in psoriasis, IL-20 is thought to be overexpressed in epidermis and to lead to characteristic keratinocyte proliferation and epidermal thickening (13). This may explain why psoriasis skin is protected from dermal fibrosis despite chronic inflammation.
Our model indicates that IL-20 may have therapeutic value for the fibrotic condition of SSc. We were able to inhibit bleomycin-induced fibrosis by using IL-20 supplementation in the mouse model. To date, steroids, cyclophosphamide, and methotrexate are considered the first-choice drugs for treating the severe skin fibrosis of SSc (46,47). However, these conventional treatments usually have limited effects. Furthermore, these treatments are often accompanied by various significant adverse effects (48). Clarifying the mechanism by which IL-20 regulates tissue fibrosis in SSc skin may lead to a better understanding of this disease and to new therapeutic approaches. However, our study has other limitations involving potential contradiction of our in vivo results with our in vitro results. For example, further studies are needed to examine the function and expression pattern of IL-20R in murine dermal fibroblasts. Similarly, the data showing that the inhibitory effect of IL-20 on collagen was blocked by Fli-1 siRNA in vitro should be confirmed in vivo (i.e., the effect of IL-20 on bleomycin-induced dermal fibrosis in Fli-1–knockout mice should be determined).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Jinnin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Jinnin, Asano, Trojanowska, Fukushima, Ihn.
Acquisition of data. Kudo, Nakayama, Inoue.
Analysis and interpretation of data. Kudo, Jinnin, Honda, Kajihara, Makino.
References
- 1.Uitto J, Kouba D. Cytokine modulation of extracellular matrix gene expression: relevance to fibrotic skin diseases. J Dermatol Sci. 2000;24:60–9. doi: 10.1016/s0923-1811(00)00143-2. [DOI] [PubMed] [Google Scholar]
- 2.Trojanowska M, LeRoy EC, Eckes B, Krieg T. Pathogenesis of fibrosis: type I collagen and the skin. J Mol Med (Berl) 1998;76:266–74. doi: 10.1007/s001090050216. [DOI] [PubMed] [Google Scholar]
- 3.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 5.Ihn H, Yamane K, Kubo M, Tamaki K. Blockade of endogenous transforming growth factor β signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor β receptors. Arthritis Rheum. 2001;44:474–80. doi: 10.1002/1529-0131(200102)44:2<474::AID-ANR67>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Asano Y, Ihn H, Yamane K, Kubo M, Tamaki K. Impaired Smad7-Smurf-mediated negative regulation of TGF-β signaling in scleroderma fibroblasts. J Clin Invest. 2004;113:253–64. doi: 10.1172/JCI16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takehara K. Hypothesis: pathogenesis of systemic sclerosis. J Rheumatol. 2003;30:755–9. [PubMed] [Google Scholar]
- 8.Overbeek MJ, Boonstra A, Voskuyl AE, Vonk MC, Vonk-Noordegraaf A, van Berkel MP, et al. Platelet-derived growth factor receptor-β and epidermal growth factor receptor in pulmonary vasculature of systemic sclerosis-associated pulmonary arterial hypertension versus idiopathic pulmonary arterial hypertension and pulmonary veno-occlusive disease: a case-control study. Arthritis Res Ther. 2011;13:R61. doi: 10.1186/ar3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brissett M, Veraldi KL, Pilewski JM, Medsger TA, Jr, Feghali-Bostwick CA. Localized expression of tenascin in systemic sclerosis–associated pulmonary fibrosis and its regulation by insulin-like growth factor binding protein 3. Arthritis Rheum. 2012;64:272–80. doi: 10.1002/art.30647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi Y. IL-1α gene expression and protein production by fibroblasts from patients with systemic sclerosis. Clin Exp Immunol. 1994;97:445–50. doi: 10.1111/j.1365-2249.1994.tb06108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of pathogenesis in scleroderma. I. Overproduction of interleukin 6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol. 1992;19:1207–11. [PubMed] [Google Scholar]
