Abstract
Purpose.
Adipose-derived stem cells (ASCs) have gained importance due to their myriad potential clinical applications. We hypothesize that progenitor cells also exist besides those conventionally isolated from the stromal vascular fraction (SVF).
Method.
Central and medial orbital adipose tissues obtained from patients during eyelid surgery were digested with collagenase for 3 or 16 hours at 37°C with or without shaking. After centrifugation, the remaining cell pellet was resuspended and filtered to yield flow through in SVF and retained cells (RC) on the filter. Single cells from RC and SVF were cultured on 5% coated Matrigel in serum-free modified embryonic stem cells medium (MESCM) for 10 passages. The progenitor status was evaluated by the expression of a number of markers by qPCR and immunofluorescence staining as well as their plasticity for endothelial and tri-lineage differentiation.
Results.
Type I collagenase digestion for 3 hours under shaking was significantly less effective in releasing progenitor cells than collagenase A digestion for 16 hours without shaking. Following filtration, cells in SVF and RC, of which the latter were tangled in collagen IV-containing matrix, expressed different markers of progenitor cells. Cells from SVF and RC could be expanded for 10 passages on coated Matrigel in MESCM and exhibited similar or better potential to differentiate into vascular endothelial cells, chondrocytes, osteocytes, and adipocytes than SVF cells expanded on plastic in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS).
Conclusions.
Different progenitor cells can be isolated and expanded from orbital adipose tissues. Further characterization of their mesodermal or neuroectodermal origin might enhance clinical outcome when used as a source of autologous stem cells for ocular surface regeneration.
Keywords: adipose stem cells, basement membrane, isolation, mesenchymal stem cells, orbital adipose tissue, stromal vascular fraction
Isolation and characterization of mesenchymal progenitor cells from human orbital adipose tissue.
Introduction
Mesenchymal stem cells (MSCs) isolated from a number of adult tissues have the ability to differentiate into multiple lineages and have attracted an increasing interest in the field of regenerative medicine.1–3 Adipose-derived mesenchymal stem cells (ASCs) may be an ideal source of autologous adult MSCs because of their abundance, surgical accessibility, and plasticity to be differentiated into cells of mesodermal and nonmesodermal origin.4–6 Although adipose tissue in the body is considered mesodermally derived,4 recent study demonstrates small fractions of heterogeneous population from ASCs may contain neural crest–derived adipocyte-restricted progenitors of which the phenotype is distinct from that of non-neural crest derivatives.7 Interestingly, ocular and orbital components have been shown to be derived from combination of mesodermal and neural crest cells.8 The central fat pad in the upper eyelid and the central and temporal fat pads in the lower eyelid are mesodermally derived, while the medial fat pads of upper and lower lids are derived from neural crest cells.9,10 Indeed, ASCs from orbital adipose tissue can differentiate into the corneal epithelial lineage,11 and smooth muscle and neuronal/glial lineages.10 It remains unclear whether there are mesoderm- and neuroectoderm-derived progenitors from orbital fat.
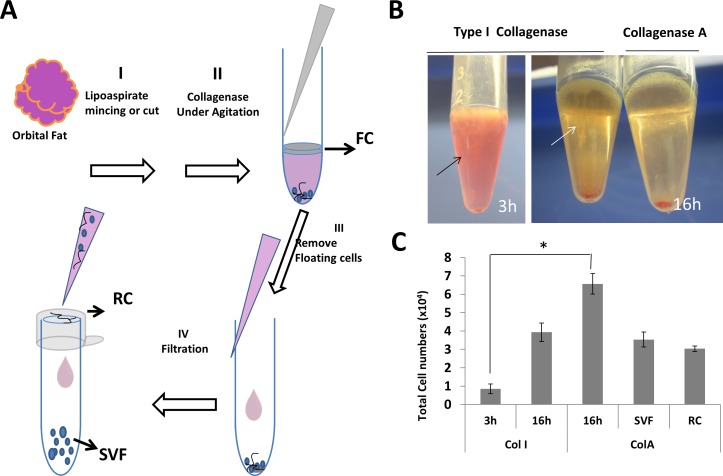
Conventionally, ASCs are isolated from the stromal vascular fraction (SVF) of human adipose tissues by the following four steps that are depicted schematically in Figure 1.4,11–15 Step I is to cut the adipose tissue obtained by surgery or liposuction into 0.5 to 1 cm3 pieces. Step II is to digest these pieces by collagenase type I (Col I), A (Col A), or II (Col II) for 30 minutes to 4 hours at 37°C. Step III is to centrifuge the digest solution to remove the floating cells (FCs) that contain mature adipose cells. Step IV is to filter the remaining digested solution to remove unwanted cellular debris via a filter with the pore size of 40 to 250 μm to collect the flow through, which after being treated with the red blood cells lysis buffer to remove red blood cells is used as SVF (see Scheme depicted in Fig. 1). Alternatively, ASCs also can be isolated by collecting cells after centrifugation following digestion of cut adipose tissues in Col I at 37°C under a water bath shaker for 1 hour without using filtration.16 It remains unclear whether these two isolation methods are compatible in isolating ASCs from human orbital fat.
Figure 1.
Different cell fractions from human orbital adipose tissue. After surgical removal, human orbital adipose tissues were cut in smaller pieces (Step I), subjected to collagenase digestion (Step II), centrifuged to remove FCs (Step III), and filtered to obtain SVF in the flow through and RC from cells caught on the filter. (Step IV) (A). Different appearances were noted after centrifugation following digestion with Col I for 3 and 16 hours, and with Col A for 16 hours (B). Such a difference was reflected by the total cell number of cells in the pellet after digestion in Col I or ColA for 3 or 16 hours, as well as SVF and RC following Step IV filtration (C).
Our recent studies have shown that collagenase, which removes interstitial, but not basement membrane collagens, can be used to isolate human limbal niche cells (LNCs), which are a subset of mesenchymal cells physically close to limbal-basal epithelial progenitors and stem cells.17 Isolated LNCs are as small as 5 μm in diameter and heterogeneously express embryonic stem cell (ESC) markers, such as Oct4, Sox2, SSEA4, and Nanog, as well as other stem cell markers, such as Nestin, N-cadherin, and CD34. Our studies further disclosed that these LNCs cannot be isolated by digestion with dispase,17 which removes the basement membrane. Hence, we hypothesized that collagenase used during conventional isolation of ASCs might have unintentionally left some progenitor cells in the fraction retained by the filter or in the FCs because they are tightly associated with the basement membrane matrix. As a first step to test this hypothesis, we demonstrated herein that the traditional method used for isolating ASCs from systemic fat, indeed, has left some progenitor cell population behind. Furthermore, we further optimize the expansion protocol by using the basement membrane substrate. Our study suggested the presence of two types of progenitor cells in human orbital fat. Further characterization of these progenitor cells may allow us to use them as a source of autologous stem cells for ocular surface regeneration.
