Abstract
Seafood consumption is the primary route of methylmercury (MeHg) exposure for most populations. Inherent uncertainties in dietary survey data point to the need for an empirical tool to confirm exposure sources. We therefore explore the utility of Hg stable isotope ratios in human hair as a new method for discerning MeHg exposure sources. We characterized Hg isotope fractionation between humans and their diets using hair samples from Faroese whalers exposed to MeHg predominantly from pilot whales. We observed an increase of 1.75‰ in δ202Hg values between pilot whale muscle tissue and Faroese whalers’ hair but no mass-independent fractionation. We found a similar offset in δ202Hg between consumed seafood and hair samples from Gulf of Mexico recreational anglers who are exposed to lower levels of MeHg from a variety of seafood sources. An isotope mixing model was used to estimate individual MeHg exposure sources and confirmed that both Δ199Hg and δ202Hg values in human hair can help identify dietary MeHg sources. Variability in isotopic signatures among coastal fish consumers in the Gulf of Mexico likely reflects both differences in environmental sources of MeHg to coastal fish and uncertainty in dietary recall data. Additional data are needed to fully refine this approach for individuals with complex seafood consumption patterns.
1. Introduction
Methylmercury (MeHg) exposure adversely affects neurodevelopment,1 can reduce cognitive function, and may impair cardiovascular health.2−4 Seafood consumption is the primary source of MeHg exposure for most human populations.5,6 Accurately characterizing MeHg exposures and associated health risks requires a detailed understanding of both the amounts and types of seafood consumed by individuals because MeHg concentrations can vary by more than 2 orders of magnitude across fish species.7 Dietary surveys that characterize seafood consumption are commonly used to assess MeHg exposure but are affected by a variety of uncertainties7−9 and provide no information about the environmental origins of MeHg in fish. Determining the impacts of environmental change on human health requires linking environmental MeHg production directly to human exposures. Here we explore the utility of Hg stable isotope ratios measured in human hair to discern and quantify MeHg exposure from different types of seafood as a first step in this linkage.
All seven stable isotopes of Hg (196–204 amu) undergo mass-dependent fractionation (MDF).10 Prior studies in human populations report a consistent ∼2‰ increase in δ202Hg values between fish and hair of fish consumers11−13 that may result from preferential in vivo demethylation of lighter Hg isotopes.11,13,14 In contrast to humans, laboratory experiments and field studies on fish suggest that no MDF occurs during trophic transfer between the food that fish consume and the MeHg in their muscle tissues.15,16 Variability in δ202Hg values among different fish species reflects differences in microbially mediated inorganic Hg(II) reduction and MeHg degradation as well as photochemical reactions prior to the mercury entering the food web.17,18
Mass-independent fractionation (MIF) of the odd-mass-number isotopes of Hg (199Hg and 201Hg) occurs primarily during photochemical reactions involving inorganic Hg species and MeHg.18−20 MIF is reported as the deviation of a measured isotope ratio from the ratio theoretically predicted to result from MDF. Positive MIF has been observed in many aquatic organisms, including plankton, fish, and marine mammals largely due to the photochemical degradation of MeHg in water.16,18,21 This isotopic signature is retained during trophic transfer of MeHg, both through the aquatic food web and into human consumers.15,16 Previous studies have revealed differences in MIF in fish due to differing magnitudes of photochemical degradation of MeHg in the water column.22,23 As a result, MIF signatures can be used to distinguish among fish living in different environments and to characterize human MeHg exposure sources.11,12,22
This study investigates the utility of Hg isotopes for identifying human exposures to MeHg from different seafood sources at an individual level. We use unique samples from individuals with a known homogeneous source of MeHg exposure (pilot whales and human hair from whalers in the Faroe Islands) to test the hypothesis that a consistent ∼2‰ increase in δ202Hg values is found in human hair compared to consumed seafood. We then evaluate the consistency between seafood consumption patterns estimated from dietary recall data and from observed isotopic compositions of hair for individuals with more complex dietary MeHg exposure. To do this, we use a priori data on fish consumption preferences of anglers from the Gulf of Mexico to identify different characteristic types of seafood consumers (e.g., predominantly coastal fish, ocean fish, or shellfish) and compare dietary recall information to observed Hg isotope ratios.
2. Methods
2.1. Sample Collection in the Faroe Islands
We analyzed Hg isotope ratios in archived hair samples collected in 1998 from six members of the Faroese whaling society with elevated MeHg exposure [mean hair total Hg = 20.6 ± 9.58 μg/g (Table S1 of the Supporting Information)]. We chose individuals with high hair total Hg concentrations to minimize the quantities of sample needed for Hg isotope ratio analysis and isolate exposures from pilot whales (Globicephala melas).24 Pilot whales have been hunted and consumed in the Faroe Islands for more than 500 years25 and represent the predominant MeHg exposure source for individuals who consume their meat.26 Individuals considered here receive virtually all of their MeHg exposure from pilot whale, as discussed by Grandjean et al.26 Dietary survey data for the general population suggest more than 80% of MeHg exposure is from pilot whale meat (mean whale total Hg = 3.3 μg g–1) and that adults consume on average 12 g day–1.27,28 In contrast, reported consumption (72 g day–1) of ocean fish (mainly cod, Gadhus morhea) by Faroese individuals is higher by weight but constitutes less total MeHg exposure because of lower Hg concentrations in the fish (mean cod total Hg = 0.07 μg g–1).27,28 Given that the Faroese whalers are overwhelmingly exposed to MeHg from pilot whales, we used this population to more clearly characterize Hg isotope fractionation between humans and their diet.
