Abstract
Dematiaceous fungi (black fungi) are a heterogeneous group of fungi present in diverse environments worldwide. Many species in this group are known to cause allergic reactions and potentially fatal diseases in humans and animals, especially in tropical and subtropical climates. This study represents the first survey of dematiaceous fungi in Malaysia and provides observations on their diversity as well as in vitro response to antifungal drugs. Seventy-five strains isolated from various clinical specimens were identified by morphology as well as an internal transcribed spacer (ITS)-based phylogenetic analysis. The combined molecular and conventional approach enabled the identification of three classes of the Ascomycota phylum and 16 genera, the most common being Cladosporium, Cochliobolus and Neoscytalidium. Several of the species identified have not been associated before with human infections. Among 8 antifungal agents tested, the azoles posaconazole (96%), voriconazole (90.7%), ketoconazole (86.7%) and itraconazole (85.3%) showed in vitro activity (MIC ≤1 µg/mL) to the largest number of strains, followed by anidulafungin (89.3%), caspofungin (74.7%) and amphotericin B (70.7%). Fluconazole appeared to be the least effective with only 10.7% of isolates showing in vitro susceptibility. Overall, almost half (45.3%) of the isolates showed reduced susceptibility (MIC >1 µg/mL) to at least one antifungal agent, and three strains (one Pyrenochaeta unguis-hominis and two Nigrospora oryzae) showed potential multidrug resistance.
Introduction
Dematiaceous fungi are a heterogeneous group of fungi with dark colonies and pigmented fungal elements. They are typically soil saprophytes, plant pathogens, and laboratory contaminants with a worldwide distribution in humid environments. Until 2008, more than 130 species from 70 genera have been recorded to be associated with infections in humans and animals [1], a vast increase from the 59 species belonging to 28 genera reported in 1996 by Rossmann et al. [2]. The genera most frequently involved in human infections include Bipolaris, Curvularia, Exserohilum, and Alternaria [3]. Many of the fungi are common allergens growing indoors. Besides causing hypersensitivity reactions in susceptible individuals that sometimes lead to acute exacerbation of asthma, they are also important opportunistic pathogens in immunocompromised patients [4], [5]. Although many of the cutaneous, subcutaneous, and corneal infections associated with dematiaceous fungi have been reported to be common in tropical and subtropical countries [6], to our knowledge, there has not been any extensive report on the diversity and in vitro antifungal susceptibility patterns of dematiaceous fungi isolated from clinical samples in a tropical country like Malaysia.
The spectrum of diseases associated with dematiaceous fungi ranges from superficial skin and soft tissue infections to disseminated sepsis with high mortality. The most common infections are phaeohyphomycosis [7], chromoblastomycosis [8], and eumycetoma [9], [10]. In a retrospective analysis of 101 cases of central nervous system (CNS) phaeohyphomycosis, over half occurred in immunocompetent patients [11]. Chromoblastomycosis is mainly associated with Fonsecaea, Phialophora, Cladosporium, Exophiala and Rhinocladiella species [8]. Eumycetoma is caused primarily by Madurella mycetomatis, but the aetiological agents of this disease vary with geographical regions [9]. In the tropics, Curvularia species are significant causes of fungal keratitis associated with trauma from fungus-contaminated plant materials [12], [13] while the Bipolaris, Curvularia, Exserohilum, Alternaria and Drechslera are frequently reported to be involved in invasive sinusitis [14]–[17]. Systemic dematiaceous fungal infections are rare compared to systemic candidiasis and aspergillosis. However, dematiaceous fungi are being increasingly recognized as invasive human pathogens [18] especially in organ transplant recipients [7].
The identification of dematiaceous fungi is traditionally based on the observation of differentiating morphological structures such as annellides or phialides, the presence or absence of collarettes on adelophialides, the differentiation of conidiophores, and septation of macroconidia. Molecular tests that are available today offer an alternative approach to the identification of dematiaceous fungi [19], [20]. The molecular test strategy most often used is DNA amplification, followed by sequence analysis of variable regions within pan-fungal conserved genes (18S rRNA, 5.8S rRNA and the 5′ end of the 28S rRNA) or the internal transcribed spacers (ITS1 and ITS2) [21]–[23]. In the past decade, the ITS1-5.8S-ITS2 region has become a useful alternative diagnostic tool for the identification of fungi of agricultural and clinical importance [24], [25].