Materials and Methods
Human Adipose Tissue Collection
Central and medial orbital fats were collected from 10 patients aged 49 to 81 (64.8 ± 6.6, n = 10) years old following routine blepharoplasty. All patients consented to the study approved by the Institutional Review Board at University of Miami (Protocol #20110692) and followed the tenets of the Declaration of Helsinki. Immediately after surgery, these adipose tissues, typically discarded at the time of surgery, were preserved on ice and transported within 4 hours to the laboratory and processed upon receipt.
Cell Isolation
Detail materials used for cell culturing are listed as Supplemental Table S1. In brief, after washing three times with PBS containing 50 μg/mL gentamicin and 1.25 μg/mL amphotericin B, fat tissues were cut into pieces of less than 5 mm in size. The same weight of tissues 0.5% (wt/vol) was subjected to digestion with 1 mg/mL of Col I (Worthington Biochemical Corp, Lakewood, NJ, USA) in modified embryonic stem cell medium (MESCM)18 or Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) for 3 hours on a shaker with intermittent manual shaking every 20 minutes and vigorous manual shaking for 10 seconds at the end of 3 hours before centrifugation at 300g for 5 minutes to collect cell pellets.16 Alternatively, cut tissues were digested with 1 mg/mL of Col A (Roche Applied Science, Indianapolis, IN, USA) in the same medium for 16 hours at 37°C without shaking. Digested tissues were pipette up and down 10 times before centrifugation at 300g for 5 minutes to remove floating adipocytes. The pellets were resuspended in MESCM and filtered through a 70 μm nylon strainer (BD Bioscience, Franklin Lakes, NJ, USA) to yield cells in the flow through as SVF and cells retained on the filter (RC). Cells in SVF and RC were treated with red cell blood cells lysis buffer to remove red blood cells and with 0.25% trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA) to yield a single cell suspension at 37°C for 5 minutes.
Phenotypic Characterization
Immediately after isolation, cells from RC and SVF were dried to adhere on the slides and fixed with 100% cold methanol at 20°C. Alternatively, cells freshly isolated or undergoing serial passages were treated with trypsin-EDTA at 37°C for 10 minutes and centrifugation at 55g for 8 minutes at the density of 2 to 4.0 × 104 cells/chamber using Cytofuge (StatSpin, Inc., Norwood, MA, USA). The cytospin preparation was dried at the room temperature for 5 minutes and then fixed with either 100% cold methanol at −20°C or 4% paraformaldehyde for 15 minutes at room temperature. For immunofluorescence staining, samples were permeabilized with 0.2% Triton X-100 in PBS for 15 to 30 minutes and blocked with 0.2% BSA in PBS for 1 hour at room temperature before addition of the primary antibody overnight at 4°C. Isotype-matched nonspecific IgG antibodies were used as controls. Image analysis was performed using confocal laser microscopy (LSM700; Carl Zeiss, Inc., Thornwood, NY, USA). All monoclonal antibodies used in this study are listed in Table 1.
Table 1.
Primary and Secondary Antibodies Used for Immunofluorescence Staining
|
Primary Antibodies | |||
|
Antibody |
Supplier |
Source |
Dilution |
| CD31 [89C2] | Cell Signaling Technology, Beverly, MA, USA | Mouse | 1:100 |
| CD34 [MEC14.7] | Abcam, Cambridge, UK | Rat | 1:100 |
| CD45 | Abcam | Rabbit | 1:100 |
| CD146 [P1H12] | Abcam | Mouse | 1:100 |
| CD44 [IM7] | Sigma-Aldrich, St. Louis, MO, USA | Rat | 1:100 |
| CD73 [EPR6114] | Abcam | Rabbit | 1:100 |
| CD90 [EPR3132] | Abcam | Rabbit | 1:100 |
| CD105 [3A9] | Abcam | Mouse | 1:100 |
| Collagen type IV clone 23IIC3 | Millipore Corp., Billerica, MA, USA | Mouse | 1:100 |
| NG2 [9.2.27] | Abcam | Mouse | 1:100 |
| Nanog | Abcam | Rabbit | 1:100 |
| Oct4 (POU5F1) [7F9.2] | Millipore Corp. | Mouse | 1:100 |
| PDGFRβ [Y92] | Abcam | Rabbit | 1:100 |
| Sox2 | Abcam | Rabbit | 1:100 |
| vWF | Abcam | Rabbit | 1:100 |
| Hoechst 33342 | Sigma-Aldrich | – | 1:500 |
| Alexa Fluor 488 Anti-Rat | Invitrogen | Donkey | 1:100 |
| Alexa Fluor 555 Anti-Mouse | Invitrogen | Donkey | 1:100 |
| Alexa Fluor 488 Anti-Mouse | Invitrogen | Donkey | 1:100 |
| Alexa Fluor 555 Anti-Rabbit | Invitrogen | Donkey | 1:100 |
Cell Culture and Cell Doubling
Single cells from RC and SVF isolated from medial orbital fat were obtained by trypsin-EDTA at 37°C for 10 minutes, seeded at 2 × 104/cm2 in 6-well plates coated with or without 5% Matrigel (MG), and cultured in MESCM containing 4 ng/mL basic fibroblast growth factor (bFGF) and 10 ng/mL leukemia inhibitory factor (LIF) as described previously.18 Media were changed every 3 to 4 days. At 70% to 80% confluence, cells were seeded at 5 × 103/cm2 per passage for a total of 10 passages. The extent of total expansion was measured by the number of cell-doubling (NCD) using the following formula: NCD = log10(y/x)/log102, where “y” is the final density of the cells and “x” is the initial seeding density of the cells. In parallel, cells were cultured in the 6-well plastic (PL) plate in DMEM with 10% FBS as a control to replicate the conventional method of expanding ASCs19. The details of materials used for culturing are listed in Table S1 of Supplemental Data.
Quantitative Real-Time PCR
Total RNAs were extracted from cells freshly isolated or undergoing serial passages from either RC or SVF by RNeasy Mini RNA isolation kit (QIAGEN, Valencia, CA, USA) and reverse-transcribed to cDNAs by high capacity cDNA transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR (qPCR) was performed in triplicate in four of six donors. The qPCR amplification of different genes was done in a 20 μL solution containing cDNA, TaqMan Gene Expression Assay Mix and universal PCR master Mix (Applied Biosystems). All TaqMan Gene Expression Relative Quantification was performed using the 7300 Real-time RT-PCR system (Applied Biosystems) according to the manufacturer's description using the following thermo cycler parameters: 10 minutes of initial activation at 95°C, 40 cycles of 15 seconds denaturation at 95°C, and 1 minute annealing and extension at 60°C. The relative gene expression data were analyzed by the comparative CT method (ΔΔCT). The results were normalized to an internal control, glceraldehyde-3-phosphate dehydrogenase (GAPDH). The probe sequences used are listed in Table 2.