Samples of muscle tissue from nine male pilot whales were obtained from specimens harvested in 2006 and archived by the Faroese Museum of Natural History. Total Hg concentrations in these tissues ranged from 2.8 to 5.7 μg/g of wet weight [mean = 4.0 ± 0.96 μg/g; n = 9 (Table S1 of the Supporting Information)]. We do not expect that the Hg isotopic composition of pilot whale tissue varied significantly between 1998 and 2006 because pilot whales harvested by the Faroese show site fidelity to ice-free deep waters29,30 beyond the continental shelf31 and shifts in Hg photochemistry32 due to global changes have likely had minimal impact on their habitat.
2.2. Sample Collection in the Gulf of Mexico
Lincoln et al.33 collected detailed dietary recall data and corresponding hair samples from Gulf of Mexico anglers who consume highly varied seafood diets. We analyzed archived hair samples for Hg isotope ratios in this study. As expected, total Hg concentrations in hair samples from Gulf of Mexico anglers considered here [mean = 1.2 ± 0.87 μg/g; n = 15 (Table S1 of the Supporting Information)] are much lower than those measured in hair from the Faroese whalers who are exposed to MeHg mainly from pilot whales. The dominant fraction of MeHg exposure for anglers is from fish harvested from the Gulf of Mexico (∼74%), with additional exposure from the commercial market and other locally caught seafood.33
To identify the isotopic composition of Hg in the seafood eaten by Gulf of Mexico anglers,33 we considered four coastal fish species (red snapper, gray snapper, red drum, and speckled trout) and two oceanic fish species (yellowfin tuna and blackfin tuna).23 These fish were previously analyzed for total Hg concentrations and isotopic compositions by Senn et al.,23 who identified distinct differences in Hg isotopic composition between oceanic and coastal fish. On average, oceanic fish displayed ∼1‰ higher δ202Hg values and ∼1.5‰ higher Δ199Hg values than coastal fish.23 Several other studies have analyzed Hg isotope ratios in shellfish and freshwater fish from the Gulf of Mexico region, including shrimp harvested from the Gulf34 and largemouth bass collected in central Florida lakes.22
2.3. Hg Stable Isotope Analyses
Pilot whale muscle tissue samples were freeze-dried for 24 h (VirTis Sentry Freezemobile 12SL) and then ground to a fine powder using an alumina ball mill mixer (SPEX SamplePrep Mixer/Mill) as described previously.22 After being ground, ∼200 mg of fish tissue or ∼15–150 mg of hair per sample was weighed into a ceramic boat and covered with Hg-free activated alumina and sodium carbonate powders.11 The Hg in these samples was thermally released in a two-stage furnace using previously published methods and trapped in an oxidizing 1% KMnO4 solution.22 The Hg in these solutions was then transferred into secondary KMnO4 solutions to separate it from any other combustion products.22 Mercury concentrations were measured in the final solutions by atomic absorption spectrometry and were used to calculate total Hg concentrations in the muscle tissue (wet weight) and hair samples (Table S1 of the Supporting Information). Mercury standards (NIST SRM 3133) measured during these concentration analyses were reproducible within ±5% standard deviation (SD). Total Hg concentrations in replicate samples had a mean relative percent difference (RPD) of 6.4 ± 1.1% (n = 7). Human hair standards and blanks were processed using the same methods (see the Supporting Information for further details).
Hg isotope ratios were measured in samples and standards using multicollector inductively coupled plasma mass spectrometry (MC-ICP-MS) according to established methods.35 During each analytical session, the UM-Almadén secondary standard was analyzed five times. Sample analytical uncertainty for each isotope ratio was estimated as the larger of 2 times the SD of the same ratio measured either in the relevant procedural standards or in the UM-Almadén secondary standard. The Hg isotopic compositions of samples are reported using delta notation (δxxxHg), which is the per mil (‰) deviation from a National Institute of Standards and Technology (NIST) Standard Reference Material (SRM 3133):
| 1 |
MDF is reported here using δ202Hg, as suggested elsewhere.36 MIF is calculated as the difference between the measured δxxxHg value and the theoretical value predicted on the basis of kinetic MDF. MIF is reported using capital delta notation as
| 2 |
where β is equal to 0.252 for 199Hg and 0.752 for 201Hg.18
2.4. Data Analysis and Isotope Mixing Model
Isotopic differences between the pilot whales and Faroese whalers’ hair samples were assessed using two sample t tests (R version 2.14.1, R Development Core Team). The Δ199Hg/Δ201Hg ratios in human hair and consumed seafood were analyzed using York regression, which incorporates error in both the dependent and independent variables.37 We used the quantitative relationship between the Hg isotopic composition of Faroese whalers’ hair and seafood consumed (i.e., pilot whales) to construct a deterministic two-end-member isotope mixing model for Gulf of Mexico anglers. Results of this analysis provide an isotope-based estimate of dietary MeHg exposure from oceanic and coastal fish species. We compare these results to dietary recall data on seafood consumption for the same individuals. Details of our application are provided in the Results.