The aim of this study was to appraise the diversity of dematiaceous fungi isolated from patients with signs and symptoms of fungal infection and the antifungal drug susceptibility profiles of these isolates. The information from this survey could be useful for the formulation of appropriate drug therapy for patients with suspected fungal infections in a tropical setting.
Materials and Methods
Ethics Statement
This study involved only the phenotypic and phylogenetic analysis of dematiaceous fungi isolated from routine cultures in the mycology laboratory. As no information was used that could lead to patient identification, it was considered unnecessary to apply for approval from the university's Medical Ethics Committee (http://www.ummc.edu.my/index.php/2011-09-28-08-46-26/2011-10-03-03-14-40/158-ummc-medical-ethics).
Sample Collection and Processing
The fungal isolates examined in this study were obtained from clinical specimens collected from patients attending the University Malaya Medical Centre (UMMC), Malaysia. Skin scrapings and nail clippings were collected from patients with suspected dermatomycosis. Respiratory specimens were routinely screened for fungal pathogens in patients presenting with respiratory tract infection. Other tissue fluids and tissues were processed for fungal isolation only on request by physicians when patients had clinical manifestations of fungal infection. All specimens were processed according to the laboratory's standard operating procedures (SOP). Direct microscopic examinations were performed on skin scrapings, hair and nail clippings treated with 40% potassium hydroxide (KOH), and on tissue smears after staining with Gram and Gomori's methenamine-silver nitrate stains. Cultures were put up on Sabouraud Dextrose Agar (SDA) with chloramphenicol (0.25 g/mL) and sheep blood agar. Blood specimens were placed into BD BACTEC Myco/F Lytic Medium for incubation in the BD BACTEC 9240 Blood Culture System (Becton Dickinson, USA). Positive blood samples were sub-cultured onto SDA with chloramphenicol and sheep blood agar. Swabs and nasopharyngeal secretions were inoculated directly onto SDA with chloramphenicol and sheep blood agar.
Fungal Isolates
Fungal isolates were grown on SDA incubated at 30°C for up to 4 weeks with alternate day examination for growth. When mixed colonies were observed, each colony type was sub-cultured for purity. Each fungal culture was observed macroscopically for colonial characteristics such as colour, texture, and topology. Tease mounts and slide cultures were carried out to study the arrangement of conidia under the light microscope. Slide cultures were prepared by growing the fungi on Potato Dextrose Agar (PDA) to encourage mould sporulation for 7 to 14 days at 30°C. The slides were examined periodically, with lactophenol cotton blue staining carried out when sufficient growth was attained.
DNA Extraction
Pure cultures on SDA plates were harvested by flooding each plate with 3 mL of phosphate buffered saline (PBS, pH 7.4) followed by gentle scraping of the agar surface with an L-shaped glass Pasteur pipette. The suspension was then collected into a 15 mL centrifuge tube with PBS washed glass beads and vortex-mixed for 5 min. For DNA extraction, 200 µL of suspension was processed with the ZR Fungal/Bacterial DNA MiniPrep™ (Zymo Research, USA) according to the protocol provided by the manufacturer.
PCR and Sequencing
For the amplification of the ITS1-5.8S-ITS2 region, the ITS1 (5'- TCC GTA GGT GAA CCT GCG G -3') and ITS4 (5'- TCC TCC GCT TAT TGA TAT GC -3') primer sets were used as forward and reverse primers respectively [26]. The 20 µL reaction volume contained 0.2 µM of each primer, 0.2 mM of deoxynucleotides, 1.5 mM of MgCl2, 1X PCR buffer, and 1 unit of DyNAzyme™ EXT DNA polymerase (Finnzymes, Finland) together with 5 µL extracted DNA. The PCR parameters consisted of an initial denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. The PCR product was then electrophoresed in 1% (w/v) agarose gel at 120 V for 30 min, purified and sent for Sanger sequencing (1st Base Laboratories Kuala Lumpur, Malaysia). Base and quality calling of sequenced ITS was performed using TraceTuner v3.0.6 [27]. Lucy version 1.20 [28] and the included zapping.awk script was then run to trim low quality bases (Phred value <20) called by TraceTuner from both ends of the sequences. Cleaned sequences with complete ITS1-5.8S-ITS2 regions and consensus sequences of duplicated ITS copies were subjected to further analysis. The sequences obtained in this study were deposited in GenBank under accession numbers shown in Table S1.