Table 2.
Assay ID and Probes Sequence Use for Real-Time PCR
|
Gene |
Assay ID, TaqMan Gene Expression |
Uni Gene |
Product Length |
| GAPDH | Hs02758991_g1 | Hs.544577 | 93 |
| α-SMA | Hs00426835_g1 | Hs.500483 | 105 |
| CD31 | Hs00169777_m1 | Hs.376675 | 65 |
| CD34 | Hs00990734_g1 | Hs.374990 | 101 |
| FLK-1 | Hs00911700_m1 | Hs.479756 | 83 |
| Nanog | Hs02387400_g1 | Hs.635882 | 109 |
| Nestin | Hs00707120_s1 | Hs.527971 | 81 |
| NG2 | Hs00361541_g1 | Hs.513044 | 60 |
| OCT4 | Hs00999634_g1 | Hs.249184 | 64 |
| PDGFRβ | Hs01019589_m1 | Hs.509067 | 62 |
| Rex-1 | Hs00381890_m1 | Hs.192477 | 109 |
| Sox-2 | Hs01053049_s1 | Hs.518438 | 91 |
Differentiation Into Vascular Endothelial Cells
To induce differentiation into vascular endothelial cells, single cells from Passage 5 of RC or SVF were seeded at the density of 104 cells per cm2 in 24-well PL plates for 3 days in the Endothelial Cell Growth Medium 2 (EGM2) supplemented with10 ng/mL VEGF (also see eTable S1 of Supplemental Data). At 80% to 90% confluence, cells were incubated with 10 μg/mL Dil-Ac-LDL (Invitrogen, Carlsbad, CA, USA) for 10 hours at 37°C in the humidified 5% CO2 incubator and fixed with 4% paraformaldehyde for immunofluorescence staining.
Tri-lineage Differentiation
For assays of adipogenesis or osteogenesis, expanded single cells during passages 3 to 5 were seeded at the density of 2.5 × 104 cells per cm2 in 24-well PL plates in DMEM with 10% FBS. At 90% confluence, the medium was switched to the Adipogenesis Differentiation Medium or the Osteogenesis Differentiation Medium, respectively (Invitrogen) and changed every 3 days. After 21 days of culturing, cells were fixed with 4% formaldehyde and stained with Oil Red O for adipocytes by adipogenesis Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA) or with 2% Alizarin Red for osteocytes following the manufacturer's protocol. Cells with oil droplet stained by Oil Red were quantified by measuring at OD at 492 nm in triplicate cultures. Mineralized cells with positive Alizarin Red staining were quantified by measuring OD at 405 nm in triplicate cultures. For the chondrogenesis assay, pellets were prepared by spinning down 1 × 105 cells and incubating in a 15 mL conical tube in Chondrogenesis Differentiation Medium (Invitrogen) with the medium changed every 3 days. After 28 days of culturing, pellets were fixed with 4% formaldehyde, embedded in the Optimal Cutting Temperature Compound, prepared for 6 μm frozen cross-sections, and stained with Alcian Blue.
Statistical Analysis
All assays were performed in triplicate, each with a minimum of three donors except for cell counting of Oct4+ cells. The data are reported as means ± SD. Analysis between two groups was performed by Student's unpaired t-test, but between three or more groups by 1-way ANOVA. Test results were reported as 2-tailed P values, where P less than 0.05 was considered statistically significant.
Results
Cell Isolation Is More Effective by Prolonged Collagenase Digestion
Two general methods have been adopted to isolate ASCs from adipose tissues.4,16 Using human medial orbital fat, we noted that the method based on digestion with Col I for 3 hours under constant shaking16 was not successful in complete digestion as evidenced by leaving undigested tissue in the floating layer (FL) after centrifugation (Fig. 1B, black arrow) and by resulting in the lowest cell yield in the pellet (Fig. 1C). Prolonged digestion with the same concentration of Col I or Col A for 16 hours was necessary to generate a clearer supernatant after centrifugation, with Col A being better than Col I (Fig. 1B, white arrow, indicating the cloudier supernatant in Col I), as evidenced by resulting in a significant higher cell yield from both pellets (Fig. 1C). Similar numbers of cells obtained from the pellet in SVF and RC after digestion with Col A for 16 hours were resuspended and filtered (Fig. 1C). Results showed that there were significant differences in cell yields between five groups (P < 0.001, n = 3) and between two groups, that is, Col I at 3 hours and Col A, at 16 hours (Fig. 1C, *P < 0.01, n = 3). Similar result of cell isolation yield was noted for human orbital central fat (not shown). There was no significant difference in cell viability between 3 hours versus 16 hours of digestion based on trypan blue staining (data not shown). Hence, we concluded that prolonged digestion was necessary to isolate cells from human orbital adipose tissues.
Expression of Progenitor Cell Markers by Different Cell Fractions
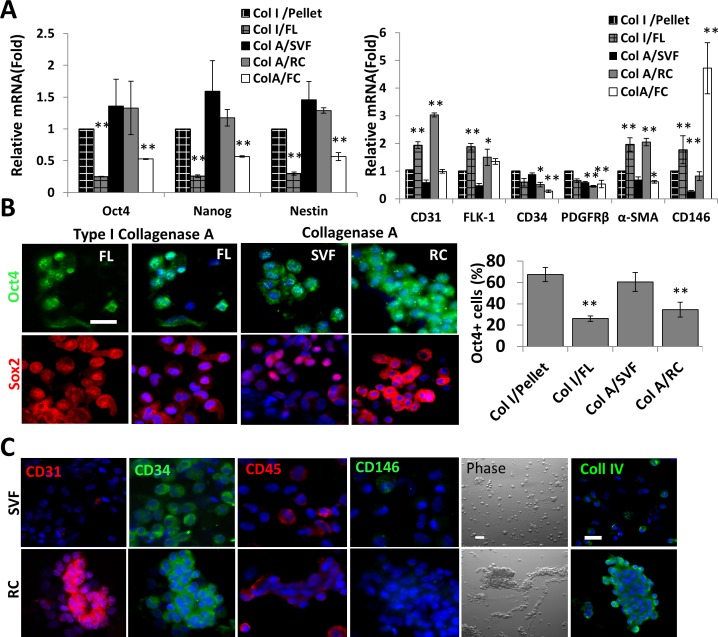
To test the hypothesis that the traditional method used for isolating ASCs might have left some progenitor cell population behind, we used quantitative RT-PCR to study transcript expression of Oct4, an ESC marker, because it is expressed in inner endothelial and outer region of arterioles and capillaries, but not arteries.13 We also examined transcript expression of CD31, FLK-1, or CD146 for endothelial progenitors, PDGFRβ, NG2, α-smooth muscle actin (α-SMA) for pericytes, and Nestin for neural stem cells (NSCs).20 Compared to the level of expression by cells in the pellet after digestion with Col I for 3 hours (set as 1), undigested cells in FL consistently exhibited significantly lower expression levels of Oct4, Nanog, and Nestin (Fig. 2A, n = 3, **P < 0.01 and *P < 0.05). Immunostaining showed that nuclear positive Oct4+ cells were found in pellets and undigested FL (Fig. 2B, FL, not shown for pellets as few