3. Results
3.1. Mass-Independent Fractionation in Hair and Seafood
Figure 1 shows Hg isotope ratios measured in all hair, fish, and whale samples considered in this study. Experimental data15 and previous field studies11−13,16 indicate that MIF does not occur during trophic transfer in humans, fish, or marine mammals. Δ199Hg values of pilot whale tissues were very consistent (mean = 1.19 ± 0.03‰; n = 9), suggesting similarity in diet and foraging areas. Values of Δ199Hg measured in Faroese whalers’ hair (mean = 1.27 ± 0.03‰; n = 6) were indistinguishable from those of the pilot whales.
Figure 1.
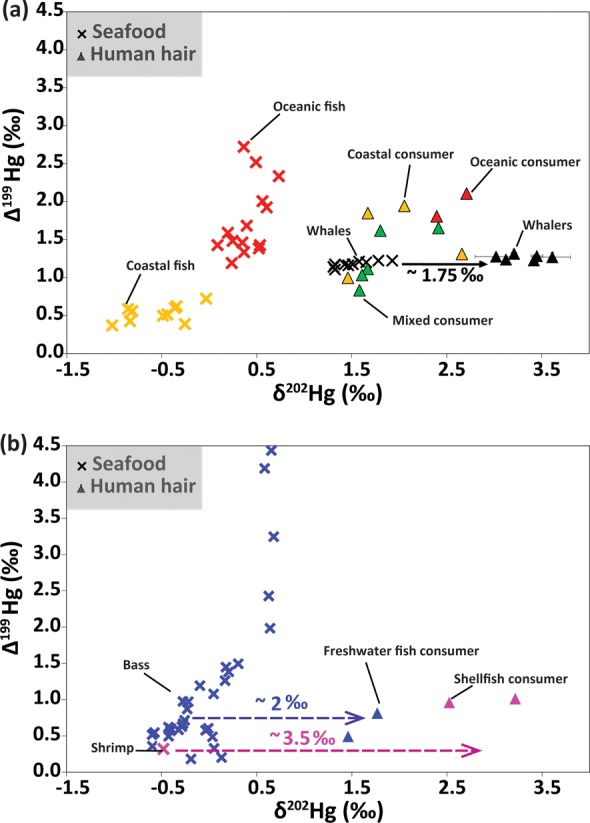
δ202Hg (‰) and Δ199Hg (‰) values in human hair (triangles) and seafood (times signs) from the Faroe Islands and Gulf of Mexico regions. Hair samples of Faroese whalers (black) have δ202Hg values on average 1.75‰ (95% confidence interval, 1.50–2.00‰) higher than those of pilot whales. Data for Gulf of Mexico coastal (yellow) and oceanic fish (red) are from ref (23). Isotopic compositions of freshwater fish (blue) and shrimp (pink) are from refs (22) and (34), respectively.
Senn et al.23 found higher MIF in oceanic fish than in coastal species from the Gulf of Mexico (Figure 1a). We also measured high positive Δ199Hg values in hair from anglers who reported consuming predominantly oceanic fish (Figure 1a). Values for these two individuals (Δ199Hg = 2.11 and 1.81‰) were in the same range as those of the oceanic fish {mean = 1.74 ± 0.48‰},23 reinforcing the hypothesis that MIF does not occur during trophic transfer. Similarly, Δ199Hg values for the mixed seafood consumers fell in an intermediate range [0.83–1.65‰ (Figure 1a)] between those of coastal and oceanic fish (Figure 1a). Hair from the two freshwater fish consumers measured here showed low Δ199Hg values (0.49 and 0.81‰) that were similar to those measured in fish from some low-clarity shallow freshwater lakes in Florida (Figure 1b).22 Hair from two shellfish consumers (Figure 1b) exhibited Δ199Hg values (0.96 and 1.01‰) slightly higher than those of shrimp34 and coastal fish10 from the Gulf of Mexico.
A discrepancy in our findings is apparent for anglers who reported primarily consuming coastal fish from the Gulf of Mexico. Hair from those individuals exhibited Δ199Hg values (1.00–1.94‰) higher than expected on the basis of those measured in coastal fish23 [0.37–0.72‰ (Figure 1a)], suggesting an oceanic source of MeHg. We attribute this anomaly to a combination of possible misreporting in dietary recall and differences in the sources of the MeHg entering coastal food webs, as discussed further below (section 4).
The Δ199Hg/Δ201Hg ratio in hair of Gulf of Mexico anglers {slope = 1.19 ± 0.03 [standard error (SE)]} observed here is the same as that previously observed in marine fish from the Gulf of Mexico (slope = 1.19).23 Experimental18−20 and field studies in coastal and open ocean environments23,38,39 suggest that Δ199Hg/Δ201Hg ratios between 1.20 and 1.36 result from photochemical degradation of MeHg before it is incorporated into food chains. Our results suggest this signature is conserved in both hair and seafood, supporting the premise that MIF is mainly due to photochemical MeHg degradation.
3.2. Mass-Dependent Fractionation in Hair and Seafood
On the basis of the results of previous studies,11,13,14 we hypothesized that MDF between seafood and human hair would result in ∼2‰ higher δ202Hg values in hair. We found that hair from the Faroese whalers displayed δ202Hg values (δ202Hg = 3.31 ± 0.22‰; n = 6) 1.75‰ higher than those of pilot whales (δ202Hg = 1.55 ± 0.21‰; n = 9) (Figure 1a). This offset is very similar to that of previous studies.11,13,14 Hair from Gulf of Mexico anglers who consumed predominantly oceanic fish also showed a similar offset in δ202Hg (2.30 and 1.98‰) compared to values measured in oceanic fish from the Gulf of Mexico (Figure 1a). For the five anglers who consumed a mixture of coastal and oceanic fish, average hair δ202Hg values were 1.40‰ higher than those of oceanic fish and 2.35‰ higher than those of coastal fish (Figure 1a).