- 12.Makino T, Fukushima S, Wakasugi S, Ihn H. Decreased serum IL-7 levels in patients with systemic sclerosis. Clin Exp Rheumatol. 2009;27:68–9. [PubMed] [Google Scholar]
- 13.Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 14.Wegenka UM. IL-20: biological functions mediated through two types of receptor complexes. Cytokine Growth Factor Rev. 2010;21:353–63. doi: 10.1016/j.cytogfr.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, et al. Cloning, expression and initial characterisation of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–50. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 16.Liao YC, Liang WG, Chen FW, Hsu JH, Yang JJ, Chang MS. IL-19 induces production of IL-6 and TNF-α and results in cell apoptosis through TNF-α. J Immunol. 2002;169:4288–97. doi: 10.4049/jimmunol.169.8.4288. [DOI] [PubMed] [Google Scholar]
- 17.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–86. [PubMed] [Google Scholar]
- 18.Hsu YH, Li HH, Hsieh MY, Liu MF, Huang KY, Chin LS, et al. Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum. 2006;54:2722–33. doi: 10.1002/art.22039. [DOI] [PubMed] [Google Scholar]
- 19.Li HH, Cheng HH, Sun KH, Wei CC, Li CF, Chen WC, et al. Interleukin-20 targets renal mesangial cells and is associated with lupus nephritis. Clin Immunol. 2008;129:277–85. doi: 10.1016/j.clim.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Wei CC, Chang MS. A novel transcript of mouse interleukin-20 receptor acts on glomerular mesangial cells as an aggravating factor in lupus nephritis. Genes Immun. 2008;9:668–79. doi: 10.1038/gene.2008.61. [DOI] [PubMed] [Google Scholar]
- 21.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178:2229–40. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 22.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. . Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 23.Maricq HR, McGregor AR, Diat F, Smith EA, Maxwell DB, LeRoy EC, et al. Major clinical diagnoses found among patients with Raynaud phenomenon from the general population. J Rheumatol. 1990;17:1171–6. [PubMed] [Google Scholar]
- 24.Maricq HR, Weinrich MC, Keil JE, Smith EA, Harper FE, Nussbaum AI, et al. Prevalence of scleroderma spectrum disorders in the general population of South Carolina. Arthritis Rheum. 1989;32:998–1006. doi: 10.1002/anr.1780320809. [DOI] [PubMed] [Google Scholar]
- 25.Ihn H, Sato S, Tamaki T, Soma Y, Tsuchida T, Ishibashi Y, et al. Clinical evaluation of scleroderma spectrum disorders using a points system. Arch Dermatol Res. 1992;284:391–5. doi: 10.1007/BF00372068. [DOI] [PubMed] [Google Scholar]
- 26.Honda N, Jinnin M, Kira-Etoh T, Makino K, Kajihara I, Makino T, et al. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin β3. Am J Pathol. 2013;182:206–16. doi: 10.1016/j.ajpath.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Jinnin M, Makino T, Kajihara I, Honda N, Makino K, Ogata A, et al. Serum levels of soluble vascular endothelial growth factor receptor-2 in patients with systemic sclerosis. Br J Dermatol. 2010;162:751–8. doi: 10.1111/j.1365-2133.2009.09567.x. [DOI] [PubMed] [Google Scholar]
- 28.Noda S, Asano Y, Akamata K, Aozasa N, Taniguchi T, Takahashi T, et al. Constitutive activation of c-Abl/protein kinase C-δ/Fli1 pathway in dermal fibroblasts derived from patients with localized scleroderma. Br J Dermatol. 2012;167:1098–105. doi: 10.1111/j.1365-2133.2012.11055.x. [DOI] [PubMed] [Google Scholar]
- 29.Asano Y, Trojanowska M. Phosphorylation of Fli1 at threonine 312 by protein kinase C δ promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor β. Mol Cell Biol. 2009;29:1882–94. doi: 10.1128/MCB.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ihn H, Ohnishi K, Tamaki T, LeRoy EC, Trojanowska M. Transcriptional regulation of the human α2(I) collagen gene: combined action of upstream stimulatory and inhibitory cis-acting elements. J Biol Chem. 1996;271:26717–23. doi: 10.1074/jbc.271.43.26717. [DOI] [PubMed] [Google Scholar]