cells could be isolated). The percentage of Oct4+ cells in pellets was higher than that of FL, suggesting that digestion of Col I for 3 hours indeed enriched isolation of Oct4+ cells. However, because fewer cells were isolated in pellets (Fig. 1C), the majority of Oct4+ cells still remained in the undigested FL. The same result was noted in nuclear Sox2+ cells (Fig. 2B). This interpretation was supported by significantly higher expression levels of CD31, FLK-1, α-SMA, and CD146 in the undigested FL (Fig. 2A, n = 3, **P < 0.01, *P < 0.05), while no difference in the expression level of CD34 and PDGFRβ (Fig. 2A, n = 3), indicative of the presence of endothelial cells and pericytes in the undigested tissue. When compared to the control Col I/Pellet, cells in SVF and RC after digestion with Col A for 16 hours had a similar expression level of Oct4, Nanog, and Nestin, while cells in FCs (FC) has significantly lower expression levels (Fig. 2A, **P < 0.01). Immunostaining showed nuclear and cytoplasmic Oct4+ cells were found in SVF as well as RC and the percentage of Oct4+ cells in SVF was higher than that in RC (Fig. 2B, n = 1152 and 1168, *P < 0.05). Nuclear positive Sox2+ cells were predominantly in SVF, while nuclear and cytoplasmic Sox2 were in RC. This result suggested that the method based on digestion of ColA for 16 hours was more effective in isolating sufficient numbers of Oct4+ or Sox2+ progenitors than digestion of Col I for 3 hours, and such progenitor cells could be fractionated further in SVF and RC by filtration. Furthermore, compared to cells in Col I/Pellet, cells in SVF had no significant differences in angiogenic markers. In contrast, cells in RC had a significantly higher expression level of CD31, FLK-1, α-SMA (Fig. 2A, n = 3, **P < 0.01, *P < 0.05), but no difference in the expression level of CD34, PDGFRβ, and CD146 (Fig. 2A, n = 3, *P < 0.05, **P < 0.01). Immunostaining showed that CD31+ cells were predominantly in RC, while CD34+, CD45+, and CD146+ cells were found similarly in SVF and RC (Fig. 2C). This finding was consistent with the previously reported one, that SVF contains mostly CD34+/CD31− cells.21 Phase contrast microscopy revealed single cells in SVF versus cells tangled with matrix strands in RC (Fig. 3C, Phase). Immunostaining confirmed the presence of collagen IV, a basement membrane component, in RC (Fig. 3C, Coll IV). Collectively, these results indicated that the method of Col A digestion for 16 hours, indeed, left substantial numbers of progenitor cells in RC by filtration due to collagenase's limitation of digesting basement membrane matrix.
Figure 2.
Expression of ESCs and angiogenesis markers by different cell fractions. Human medial orbital fat was subjected to digestion with Col I for 3 hours or ColA for 16 hours to generate pellets and FCs in FLs for the former and FC for the latter by centrifugation. Pellets from the latter digestion were fractionated further into SVF and RC by filtration (Fig. 1). Quantitative RT-PCR was used to compare the transcript expression level of Oct4, Nanog, Nestin, CD31, CD34, FLK-1, α-SMA, PDGFRβ, and CD146 ([A], n = 3, *P < 0.01, **P < 0.05 when compared to pellets after Col I for 3 hours set as 1). Immunofluoresence staining was performed for Oct4 (green), Sox2 (red), CD31 (red), CD34 (green), CD45 (red), CD146 (green), and Coll IV (green, [B, C], nuclear counterstaining by Hoechst 33342 (blue), scale bars = 50 μm). Percentage of nuclear Oct4+ cells from pellet versus FL after Col I for 3 hours and SVF versus RC after ColA for 16 hours were compared (*P < 0.05, n = 81 and 710, n = 1152 and 1168, respectively).
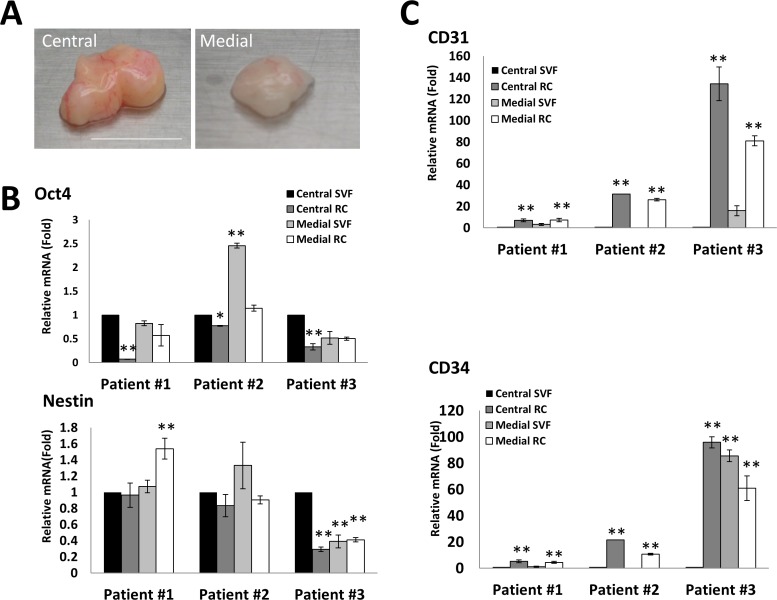
Figure 3.
Comparison of expression of ESC and angiogenic markers by central and medial fat. Human orbital fat was obtained from central and medial compartments from three patients. The central fat appeared yellow and had more blood vessels, the medial fat appeared smaller and paler in color ([A], scale bar = 1 cm). Both tissues were digested with ColA for 16 hours. The transcript expression level of Oct4 and Nestin ([B], *P < 0.05, n = 3) and that of CD31 and CD34 ([C], **P < 0.01, n = 3) were compared by quantitative RT-PCR (setting Central SVF as 1).
Expressions of Progenitor Markers by Central and Medial Orbital Fat
Because central and medial orbital fat are derived from different origins,10,22 we wondered whether progenitor cells might behave differently through isolation into SVF and RC following digestion with Col A for 16 hours. As reported, central fat appeared yellow with more blood vessels, while medial fat appeared smaller and paler in color from the same patient (Fig. 3A). For central fat, the transcript expression level of Oct4 in SVF was significantly higher than that in RC in three patients (Fig. 3B, n = 3, *P < 0.01, *P < 0.05). The same result was noted in expression of Sox2 transcript (not shown). The level of Nestin transcript showed no significant differences between SVF and RC in three patients (Fig. 3B). For medial fat, the transcript expression of Oct4, Nestin, and Sox2 (not shown) in SVF was not different from RC (Fig. 3B). For central and medial fat, expression of CD31 transcript was consistently higher in RC than SVF (Fig. 3C, n = 3, **P < 0.01). Expression of CD34 transcript was higher in RC than SVF in two of three patients. These results suggest that progenitor cells from the central and medial fat might have unique characteristics when isolated into SVF and RC.