For other types of seafood consumers, the δ202Hg values in hair were less consistently offset from those observed in seafood from the Gulf of Mexico. Values in hair from coastal fish consumers were offset by an average of 2.27‰ compared to those of coastal fish from the Gulf of Mexico, with the exception of one outlier (3.20‰ offset). The two freshwater fish consumers had hair δ202Hg values of 1.46 and 1.77‰, which are approximately 2‰ higher than those measured in Florida largemouth bass22 (Figure 1b). δ202Hg values from the two shellfish consumers (2.52 and 3.22‰) were much higher than that of Gulf of Mexico shrimp (−0.48 ± 0.05‰)34 (Figure 1b). We discuss potential causes for these differences in section 4.
3.3. MeHg Exposure Sources Calculated from Hair Hg Isotopes
We developed an empirical two-end-member isotope mixing model (eqs 3 and 4) using the results of the Faroese analysis (MDF = 1.75‰ in δ202Hg between human hair and diet). We used this model to estimate the fraction of MeHg exposure (fMIF and fMDF) resulting from the consumption of oceanic fish by Gulf of Mexico anglers, based on Hg isotope ratios measured in their diet and hair. We did not include shellfish or freshwater fish consumers in this analysis because we do not have sufficient data on Hg isotopes in these organisms to parameterize the model.
| 3 |
| 4 |
where fMIF and fMDF represent the expected fractions of MeHg exposure from oceanic fish based on observed Δ199Hg and δ202Hg values in hair, respectively, and Δ199Hgh and δ202Hgh are the measured Hg isotope ratios in hair from Gulf of Mexico anglers. The reported average Hg isotope ratios in oceanic (δ202Hgoc and Δ199Hgoc) and coastal (δ202Hgc and Δ199Hgc) fish from the northern Gulf of Mexico are as follows:23 δ202Hgoc = 0.41‰, Δ199Hgoc = 1.74‰, Δ199Hgc = 0.53‰, and δ202Hgc = −0.54‰.
We used dietary recall data to develop a comparable estimate of the fraction of an individual’s MeHg exposure from oceanic fish using eq 5. The summed product of individual consumed masses (m) of oceanic (oc) and coastal (c) fish species reported over a period of 3 months and their respective MeHg concentrations (C) were used to estimate the fraction of MeHg from ocean fish consumed by each angler. The 3 month recall period represents the same exposure window recorded in hair samples analyzed for Hg isotopes.33
| 5 |
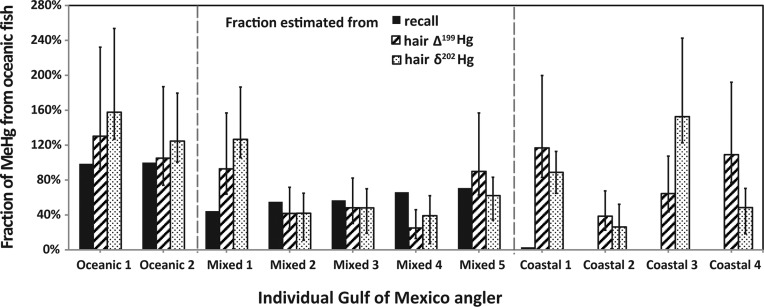
Figure 2 compares the fraction of individual MeHg exposure from oceanic fish consumption based on isotope concentrations (fMIF and fMDF) and dietary survey recall data (fr). The sensitivity of fMIF and fMDF to the observed variability in the fish Hg isotope ratios (±1 SD) is shown by error bars in Figure 2. This analysis shows that for oceanic fish consumers, there is good agreement between the estimated fraction of MeHg from oceanic fish based on the isotope mixing model and the dietary recall data. For anglers who consumed both coastal and oceanic fish, there is also good agreement for three individuals (<15% difference; mixed 2, 3, and 5) but greater discrepancies for the other two anglers (mixed 1 and 4). Full results for individuals and details of sensitivity analyses are provided in Table S2 of the Supporting Information.
Figure 2.
Fraction of total MeHg exposure due to consumption of oceanic fish for Gulf of Mexico anglers based on dietary recall data (black), hair Δ199Hg values (cross-hatched), and hair δ202Hg values (dotted). Error bars for estimates show variability that can be attributed to the ±1 SD variability in fish Hg isotope ratios.
For the coastal fish consumers, modeled MeHg exposures that can be attributed to oceanic fish are consistently higher than dietary recall data. Δ199Hg values for some coastal fish consumers (coastal 1, 3, and 4) are more consistent with those of oceanic fish (Figures 1 and 2 and Table S2 of the Supporting Information). Results of the sensitivity analysis (Figure 2 and Table S2 of the Supporting Information) indicate that moderate variability in the Δ199Hg values of coastal Gulf of Mexico fish cannot explain the high Δ199Hg values observed in the hair of these anglers (coastal 1, 3, and 4). Instead, we attribute differences to a combination of uncertainty in dietary recall data and diversity in the sources of the MeHg entering coastal food webs, as discussed further below.