Data Mining
Unique ITS nucleotide sequences with complete ITS1-5.8S-ITS2 regions were searched against National Centre for Biotechnology Information (NCBI, US) non-redundant database using local Nucleotide-BLAST program to identify the molecularly-related isolates. To avoid false identification due to the errors deposited in NCBI GenBank database, we collected the top five hits from the blast results. ITS sequences of all species collected were then randomly mined from the NCBI GenBank for their complete ITS sequences with at least two sequences verified for each species, except when there was only one record in the database.
Phylogenetic Analysis
All ITS sequences from clinical isolates, together with those retrieved from the NCBI database and two out-group strains of Saccharomyces boulardii, were subjected to phylogenetic analysis. Multiple sequence alignment of all data mined ITS sequences was generated using M-Coffee [29] which adopted other packages to compute the alignments and used T-Coffee to combine all these alignments into one unique final alignment. Phylogenetic analysis was then performed using MrBayes [30]. Bayesian MCMC analysis was started by sampling across the entire general time reversible (GTR) model space. A total of 600,000 generations were run with a sampling frequency of 100, and diagnostics were calculated every 1,000 generations. A burn-in setting of 25% was used to discard the first 1,500 trees.
Antifungal Susceptibility Testing
The Epsilometer Test (Etest, Biomerieux, France) for antifungal susceptibility was carried out to determine the minimum inhibitory concentration (MIC) of antifungal drugs according to the manufacturer's protocol. The antifungal drugs tested in this study were amphotericin B (AMB), five azoles, viz. itraconazole (ITC), voriconazole (VRC), ketoconazole (KTC), fluconazole (FLC), and posaconazole (PSC), and two echinocandins, viz. anidulafungin (ANID) and caspofungin (CAS).
Results
Fungal Isolates
A total of 75 (6%) black fungi were identified from a collection of 1,250 molds isolated in the Mycology Unit of UMMC in a 5-year period, from 2006 to 2011. These isolates were mostly obtained from superficial skin, nail, subcutaneous and nasopharyngeal specimens (62), blood (10), and tissue biopsies (3) (Table S1). All isolates formed typical dark brown, olivaceous or black colonies, appearing dark on the reverse side of the agar plate and displaying septate fungal elements under the microscope.
Morphological Identification
Identification based on morphological characteristics enabled the classification of most (89.3%) of the dematiaceous fungi at the genus level, using the criteria previously described [19], [20], [31], [32] (Figure 1). Generally, Bipolaris, Curvularia and Exserohilum were characterized by floccose and brown to black colonies. The macroconidia of Curvularia had thin cell walls and transverse septa. They often appeared as curved structures due to the swelling of the central cell, which was darker compared to the end cells. The macroconidia were thick-walled and fusiform, with three to four septations in Bipolaris, and cylindrical in shape with seven to eleven septa in Exserohilum. Alternaria colonies were greenish black with short, woolly hyphae. The conidia of this genus were longitudinally and transversely septate, brown and ovate arranged singly or in chains. Members of Neoscytalidium produced colonies that were densely fluffy with gray to dark gray aerial mycelia. These fungi were typified by arthroconidia arranged in chains in a zig-zag appearance. Cladosporium isolates were velvety with olivaceous green to black green colonies. Their characteristic features included conidiophores that were straight and branched at the apical region, conidia that were ovoid to globose, with dark scarring and arranged in chains, and ramoconidia.