Serial Passages of SVF and RC Cells
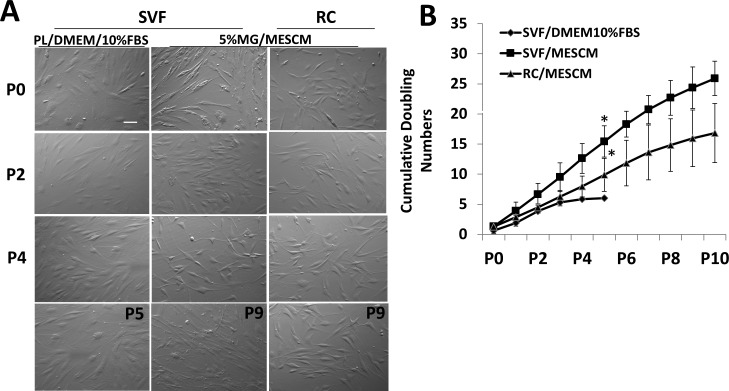
We have reported that LNCs isolated by collagenase digestion can be expanded successfully for up to 12 passages on PL coated with 5% MG in MESCM.23 We, thus, compared this same technique to expand cells from SVF and RC to the conventional method to expand cells from SVF on PL in DMEM + 10% FBS. In the primary culture, SVF cells cultured on PL in DMEM/10% FBS as well as on coated MG in MESCM were predominantly of a spindle shape. In contrast, RC cells cultured on 5% MG in MESCM were more dendritic (Fig. 4A). The SVF cells cultured on PL in DMEM/10% FBS increased in cell size and could not be passaged at P5. In contrast, SVF and RC cells cultured on coated MG in MESCM remained of the same morphology as the primary culture and could be passaged up to P10. The cumulative doubling numbers of SVF and RC cells expanded on coated MG in MESCM were significantly greater than those of SVF cells expanded on PL in DMEM/10% FBS (Fig. 4B, n = 3, P < 0.01). Additionally, the cumulative doubling numbers of SVF cells were significantly greater than that of RC cells when both were expanded on coated MG in MESCM. These results indicated that SVF and RC could be expanded successfully on 5% V in MESCM to more passages than the conventional method.
Figure 4.
Serial passages of SVF and RC on coated MG in MESCM. The SVF and RC cells isolated from medial fat by ColA for 16 hours from two patients were successively passaged to P10 on coated 5% MG in MESCM. Representative phase images on Day 8 were compared to those of SVF and RC cultured on PL in DMEM/10% FBS (A). Cumulative double numbers from SVF and RC cells cultured on coated MG in MESCM were significantly higher than SVF cells cultured on PL in DMEM/10% FBS ([B], *P < 0.01, n = 3). Scale bar: 100 μm.
Phenotypic Characterization of Expanded Cells
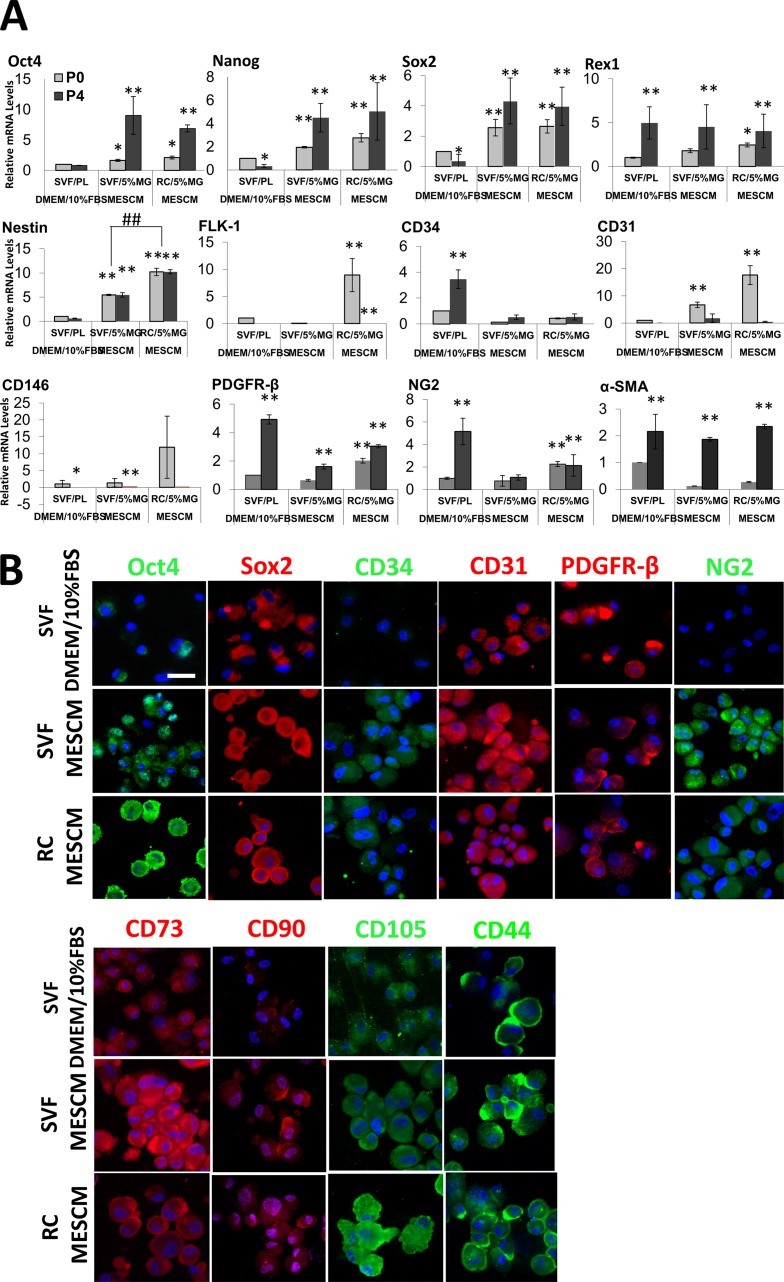
We have reported previously that limbal stromal niche cells (LNC) expanded on 5% MG in MESCM lose expression of ESC markers,18 but can be differentiated into pericyte-like angiogenic progenitors.23 Hence, we would like to find out whether ESC and/or angiogenic associated markers, such as CD31, CD34, PDGFRβ, and NG2, were maintained during expansion on 5% MG in MESCM. We also investigated expression of CD73, CD90, CD44, and CD105, which are known to be expressed by MSCs derived from adipose tissues.2 Quantitative RT-PCR analysis showed that, when compared to control SVF cells expanded on PL at P0, the expression levels of Nanog and Sox2 by SVF cells expanded on PL in DMEM + 10% FBS at P4 were significantly downregulated (Fig. 5A, *P < 0.05, n = 3), while those of CD34, PDGFRβ, NG2, and α-SMA were upregulated, suggesting a continuous shift of progenitor cells toward the pericyte phenotype under the conventional culturing condition (Fig. 5A, **P < 0.01, n = 3). In contrast, when we compared control SVF cells on PL to SVF cells expanded on coated MG in MESCM, the latter exhibited significant upregulation of all ESC markers, but not markers of angiogenesis progenitors at P0, and further upregulation of these markers at P4 except for Nestin, suggesting that the progenitor status was augmented by coated MG in MESCM (Fig. 5A, **P < 0.01, n = 3). A similar result was noted when we compared control SVF cells on PL to RC cells