4. Discussion
4.1. Utility of Hg Isotopes for Tracking Sources of MeHg
Recently, Blum et al.39 reported a relationship between fish feeding depth and Δ199Hg values that provides observational evidence of the uptake of MeHg produced in subsurface open ocean seawater by fish. The authors found that Δ199Hg values in oceanic fish were related to feeding depth and the magnitude of photochemical degradation of MeHg in the water column. Here we extend this analysis to consider the source of MeHg for pilot whales around the Faroe Islands (Figure 3).
Figure 3.
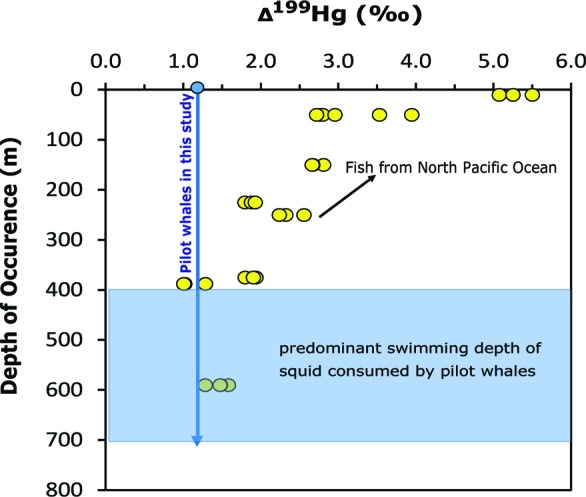
Values of Δ199Hg (‰) for pilot whales (blue arrow) from this study and nine species of marine fish (yellow circles) that feed at different depths in the central North Pacific Ocean.39 Also shown is the depth preference for the main prey item of pilot whales (squid, Todarodes sagittatus).
We find that Δ199Hg values measured in the pilot whales are comparable to those for deep-sea fish (>400 m) from the North Pacific Ocean39 (i.e., 1–2‰) (Figure 3). This is consistent with pilot whales predominantly feeding in deep waters beyond the continental shelf.40 These whales follow the abundance of their primary prey (squid, Todarodes sagittatus) to depths between 400 and 700 m (Figure 3).41 Our findings thus suggest that MeHg in the whales has been produced in the subsurface ocean water column.39,42,43
Prior studies have reported offsets in δ202Hg values between seafood and consumers similar to those observed in this study. Examples include Bolivian indigenous populations (offset = 2.0‰),13 a French urban population (offset = 2.2‰),12 and North American dentists (offset = 1.9‰).11 Similarities across multiple populations suggest that the mechanism of MDF within the human body is relatively consistent, possibly because of in vivo degradation of MeHg, and δ202Hg values may be used to identify sources of MeHg exposure for individuals.
Pharmacokinetic variability in MeHg dynamics may partially explain differences in the δ202Hg offsets between human hair and seafood among recreational anglers from the Gulf of Mexico (Figure 1). Marine mammals such as seals and some whales have the ability to demethylate MeHg in their liver14,44,45 and also to exhibit δ202Hg values higher than those of prey in their diet.16 In humans, lighter Hg isotopes are preferentially demethylated and excreted in urine.11 Variability in efficiencies of demethylation pathways in the human body would likely follow differences in other pharmacokinetic processes such as intestinal absorption of MeHg and partitioning of MeHg between blood and hair,46 leading to some variability in the offset in δ202Hg values between human hair and seafood. Additional research is needed to better characterize the demethylation pathways in the human body that are responsible for observed MDF between human hair and consumed MeHg in seafood.
4.2. Diverse Sources of MeHg in Consumed Fish
Estimated MeHg exposure from oceanic fish for Gulf of Mexico anglers derived from hair isotope data compares well to dietary survey data for consumers of predominantly oceanic and mixed fish species. Given the uncertainties in dietary recall data resulting from erroneous portion sizes, inaccurate meal frequencies, or incorrectly identified seafood species,9,47 we do not expect perfect agreement between reported consumption and isotope-based estimates. Pilot data for oceanic and mixed seafood consumers reported here suggest that Hg isotope ratios provide reasonable empirical estimates of MeHg exposure sources and may eventually be used to verify the accuracy of dietary recall data.
Results from the isotope mixing model seem to overestimate the level of MeHg derived from oceanic fish for those individuals consuming predominantly coastal fish species. Possible explanations for this departure from expectation include misreporting in dietary recall data. Because Gulf of Mexico oceanic fish have concentrations of MeHg much higher than those of coastal fish,23 consumption of even a few meals of oceanic fish may substantially affect the Hg isotopic composition of human hair. In contrast, consumption of a few meals of coastal fish is less likely to have a substantial impact on hair Hg isotope ratios of oceanic fish consumers. This may explain why we observed anomalies only among coastal fish consumers despite the fact that misreporting could occur among all individuals.
Second, we hypothesize that the MeHg in coastal fish from parts of the Gulf of Mexico and other estuaries may have an origin similar to that of oceanic fish. Environmental MeHg sources in coastal ecosystems include production in benthic sediments, rivers, and the oceanic water column.48 The relative importance of each source varies substantially across coastal systems and likely also among fish species.49−53 For example, Harris et al.48 noted that some areas of the Gulf of Mexico are highly influenced by riverine Hg inflow, while other areas are affected by large inputs from the Atlantic Ocean Loop Current. Each of these environmental sources of MeHg undergoes different photochemical reactions prior to incorporation into the food web and thus can be expected to display different Hg isotope ratios. Therefore, coastal fish harvested from the broader Gulf of Mexico region likely exhibit a range of Δ199Hg values wider than the range of those measured by Senn et al.23 Additional data are needed to test the hypothesis that distinct locations of environmental MeHg production and uptake at the base of coastal food webs can be measured using Hg isotopes in coastal fish. However, our preliminary results point to the potential utility of Hg isotopes for tracking environmental sources of MeHg production entering coastal food webs, similar to previous studies in the open ocean.39 Such research would have great value for interpreting the foraging patterns of coastal fish and associated locations of MeHg uptake.