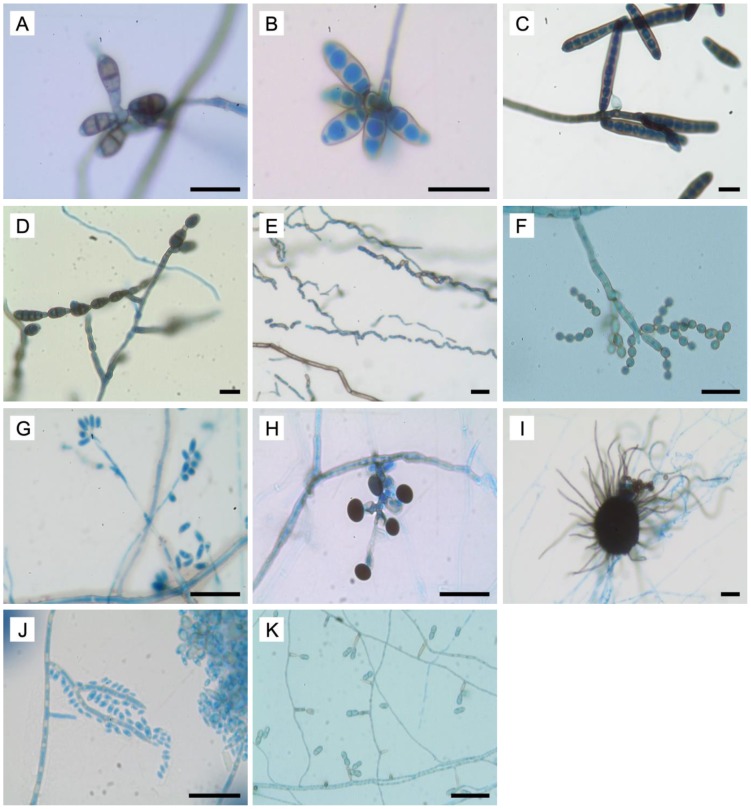
Figure 1. Microscopic features of dematiaceous fungi.
(A-D) Macroconidia of Curvularia, Bipolaris, Exserohilum and Alternaria (E) Arthroconidia of Neosyctalidium (F) Globose chain conidia and ramoconidia of Cladosporium (G) conidia of Daldinia (H) Dark conidia of Nigrospora (I) Chaetomium perithecium covered with long setae and dark ascospores (J) Spine-like conidiophore and hyaline conidia of Exophiala (K) Ochroconis two-celled clavate conidia with cylindrical conidiophore. Bars 20 µm.
Daldinia colonies appeared felty and whitish. Microscopically, the conidiophores were irregularly branched with conidiogeneous cells arising from the terminus, and conidia were ellipsoid. Nigrospora colonies were wooly and white, becoming black on aging. A single dark conidium which was spherical or oblate with a smooth wall was found at the apex of conidiogeneous cells. Chaetomium was characterized by dark colored perithecium with ostiolate and covered with long setae. The ascospores observed were mostly ovoidal, dark- colored and single-celled. The colonies were white with aerial mycelium, becoming grayish when mature. The Exophiala colonies were olivaceous-black, mucoid at the center and greenish aerial mycelium were observed at the periphery of aged colonies. Conidiophores were simple or branched, erect and spine-like with one-celled, hyaline, subglobose to ellipsoidal conidia. Lastly, the colonies of Ochroconis were dry, red-brown with red-brown pigment diffused into the growth medium. The conidiophores were cylindrical, bearing two-celled, light brown and clavate conidia.
Morphological identification was, however, not possible when there were overlapping characteristics between genera, and for strains that did not sporulate. Five mycelia sterilia and three other isolates (UM 221, UM 238 and UM 1110) could not be identified by their morphological features alone.