expanded on coated MG in MESCM except for significantly more expression of Nestin by RC than SVF (##P < 0.01, n = 3). For markers of angiogenesis progenitors, RC cells expressed more FLK-1, CD31, CD146, PDFGRβ, and NG2 than SVF at P0 on coated MG in MESCM. There was an increasing trend of expressing a higher level of PDGFRβ, NG2, and α-SMA (Fig. 5A, **P < 0.01, *P < 0.05 except for α-SMA, n = 3) by SVF and RC cells from P0 to P4 on coated MG in MESCM, also suggesting more expression of the angiogenesis markers with time. Immunofluorescence staining confirmed that SVF and RC cells expanded on coated MG in MESCM from P4 to P6 expressed stronger staining for Oct4, Sox2, CD34, CD31, PDGFRβ, and NG2 when compared to SVF cultured on PL in DMEM + 10% FBS (Fig. 5B). Furthermore, expression of MSC markers, such as CD73, CD90, CD105, and CD44, also was stronger in SVF and RC cells expanded on coated MG in MESCM than SVF cells expanded on PL in DMEM + 10% FBS. Collectively, these data supported the notion that SVF and RC contained progenitor cells, of which the phenotype could be better preserved by serial passages on coated MG in MESCM than the conventional method.
Figure 5.
Phenotypic characterization of expanded cells. Transcript expression levels of several markers by qRT-PCR were compared at P0 and P4 between SVF cells expanded on PL in DMEM/10% FBS, SVF, and RC cells expanded on coated MG in MESCM ([A], *P < 0.05, **P < 0.01, ##P < 0.01, n = 3, by setting SVF/PL at P0 in DMEM/10% FBS as 1, see Fig. 4 legend). Immunofluorescence staining of these markers also was compared among P4 SVF cells cultured on PL in DMEM/10% FBS and P6 SVF or RC cells cultured on coated MG in MESCM (B). Nuclei were counterstained by Hoechst 33342 (blue). Scale bar: 50 μm.
Vascular Endothelial Differentiation Potential
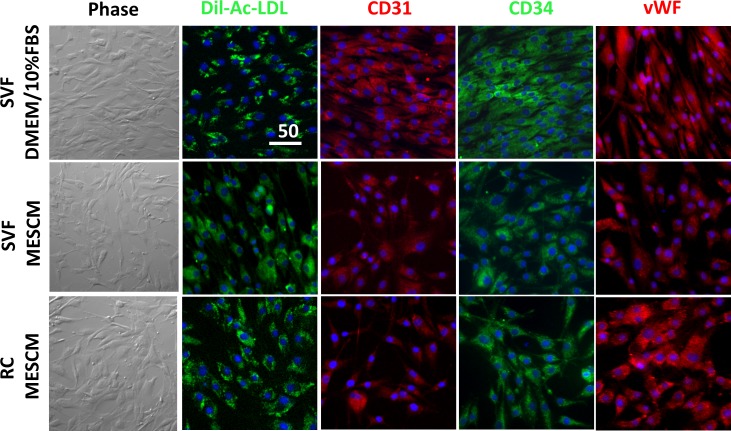
The ASCs can be differentiated into vascular endothelial cells.21,24,25 Isolated LNCs adopt angiogenic progenitor phenotype on coated MG in MESCM.23 To test whether expanded cells from SVF and RC might have angiogenic potential, we cultured P4 cells on PL in EMG2 medium supplemented with10 ng/mL VEGF-A for 4 days as reported.26 The result showed that the cell morphology changed from a spindle shape to a more rounded shape (Fig. 6, Phase), resembling human umbilical vascular endothelial cells that we have reported previously.23 Compared to SVF cells expanded on PL in DMEM/10% FBS, SVF and RC cells expanded on coated MG in MESCM expressed positive staining to CD31, CD34, and vWF, and took up Dil-Ac-LDL (Fig. 6). These results suggested that SVF and RC cells expanded on coated MG in MESCM maintained similar potential better than the conventional method to differentiate into angiogenesis progenitors.
Figure 6.
Endothelial differentiation and supporting function. P4 SVF cells expanded on PL in DMEM/10% FBS or 5% MG in MESCM and RC cells expanded on 5% MG in MESCM (see Fig. 4 legend) subsequently were cultured on PL in EGM2 supplement with VEGF-A for 4 days before being tested for uptake of Dil-Ac-LDL (green) and immunostaining of CD31 (red), CD34 (green), and vWF (red). Fluorescence prelabeled (red) HUVEC and expanded adipose SVF or RC (green) in DMEM/10% FBS or MESCM cells were seed alone on 100% MG in EGM2. Nuclei were counterstained by Hoechst 33342 (blue). Scale bar: 50 μm.