The δ202Hg values measured in hair from anglers who were predominantly shellfish consumers (Figure 1b and Table S3 of the Supporting Information) are ∼3.5‰ higher than recent measurements of δ202Hg values in shrimp from the Gulf of Mexico.34 This larger offset, compared with the previously observed ∼2‰ offset for finfish consumers, may result from the larger and more variable proportion of total Hg that is inorganic Hg in shellfish compared to finfish.54 Inorganic Hg in seafood is known to have δ202Hg values lower than those of MeHg in the same organism.54 Preferential uptake of MeHg and excretion of inorganic Hg combined with in vivo demethylation in the human body may lead to the larger offset in δ202Hg values observed here among shellfish consumers. Additional measurements of the isotopic composition of both inorganic Hg and MeHg in frequently consumed shellfish species (e.g., crab and shrimp) are needed to confirm these hypotheses. Moreover, we expect that shellfish consumed by the Gulf of Mexico anglers are more variable in isotopic composition than the data presented in Figure 1 because more than 90% of shrimp consumed in the United States is imported.8
In summary, these data demonstrate that no MIF, but significant MDF, occurs between consumed seafood and human hair. We measured an offset in δ202Hg values between consumed seafood and human hair of ∼2‰, which is consistent with prior studies.11−13 The mechanism responsible for this observation may be demethylation of MeHg within mammals and warrants further investigation. Previous studies have identified differences in Hg isotopic composition in human biomarkers that can be used to distinguish between exposure to elemental gaseous Hg from dental practices and gold mining and exposure to MeHg in seafood.11,12 In this study, reasonable consistency between estimated fractions of MeHg exposure from oceanic fish based on hair Hg isotope ratios and dietary recall data for oceanic and mixed fish consumers suggests that Hg isotopes show promise as a tool for estimating different MeHg exposure sources. Both Δ199Hg and δ202Hg values can be used to differentiate between MeHg exposure sources (e.g., coastal vs oceanic fish). In addition, Hg isotopes also show potential for providing a direct measurement of environmental MeHg sources taken up by biota.
Acknowledgments
We acknowledge financial support for this study from the Harvard National Institute of Environmental Health Sciences Center Grant (ES00002). We are grateful to Dorete Bloch (Faroese Museum of Natural History), Keri Godin (Harvard Medical School, Boston, MA), and Nil Basu (McGill University, Montreal, QC) for the guidance and assistance with the pilot whale samples. We are also thankful to members of the Faroese whaling society and Gulf of Mexico recreational anglers who provided hair samples for our study.
Supporting Information Available
Additional methods, results, tables, and references. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
E.M.S. and J.P.S. are co-senior authors.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Oken E.; Radesky J. S.; Wright R. O.; Bellinger D. C.; Amarasiriwardena C. J.; Kleinman K. P.; Hu H.; Gillman M. W. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 2008, 167101171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A. H. A review of the studies of the cardiovascular health effects of methylmercury with consideration of their suitability for risk assessment. Environ. Res. 2005, 981133–142. [DOI] [PubMed] [Google Scholar]
- Auger N.; Kofman O.; Kosatsky T.; Armstrong B. Low-Level Methylmercury Exposure as a Risk Factor for Neurologic Abnormalities in Adults. Neurotoxicology 2005, 262149–157. [DOI] [PubMed] [Google Scholar]
- Edna Y.; Joaquim V.; Lynn G.; Illeane P.; Ellen S. Low level methylmercury exposure affects neuropsychological function in adults. Environ. Health 2003, 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D.; Anderson H. A.; Chan L. H. M.; Mahaffey K. R.; Murray M.; Sakamoto M.; Stern A. H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio 2007, 3613–11. [DOI] [PubMed] [Google Scholar]
- Global Mercury Assessment; United Nations Environment Programme: Nairobi, Kenya, 2002. [Google Scholar]
- Karimi R.; Fitzgerald T. P.; Fisher N. S. A Quantitative Synthesis of Mercury in Commercial Seafood and Implications for Exposure in the United States. Environ. Health Perspect. 2012, 120111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland E. M. Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environ. Health Perspect. 2007, 1152235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intake of Fish and Shellfish. In Exposure Factors Handbook; U.S. Environmental Protection Agency: Washington, DC, 2011; Chapter 10. [Google Scholar]