Phylogenetic Analysis and Taxonomic Classification
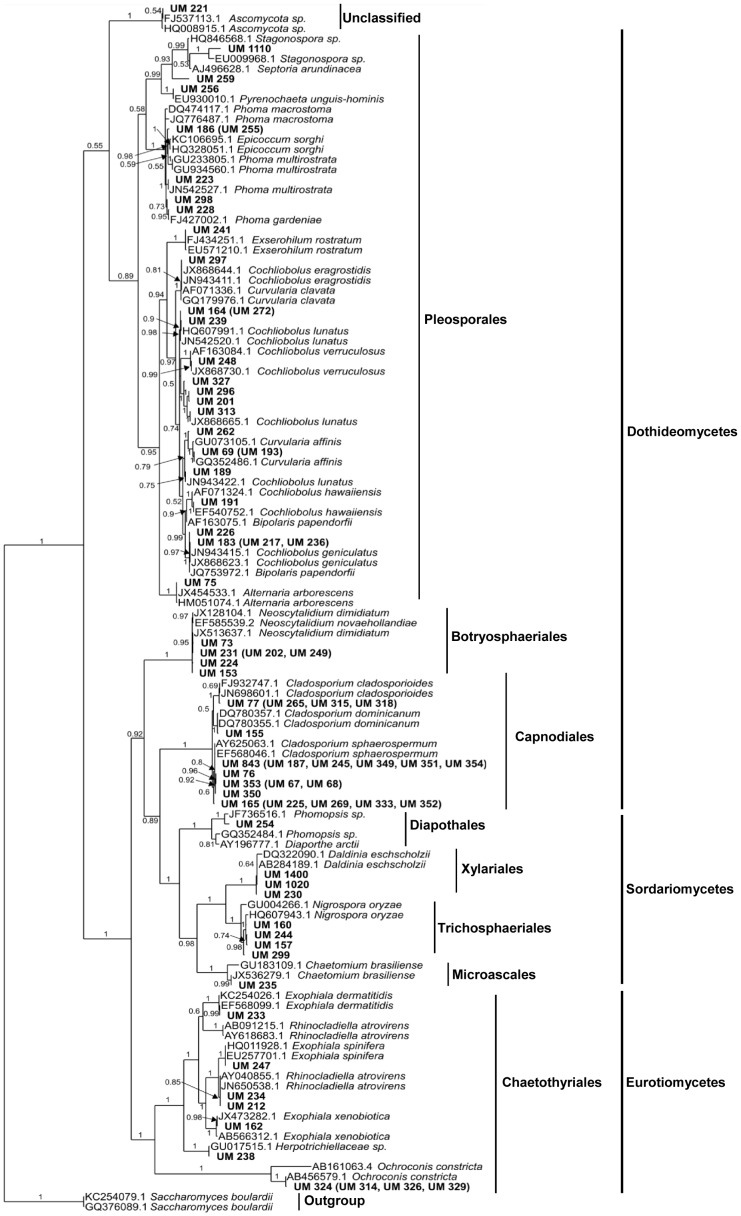
A total of 120 ITS sequences were used for the Bayesian tree construction (Figure 2). These comprised 41 unique sequences from UM isolates, 10 representative sequences from 34 UM isolates with shared sequences, 67 from reference dematiaceous fungi and two out-group strains of Saccharomyces boulardii. The phylogenetic tree resolved the 75 UM isolates into four distinct classes of Dothideomycetes (74.7%), Sordariomycetes (12.0%), Eurotiomycetes (13.3%), and one unclassified cluster made up of UM 221 and two reference Ascomycota species. Each class encompassed one to four members of orders comprising one to 28 UM isolates. Most of the isolates (65.3%) were in the two largest orders Pleosporales (28) and Capnodiales (21). From their clustering with reference strains, the majority of the isolates (86.7%) were resolved to the species level.
Figure 2. Classifications of fungal isolates.
Bayesian tree generated with general time reversible (GTR) model space based on unique ITS1-5.8S-ITS2 gene sequences with two strains of Saccharomyces boulardii as out-group. Isolate sequence duplicates are listed in parentheses next to their representative. Clinical isolates from UMMC used in this study are printed in bold. Bayesian posterior probability values for every clustering are printed on each node.
Congruence between morphological and molecular identification was observed for all Cladosporium, Neoscytalidium, Ochroconis, Daldinia and Nigrospora isolates (Table S1). Among the incongruent classifications were six of seven Bipolaris spp. and seven of 11 Curvularia spp., all resolved in the phylogenetic analysis as Cochliobolus spp.. UM 256 was identified as Phoma by morphology but Pyrenochaeta by molecular analysis, while UM 212 and UM 234 were two isolates with morphological features of Exophiala species clustered with Rhinocladiella atrovirens in the phylogenetic tree. Of the five mycelia sterilia, only one was identified as Nigrospora oryzae; the other 4 remained unclassified even after molecular analysis. Similarly, of the three isolates with no identifiable morphological features, UM 238 was resolved as a Herpotrichiellaceae sp., UM 221 showed 100% ITS sequence similarity with Ascomycota sp. FJ537113, while UM 1110 was found to be closely related to Stagonospora sp. or Septoria arundinacea.