Tri-lineage Differentiation Potential
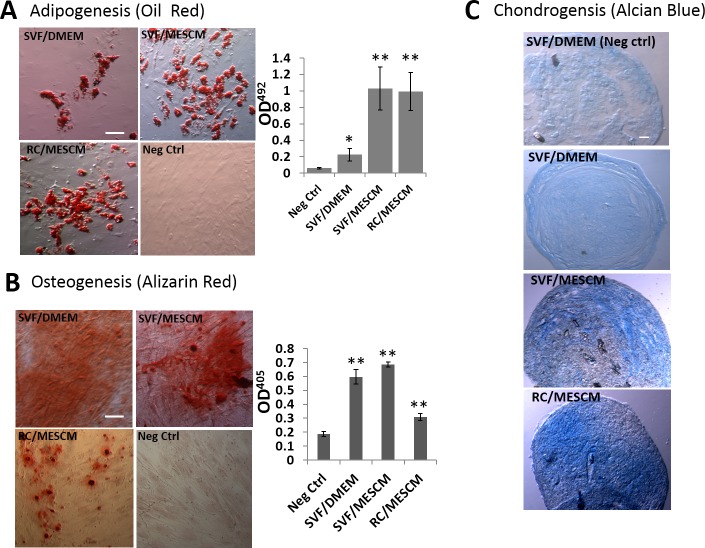
A number of studies have shown that human ASCs expanded on PL in DMEM + 10% FBS exhibit tri-lineage differentiation with adipogenesis, osteogenesis, and chondrogenesis.27 We, thus, examined whether it also was true for the aforementioned SVF and RC that were expanded on coated MG in MESCM at P4. Under adipogenic induction, Oil Red O–stained oil droplets were detected at the end of 3-week induction in SVF and RC cells expanded in MESCM. The degree of adipose differentiation in RC and SVF cells expanded on coated MG in MESCM was significantly higher than the control, that is, SVF cells expanded on PL in DMEM/10% FBS (Fig. 7B, n = 3, **P < 0.01). Under the osteogenic induction, RC expanded on MG achieved significantly higher osteogenic potential than the negative control. The SVF cells expanded on coated MG in MESCM achieved similar osteogenic potential to those expanded on PL in DMEM/10% FBS and both were greater than the negative control (Fig. 7B, n = 3, **P < 0.01). Under the chondrogenic induction for 4 weeks, SVF and RC cells expanded on MG in MESCM showed a stronger Alcian blue staining than SVF cells expanded on PL in DMEM/10% FBS (Fig. 7C). These results indicated that SVF and RC contained progenitor cells that exhibited similar or better potential in tri-lineage differentiation when expanded on coated MG in MESCM than SVF expanded by the conventional method.
Figure 7.
Comparison of tri-lineage differentiation. Cells cultured at P4 wee induced for adipogenesis, osteogenesis, or chondrogenesis (see Fig. 4 legend). The control P4 cultures were cultured in DMEM/10% FBS. The presence of lipids was detected by Oil Red O staining and quantified by absorbance measured at OD492nm (A). The presence of calcium mineralization was detected by Alizarin Red staining and quantified by absorbance measured at OD405nm (B). Chondrogenesis was measured by positive Alcian Blue staining of a cross-section of cell pellets (C). *P < 0.05, **P < 0.01, n = 3. Scale bars: 50 μm.
Discussion
Herein we made two steps forward in optimizing the recovery and expansion of orbital ASCs, by identifying significant cell populations normally left behind in the RC fractions, and by promoting greater passaging growth in a serum-free supplemented MESCM using MG as the substrate. Although ASCs can be isolated from adipose tissues in the body using two different methods using collagenase,4,16 our study revealed that the method based on digestion with Col I for 3 hours under continuous shaking failed to release the majority of Oct4+ cells from the orbital adipose tissue. The isolated cells in the pellet were enriched (∼70%) in expression of Oct4, but the overall cell yield was too low because the majority of Oct4+ cells still remained in the FC layer due to entrapment by mature adipocytes in the undigested tissue (Fig. 1). This low cell yield might not be an issue for adipose tissues obtained from the rest of the body, because it is compensated for the tissue abundance, but does present a challenge when applied to orbital adipose tissue. In addition, it highlights the variations in cell yield that may be derived from the conventional approach. We further found that the method based on 16 hours of digestion with ColA was better than Col I for 16 hours in fully digesting the entire orbital adipose tissue, resulting in a significantly higher cell yield (Fig. 1). We confirmed that cells in the SVF contained progenitor cells because they were enriched (∼60%) in Oct4+ cells and expressed Sox2, Nanog, and Nestin (Fig. 2). However, cells in RC also contained similar progenitor cells, albeit to a slightly lower extent. This finding supports our hypothesis that the traditional method used for isolating ASCs from systemic fat is not applicable for orbital fat, because it will leave some progenitor cell population behind due to entrapment with collagen IV-containing basement membrane (Fig. 2). It also is in agreement with our previous finding that collagenase does not digest such basement membrane components as collagen IV and laminin 5 in limbal tissues.17 Addition of Dispase II follow by ColA may further release the close associated progenitor cells as we have reported.28
Histological, immunohistochemical, and immunofluorescence studies have demonstrated that SVF contains cells derived from the perivascular region of the adipose tissue,12,13,15 and can be subdivided further into the following four different cell types: ASCs (CD31−/CD34+/CD45−/CD90+/CD105−/CD146−), endothelial progenitors cells (CD31+/CD34+/CD45−/CD90+/CD105low/CD146+), pericytes (CD31−/CD34−/CD45−/CD90+/CD105−/CD146+), and CD45+ blood derived cells.29 They also may contain other cell types, such as granulocytes, lymphocytes, and macrophages.5,30,31 Our study suggested that there were two types of progenitor cells in SVF and RC, respectively. Their characterization is summarized in Table 3. Progenitor cells in RC demonstrated a significantly higher expression of CD31, FLK-1, and α-SMA than those in SVF (Fig. 2A). Immunostaining confirmed that CD31+ cells were predominantly in RC, while CD34+ cells were similarly found in SVF and RC (Fig. 2C). The finding that cells in RC expressed more CD31 than those in SVF was in agreement with other reports showing that ASCs from SVF in human fat tissues express more CD347,24,32,33 and very few CD31.31 The aforementioned phenotype, that is, CD34+/CD31−/CD146−/a-SMA−/CD90+, lends support to the premise that the SVF preferentially isolates cells from the outer adventitial stromal ring of blood vessels.12 This finding was consistent with the report showing that SVF contains mostly CD34+/CD31− cells.21 Our findings were consistent between medial orbital fat derived from mesoderm and central orbital fat derived from neural crest (Fig. 3). We, thus, concluded that the conventional method excludes progenitor cells that might lie closer to the endothelium because of their propensity to be associated with the basement membrane in adipose tissues in the entire body, including the orbit. These progenitor cells might consist of inner layer endothelial progenitor CD34+/CD31+ cells and CD146+/α-SMA+/CD90±/CD34−/CD31− pericytes.12
Table 3.