- Blum J. D.Applications of stable mercury isotopes to biogeochemistry. In Handbook of Environmental Isotope Geochemistry; Springer: Berlin, 2011; Chapter 12, pp 229–245. [Google Scholar]
- Sherman L. S.; Blum J. D.; Franzblau A.; Basu N. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ. Sci. Technol. 2013, 4773403–3409. [DOI] [PubMed] [Google Scholar]
- Laffont L.; Sonke J. E.; Maurice L.; Monrroy S. L.; Chincheros J.; Amouroux D.; Behra P. Hg speciation and stable isotope signatures in human hair as a tracer for dietary and occupational exposure to mercury. Environ. Sci. Technol. 2011, 45239910–9916. [DOI] [PubMed] [Google Scholar]
- Laffont L.; Sonke J. E.; Maurice L.; Hintelmann H.; Pouilly M.; Sanchez Bacarreza Y.; Perez T.; Behra P. Anomalous mercury isotopic compositions of fish and human hair in the Bolivian Amazon. Environ. Sci. Technol. 2009, 43238985–8990. [DOI] [PubMed] [Google Scholar]
- Wagemann R.; Trebacz E.; Boila G.; Lockhart W. L. Methylmercury and total mercury in tissues of arctic marine mammals. Sci. Total Environ. 1998, 218119–31. [DOI] [PubMed] [Google Scholar]
- Kwon S. Y.; Blum J. D.; Carvan M. J.; Basu N.; Head J. A.; Madenjian C. P.; David S. R. Absence of fractionation of mercury isotopes during trophic transfer of methylmercury to freshwater fish in captivity. Environ. Sci. Technol. 2012, 46147527–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot V.; Pastukhov M. V.; Epov V. N.; Husted S.; Donard O. F.; Amouroux D. Higher Mass-Independent Isotope Fractionation of Methylmercury in the Pelagic Food Web of Lake Baikal (Russia). Environ. Sci. Technol. 2012, 46115902–5911. [DOI] [PubMed] [Google Scholar]
- Kritee K.; Barkay T.; Blum J. D. Mass dependent stable isotope fractionation of mercury during mer mediated microbial degradation of monomethylmercury. Geochim. Cosmochim. Acta 2009, 7351285–1296. [Google Scholar]
- Bergquist B. A.; Blum J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 2007, 3185849417–420. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Hintelmann H. Mercury isotope fractionation during photoreduction in natural water is controlled by its Hg/DOC ratio. Geochim. Cosmochim. Acta 2009, 73226704–6715. [Google Scholar]
- Zheng W.; Hintelmann H. Isotope fractionation of mercury during its photochemical reduction by low-molecular-weight organic compounds. J. Phys. Chem. A 2010, 114124246–4253. [DOI] [PubMed] [Google Scholar]
- Gantner N.; Hintelmann H.; Zheng W.; Muir D. C. Variations in stable isotope fractionation of Hg in food webs of arctic lakes. Environ. Sci. Technol. 2009, 43249148–9154. [DOI] [PubMed] [Google Scholar]
- Sherman L.; Blum J. Mercury stable isotopes in sediments and largemouth bass from Florida lakes, USA. Sci. Total Environ. 2013, 448, 163–175. [DOI] [PubMed] [Google Scholar]
- Senn D. B.; Chesney E. J.; Blum J. D.; Bank M. S.; Maage A.; Shine J. P. Stable isotope (N, C, Hg) study of methylmercury sources and trophic transfer in the Northern Gulf of Mexico. Environ. Sci. Technol. 2010, 4451630–1637. [DOI] [PubMed] [Google Scholar]
- Choi A. L.; Weihe P.; Budtz-Jørgensen E.; Jørgensen P. J.; Salonen J. T.; Tuomainen T.-P.; Murata K.; Nielsen H. P.; Petersen M. S.; Askham J. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ. Health Perspect. 2009, 1173367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensen J. P.Pilot Whaling in the Faroe Islands: History. Ethnography; Symbol In Faroe University Press: Tórshavn, Faroe Islands, 2009; p 59. [Google Scholar]
- Grandjean P.; Weihe P.; Jørgensen P.; Clarkson T.; Cernichiari E.; Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch. Environ. Health 1992, 473185–195. [DOI] [PubMed] [Google Scholar]
- Julshamn K.; Andersen A.; Ringdal O.; Mørkøre J. Trace elements intake in the Faroe Islands I. Element levels in edible parts of pilot whales (Globicephalus meleanus). Sci. Total Environ. 1987, 65, 53–62. [DOI] [PubMed] [Google Scholar]
- Vestergaad T.; Zachariassen P.. Dietary survey 1981–1982 in Faroese. Frodskaparrit; 1987. [Google Scholar]
- The Cryosphere Today. Polar Research Group at the University of Illinois at Urbana-Champaign: Urbana, IL: (http://arctic.atmos.uiuc.edu/cryosphere/) (accessed October 12, 2013). [Google Scholar]
- Northern Hemisphere Snow. National Snow and Ice Data Center (NSIDC): Boulder, CO: (http://nsidc.org/cryosphere/sotc/snow_extent.html) (accesed October 12, 2013).
- Long-Finned Pilot Whale (Globicephala melas). North Atlantic Marine Mammal Commission (NAMMCO): Tromsø, Norway: (http://www.nammco.no/Nammco/Mainpage/MarineMammals/long-finned_pilot_whale.html) (accessed September 15, 2013).