Antifungal Susceptibility Testing
The results from the in vitro antifungal susceptibility tests varied among the various genera and species of dematiaceous fungi in this study (Table S1). As currently, there are no established guidelines on MIC breakpoints for dematiaceous fungi, interpretive comments in this study are based upon an MIC of ≤1 µg/mL being considered an indicator of potential susceptibility for most of the drugs used to treat infections by dematiaceous fungi [3]. Going by this guideline, PSC showed the highest in vitro activity (96% with MIC ≤1 µg/mL), followed by VRC (90.7%), ANID (89.3%), KTC (86.7%), ITC (85.3%), CAS (74.7%) and AMB (70.7%). FLC appeared to be the least active with 10.7% of isolates showing potential susceptibility and 34.7% having MIC>256 µg/mL. Among the isolates, Pyrenochaeta unguis-hominis and N. oryzae showed reduced susceptibility (MIC >1 µg/mL) to the largest number of antifungals with the former showing potential susceptibility to only ANID (Table 1). Overall, 45.3% of isolates showed reduced susceptibility to at least one antifungal.
Table 1. In vitro susceptibility of dematiaceous fungal isolates to antifungal agents, grouped according to MICa categories.
| Fungal Identity, (nb) | Antifungal Drugs and MICa Categories | |||||||||||||||||||||||
| AMBc | KTCd | FLCe | ITCf | VRCg | PSCh | ANIDi | CASj | |||||||||||||||||
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | |
| Alternaria arborescens (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Ascomycota sp. (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Chaetomium brasiliense (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Cladosporium cladosporioides (4) | 3 | 1 | 4 | 1 | 3 | 4 | 4 | 4 | 4 | 4 | ||||||||||||||
| Cladosporium dominicanum (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Cladosporium sphaerospermum (16) | 6 | 9 | 1 | 14 | 2 | 1 | 7 | 8 | 15 | 1 | 15 | 1 | 16 | 15 | 1 | 14 | 2 | |||||||
| Bipolaris papendorfii/Cochliobolus geniculatus (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Cochliobolus geniculatus (3) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||||||
| Cochliobolus hawaiiensis (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Cochliobolus lunatus (8) | 8 | 8 | 2 | 5 | 1 | 8 | 7 | 1 | 8 | 8 | 8 | |||||||||||||
| Cochliobolus verruculosus (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Curvularia affinis (3) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||||||
| Curvularia eragrostidis (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Daldinia eschscholzii (3) | 3 | 3 | 1 | 2 | 3 | 3 | 3 | 3 | 1 | 2 | ||||||||||||||
| Exophiala dermatitidis (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Exophiala spinifera (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Exophiala xenobiotica (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Exserohilum rostratum (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Herpotrichiellaceae sp. (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Neoscytalidium dimidiatum (4) | 4 | 4 | 1 | 3 | 3 | 1 | 4 | 4 | 4 | 4 | ||||||||||||||
| Neoscytalidium dimidiatum/Neoscytalidium novaehollandiae (2) | 2 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | ||||||||||||||
| Nigrospora oryzae (4) | 4 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 3 | 2 | 2 | 3 | 1 | 1 | 3 | |||||||
| Ochroconis constricta (4) | 4 | 3 | 1 | 4 | 4 | 4 | 4 | 4 | 4 | |||||||||||||||
| Phoma gardeniae (2) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||||||||||||||||
| Phoma multirostrata (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Phoma multirostrata/Epicoccum sorghi (2) | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | |||||||||||||||
| Phomopsis sp./Diaporthe arctii (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Pyrenochaeta unguis-hominis (1) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||
| Rhinocladiella atrovirens (2) | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | ||||||||||||||
| Stagonospora sp./Septoria arundinacea (2) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | |||||||||||||||
| Total | 53 | 17 | 5 | 65 | 7 | 3 | 8 | 41 | 26 | 64 | 7 | 4 | 68 | 6 | 1 | 72 | 0 | 3 | 67 | 4 | 4 | 56 | 5 | 14 |
minimum inhibitory concentration.
Number of fungal isolates.
Amphotericin B.
Ketoconazole.
Fluconazole.
Itraconazole.
Voriconazole.
Posaconazole.
Anidulafungin.
Caspofungin.
MIC categories:
Category A: ≤1 µg/mL (FLC: ≤1 µg/mL).
Category B: >1–32 µg/mL (FLC: >1–256 µg/mL).
Category C: >32 µg/mL (FLC: >256 µg/mL).