Expression of Markers for ESCs, NSCs, Endothelium Progenitor, and Pericytes by Progenitors in SVF and RC From Human Orbital Fat
|
Gene Expression |
SVF |
RC |
|
| ESCs | Oct4 | ++ | ++ |
| Sox2 | ++ | ++ | |
| NSCs | Nestin | ++ | ++ |
| FLK-1 | + | +++ | |
| Endothelium progenitors | CD31 | + | +++ |
| CD146 | + | + | |
| Pericytes | PDGFRβ | ++ | ++ |
| α-SMA | + | ++ | |
Conventionally, ASCs, like other adult MSCs, are expanded on PL in DMEM with 10% FBS or bovine serum. However, this expansion method tends to result in the loss of cell proliferative potential after 4 to 6 passages.34,35 Others have tried to circumvent this problem by using serum-free media36 or media containing lower serum concentrations, but supplemented with other growth factors.34,37,38 Nonetheless, little is known whether these attempts have successfully retained expression of ESC markers and markers of angiogenesis progenitors. As shown in our recent report, we have successfully devised a new strategy to expand LNCs on coated MG in MESCM containing bFGF and LIF for up to 12 passages.18,23 Because of the propensity of progenitor cells in RC to be associated with the basement membrane, we expanded them on coated MG, and also noted a similar success in expanding cells from SVF and RC for at least 10 passages while SVF cells expanded by the conventional method only reached 4 passages (Fig. 4B). Furthermore, similar to LNCs,18,23 such expanded SVF and RC retained significantly higher expression of markers for ESC, neural crest, and angiogenesis progenitors than SVF expanded by the conventional method at the same P4 (Fig. 5). Progenitor cells from SVF and RC expanded on coated MG in MESCM exhibited similar potentials for differentiating into angiogenesis progenitors (Fig. 6) and similar or better potential for tri-lineage differentiation into adipocytes, osteocytes, and chondrocytes (Fig. 7) than cells from SVF expanded by the conventional method.
Hence, we concluded that there are, indeed, different sources of progenitor cells in ASCs, of which the isolation can be achieved via SVF and RC fractions, respectively, and the expansion method can be optimized further by the use of the basement membrane substrate. Future studies are needed to determine whether their plasticity can be extended beyond tri-lineage and endothelial differentiation to see if they are differentially derived from mesoderm or neuroectoderm. These studies will help us unravel the therapeutic potential of using these cells as a source of autologous stem cells for ocular surface regeneration.
Acknowledgments
Supported by Grants EY06819 (SCGT), and P30 EY022589 and EY014081 from the National Eye Institute, National Institutes of Health (Bethesda, MD, USA), the Walter G. Ross Distinguished Chair in Ophthalmic Research (JLG), unrestricted grants from Research to Prevent Blindness, Inc., and research grants from TissueTech, Inc.
Disclosure: S. Chen, None; M. Mahabole, None; E. Horesh, None; S. Wester, None; J.L. Goldberg, None; S.C.G Tseng, None
References
- 1. Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-- current views stem cells. 2007; 25: 2896–2902 [DOI] [PubMed] [Google Scholar]
- 2. Garcia-Castro J, Trigueros C, Madrenas J, Perez-Simon JA, Rodriguez R, Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 2008; 12: 2552–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011; 9: 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7: 211–228 [DOI] [PubMed] [Google Scholar]
- 5. Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005; 330: 142–150 [DOI] [PubMed] [Google Scholar]
- 6. Sgodda M, Aurich H, Kleist S, et al. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007; 313: 2875–2886 [DOI] [PubMed] [Google Scholar]
- 7. Sowa Y, Imura T, Numajiri T, et al. Adipose stromal cells contain phenotypically distinct adipogenic progenitors derived from neural crest. PLoS One. 2013; 8: e84206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Billon N, Monteiro MC, Dani C. Developmental origin of adipocytes: new insights into a pending question. Biol Cell. 2008; 100: 563–575 [DOI] [PubMed] [Google Scholar]
- 9. Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979; 29: 27–43 [DOI] [PubMed] [Google Scholar]
- 10. Korn BS, Kikkawa DO, Hicok KC. Identification and characterization of adult stem cells from human orbital adipose tissue. Ophthal Plast Reconstr Surg. 2009; 25: 27–32 [DOI] [PubMed] [Google Scholar]
- 11. Ho JH, Ma WH, Tseng TC, Chen YF, Chen MH, Lee OK. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Eng Part A. 2011; 17: 255–266 [DOI] [PubMed] [Google Scholar]
- 12. Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A. 2010; 77: 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin G, Garcia M, Ning H, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008; 17: 1053–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008; 102: 77–85 [DOI] [PubMed] [Google Scholar]
- 15. Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012; 21: 1299–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010; 5: 1294–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011; 17: 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie HT, Chen SY, Li GG, Tseng SC. Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012; 53: 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002; 13: 4279–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park D, Xiang AP, Mao FF, et al. Nestin is required for the proper self-renewal of neural stem cells. Stem Cells. 2010; 28: 2162–2171 [DOI] [PubMed] [Google Scholar]
- 21. Merfeld-Clauss S, Gollahalli N, March KL, Traktuev DO. Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng Part A. 2010; 16: 2953–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp Eye Res. 1979; 29: 27–43 [DOI] [PubMed] [Google Scholar]
- 23. Li GG, Chen SY, Xie HT, Zhu YT, Tseng SC. Angiogenesis potential of human limbal stromal niche cells. Invest Ophthalmol Vis Sci. 2012; 53: 3357–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004; 110: 349–355 [DOI] [PubMed] [Google Scholar]
- 25. Zannettino AC, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008; 214: 413–421 [DOI] [PubMed] [Google Scholar]
- 26. Park SW, Jun KY, Jeon J, et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010; 116: 5762–5772 [DOI] [PubMed] [Google Scholar]
- 27. Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003; 5: 362–369 [DOI] [PubMed] [Google Scholar]
- 28. Espana EM, Romano AC, Kawakita T, Di Pascuale M, Smiddy R, Tseng SC. Novel enzymatic isolation of an entire viable human limbal epithelial sheet. Invest Ophthalmol Vis Sci. 2003; 44: 4275–4281 [DOI] [PubMed] [Google Scholar]
- 29. Yoshimura K, Shigeura T, Matsumoto D, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006; 208: 64–76 [DOI] [PubMed] [Google Scholar]
- 30. Varma MJ, Breuls RG, Schouten TE, et al. Phenotypical and functional characterization of freshly isolated adipose tissue-derived stem cells. Stem Cells Dev. 2007; 16: 91–104 [DOI] [PubMed] [Google Scholar]
- 31. Astori G, Vignati F, Bardelli S, et al. “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med. 2007; 5: 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004; 109: 656–663 [DOI] [PubMed] [Google Scholar]
- 33. Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004; 109: 1292–1298 [DOI] [PubMed] [Google Scholar]
- 34. Parker A, Shang H, Khurgel M, Katz A. Low serum and serum-free culture of multipotential human adipose stem cells. Cytotherapy. 2007; 9: 637–646 [DOI] [PubMed] [Google Scholar]
- 35. Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315–317 [DOI] [PubMed] [Google Scholar]
- 36. Patrikoski M, Juntunen M, Boucher S, et al. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013; 4: 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iwashima S, Ozaki T, Maruyama S, et al. Novel culture system of mesenchymal stromal cells from human subcutaneous adipose tissue. Stem Cells Dev. 2009; 18: 533–543 [DOI] [PubMed] [Google Scholar]
- 38. Skurk T, Ecklebe S, Hauner H. A novel technique to propagate primary human preadipocytes without loss of differentiation capacity. Obesity (Silver Spring). 2007; 15: 2925–2931 [DOI] [PubMed] [Google Scholar]