- Fisher J. A.; Jacob D. J.; Soerensen A. L.; Amos H. M.; Corbitt E. S.; Streets D. G.; Wang Q.; Yantosca R. M.; Sunderland E. M. Factors driving mercury variability in the Arctic atmosphere and ocean over the past 30 years. Global Biogeochem. Cycles 2013, 2741226–1235. [Google Scholar]
- Lincoln R. A.; Shine J. P.; Chesney E. J.; Vorhees D. J.; Grandjean P.; Senn D. B. Fish consumption and mercury exposure among Louisiana recreational anglers. Environ. Health Perspect. 2011, 1192245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S. Y.; Blum J. D.; Chirby M. A.; Chesney E. J. Application of mercury isotopes for tracing trophic transfer and internal distribution of mercury in marine fish feeding experiments. Environ. Toxicol. Chem. 2013, 32102322–2330. [DOI] [PubMed] [Google Scholar]
- Blum J. D.; Bergquist B. A. Reporting of variations in the natural isotopic composition of mercury. Anal. Bioanal. Chem. 2007, 3882353–359. [DOI] [PubMed] [Google Scholar]
- Bergquist B. A.; Blum J. D. The odds and evens of mercury isotopes: Applications of mass-dependent and mass-independent isotope fractionation. Elements 2009, 56353–357. [Google Scholar]
- York D. Least squares fitting of a straight line with correlated errors. Earth Planet. Sci. Lett. 1968, 5, 320–324. [Google Scholar]
- Gehrke G. E.; Blum J. D.; Slotton D. G.; Greenfield B. K. Mercury isotopes link mercury in San Francisco Bay forage fish to surface sediments. Environ. Sci. Technol. 2011, 4541264–1270. [DOI] [PubMed] [Google Scholar]
- Blum J. D.; Popp B. N.; Drazen J. C.; Choy C. A.; Johnson M. W. Methylmercury production below the mixed layer in the North Pacific Ocean. Nat. Geosci. 2013, 6, 879–884. [Google Scholar]
- Hoydal K.; Lastein L.. Analysis of Faroese catches of pilot whales (1709–1992), in relation to environmental variations. In Biology of northern hemisphere pilot whales; Donovan G. P., Lockyer C. H., Martin A. R., Eds.; International Whaling Commission: Cambridge, U.K., 1993; pp 89–106. [Google Scholar]
- Lordan C.; Collins M. A.; Key L. N.; Browne E. D. The biology of the ommastrephid squid, Todarodes sagittatus, in the north-east Atlantic. Journal of the Marine Biological Association of the UK 2001, 812299–306. [Google Scholar]
- Sunderland E. M.; Krabbenhoft D. P.; Moreau J. W.; Strode S. A.; Landing W. M. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models. Global Biogeochem. Cycles 2009, 23, DOI: 10.1029/2008GB003425. [Google Scholar]
- Cossa D.; Averty B.; Pirrone N. The origin of methylmercury in open Mediterranean waters. Limnol. Oceanogr. 2009, 543837–844. [Google Scholar]
- Wagemann R.; Trebacz E.; Boila G.; Lockhart W. Mercury species in the liver of ringed seals. Sci. Total Environ. 2000, 261121–32. [DOI] [PubMed] [Google Scholar]
- Caurant F.; Navarro M.; Amiard J.-C. Mercury in pilot whales: Possible limits to the detoxification process. Sci. Total Environ. 1996, 186195–104. [DOI] [PubMed] [Google Scholar]
- Clewell H. J.; Gearhart J. M.; Gentry P. R.; Covington T. R.; VanLandingham C. B.; Crump K. S.; Shipp A. M. Evaluation of the uncertainty in an oral reference dose for methylmercury due to interindividual variability in pharmacokinetics. Risk Analysis 1999, 194547–558. [DOI] [PubMed] [Google Scholar]
- Warner K.; Walker Timme B. L.; Hirshfield M.. Oceana Study Reveals Seafood Fraud Nationwide. Oceana: Washington, DC: (accessed December 2013).
- Harris R.; Pollman C.; Landing W.; Evans D.; Axelrad D.; Hutchinson D.; Morey S. L.; Rumbold D.; Dukhovskoy D.; Adams D. H.; Vijayaraghavan K.; Holmes C.; Atkinson R. D.; Myers T.; Sunderland E. Mercury in the Gulf of Mexico: Sources to receptors. Environ. Res. 2012, 119, 42–52. [DOI] [PubMed] [Google Scholar]
- Balcom P. H.; Hammerschmidt C. R.; Fitzgerald W. F.; Lamborg C. H.; O’Connor J. S. Seasonal distributions and cycling of mercury and methylmercury in the waters of New York/New Jersey Harbor Estuary. Mar. Chem. 2008, 10911–17. [Google Scholar]
- Mason R. P.; Choi A. L.; Fitzgerald W. F.; Hammerschmidt C. R.; Lamborg C. H.; Soerensen A. L.; Sunderland E. M. Mercury biogeochemical cycling in the ocean and policy implications. Environ. Res. 2012, 119, 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland E. M.; Amirbahman A.; Burgess N. M.; Dalziel J.; Harding G.; Jones S. H.; Kamai E.; Karagas M. R.; Shi X.; Chen C. Y. Mercury sources and fate in the Gulf of Maine. Environ. Res. 2012, 119, 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt C. R.; Fitzgerald W. F. Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ. Sci. Technol. 2004, 3851487–1495. [DOI] [PubMed] [Google Scholar]
- Sunderland E. M.; Dalziel J.; Heyes A.; Branfireun B. A.; Krabbenhoft D. P.; Gobas F. A. Response of a macrotidal estuary to changes in anthropogenic mercury loading between 1850 and 2000. Environ. Sci. Technol. 2010, 4451698–1704. [DOI] [PubMed] [Google Scholar]
- Tsui M. T. K.; Blum J. D.; Kwon S. Y.; Finlay J. C.; Balogh S. J.; Nollet Y. H. Sources and Transfers of Methylmercury in Adjacent River and Forest Food Webs. Environ. Sci. Technol. 2012, 462010957–10964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.