- 31.Filippova M, Song H, Connolly JL, Dermody TS, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. J Biol Chem. 2002;277:21730–9. doi: 10.1074/jbc.M200113200. [DOI] [PubMed] [Google Scholar]
- 32.Wolk K, Witte E, Warszawska K, Schulze-Tanzil G, Witte K, Philipp S, et al. The Th17 cytokine IL-22 induces IL-20 production in keratinocytes: a novel immunological cascade with potential relevance in psoriasis. Eur J Immunol. 2009;39:3570–81. doi: 10.1002/eji.200939687. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, et al. Animal model of sclerotic skin. I. Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999;112:456–62. doi: 10.1046/j.1523-1747.1999.00528.x. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka C, Fujimoto M, Hamaguchi Y, Sato S, Takehara K, Hasegawa M. Inducible costimulator ligand regulates bleomycin-induced lung and skin fibrosis in a mouse model independently of the inducible costimulator/inducible costimulator ligand pathway. Arthritis Rheum. 2010;62:1723–32. doi: 10.1002/art.27428. [DOI] [PubMed] [Google Scholar]
- 35.Marotta M, Martino G. Sensitive spectrophotometric method for the quantitative estimation of collagen. Anal Biochem. 1985;150:86–90. doi: 10.1016/0003-2697(85)90443-9. [DOI] [PubMed] [Google Scholar]
- 36.Czuwara-Ladykowska J, Shirasaki F, Jackers P, Watson DK, Trojanowska M. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J Biol Chem. 2001;276:20839–48. doi: 10.1074/jbc.M010133200. [DOI] [PubMed] [Google Scholar]
- 37.Czuwara-Ladykowska J, Sementchenko VI, Watson DK, Trojanowska M. Ets1 is an effector of the transforming growth factor β (TGF-β) signaling pathway and an antagonist of the profibrotic effects of TGF-β. J Biol Chem. 2002;277:20399–408. doi: 10.1074/jbc.M200206200. [DOI] [PubMed] [Google Scholar]
- 38.Jinnin M, Ihn H, Yamane K, Mimura Y, Asano Y, Tamaki K. α2(I) collagen gene regulation by protein kinase C signaling in human dermal fibroblasts. Nucleic Acids Res. 2005;33:1337–51. doi: 10.1093/nar/gki275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Phosphatidylinositol 3-kinase is involved in α2(I) collagen gene expression in normal and scleroderma fibroblasts. J Immunol. 2004;172:7123–35. doi: 10.4049/jimmunol.172.11.7123. [DOI] [PubMed] [Google Scholar]
- 40.Morris E, Chrobak I, Bujor A, Hant F, Mummery C, Ten Dijke P, et al. Endoglin promotes TGF-β/Smad1 signaling in scleroderma fibroblasts. J Cell Physiol. 2011;226:3340–8. doi: 10.1002/jcp.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clements P, Lachenbruch P, Seibold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 42.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–6. [PubMed] [Google Scholar]
- 43.Hsieh MY, Chen WY, Jiang MJ, Cheng BC, Huang TY, Chang MS. Interleukin-20 promotes angiogenesis in a direct and indirect manner. Genes Immun. 2006;7:234–42. doi: 10.1038/sj.gene.6364291. [DOI] [PubMed] [Google Scholar]
- 44.Heuze-Vourc’h N, Liu M, Dalwadi H, Baratelli FE, Zhu L, Goodglick L, et al. IL-20, an anti-angiogenic cytokine that inhibits COX-2 expression. Biochem Biophys Res Commun. 2005;333:470–5. doi: 10.1016/j.bbrc.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 45.Asano Y, Markiewicz M, Kubo M, Szalai G, Watson DK, Trojanowska M. Transcription factor Fli1 regulates collagen fibrillogenesis in mouse skin. Mol Cell Biol. 2009;29:425–34. doi: 10.1128/MCB.01278-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apras S, Ertenli I, Ozbalkan Z, Kiraz S, Ozturk MA, Haznedaroglu IC, et al. Effects of oral cyclophosphamide and prednisolone therapy on the endothelial functions and clinical findings in patients with early diffuse systemic sclerosis. Arthritis Rheum. 2003;48:2256–61. doi: 10.1002/art.11081. [DOI] [PubMed] [Google Scholar]
- 47.Pope JE, Bellamy N, Seibold JR, Baron M, Ellman M, Carette S, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44:1351–8. doi: 10.1002/1529-0131(200106)44:6<1351::AID-ART227>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 48.Haustein UF. Systemic sclerosis-scleroderma. Dermatol Online J. 2002;8:3. [PubMed] [Google Scholar]