Clinical Significance of Isolates
The ubiquitous presence of dematiaceous fungi in the environment makes it very difficult to gauge their clinical significance when they are isolated from patient samples. In this study, we believe the majority of our isolates are not contaminants or colonizers as all isolates were from symptomatic patients, most of whom had a physician's diagnosis of fungal infection or were started on antifungal therapy. Moreover, for superficial skin and nail samples, positive cultures correlated well with signs of fungal proliferation or tissue invasion under direct KOH microscopic examination.
Discussion
With increasing recognition of the important role of fungi in human infections, diagnostic laboratories are now expected to be able to rapidly detect and accurately identify fungal pathogens to ensure early and appropriate therapy for infected patients. We have utilized the ITS sequence-based phylogenetic analysis in the classification of most of the dematiaceous fungi in this study, with good congruence attained with morphological identification. The molecular species differentiation was particularly useful for the fungi with ambiguous microscopic features, such as Phoma and Pyrenochaeta, two coelomycetes with overlapping pycnidial, conidial and cultural characteristics that were difficult to distinguish [3], [33], [34]. In the Chaetothyriales order, the synanamorphs of Exophiala and Rhinocladiella make the molecular approach a better option for their identification [35], [36]. Most of the Bipolaris and Curvularia species were resolved as Cochliobolus, the teleomorph of Bipolaris and Curvularia [37], [38]. Nevertheless, there were five isolates not resolved to the genus or species level by the ITS sequence analysis due to insufficient information in the current GenBank database [39]. More extensive sampling and further studies using multi-locus phylogeny would improve the identification of these dematiaceous fungi in the future.
Some of the dematiaceous fungi isolated in this study have been previously reported to cause human infections [3], [40]–[42]. Ten of the 16 genera we identified are listed as potential aetiological agents of phaeohyphomycosis in the guidelines compiled by an expert panel of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and European Confederation of Medical Mycology (ECMM) [43] (Table 2). Interestingly, we encountered seven species that, to our knowledge, have not been reported to be associated with human infections. These are Cladosporium dominicanum, Curvularia affinis and Ochroconis constricta (from skin scrapings), Curvularia eragrostidis (from nails), Daldinia eschscholzii (from skin, nails and blood samples), Phoma gardeniae (from skin lesion swab and nails), and Stagonospora sp./S. arundinacea (from nasopharyngeal secretion and skin scrapings). The possibility that these clinical isolates might be contaminants or had been due to the patients' incidental exposure to fungal spores cannot be totally discounted. However, in all these cases, no other pathogens that could account for the patients' signs and symptoms were identified at the infected sites.
Table 2. Genera and MICa profiles of potential phaeohyphomycosis aetiological agents compiled by Chowdhary et al. [43] and isolates identified in this study.
| Genus | Fungal species | Source | Case reported | MICa (µg/mL) | ||||
| AMBb | FLCc | ITCd | VRCe | PSCf | ||||
| Chaetomium | C. brasiliense | this study | [44] | 12 | 4.0 | 0.5 | 0.012 | 0.75 |
| C. globosum | [43] | - | 0.5–8.0 | 32->64 | 0.03–0.5 | 0.5 | - | |
| C. perlucidum | [43] | - | 0.25 | - | 0.01–0.06 | 0.5 | 0.06–0.12 | |
| Bipolaris | Cochliobolus hawaiiensis | this study | [19], [45] | 0.016 | 1.5 | 0.012 | 0.023 | 0.008 |
| B. hawaiiensis | [43] | - | 0.12–0.25 | >64 | 0.03–0.5 | 0.25–2.0 | 0.03–0.5 | |
| B. australiensis | [43] | - | 0.06–0.12 | 8.0–16.0 | 0.25–0.5 | 0.05–1.0 | 0.06 | |
| B. spicifera | [43] | - | 0.03–4.0 | 4.0->64.0 | 0.03–8.0 | 0.25–4.0 | 0.03–2.0 | |
| B. papendorfii/Cochliobolus geniculatus | this study | [19], [46] | 0.012 | 1.0 | 0.012 | 0.023 | 0.006 | |
| Curvularia | Cochliobolus geniculatus (C. senegalensis) | this study | [19], [47] | <0.002–0.094 | 1.5–8 | 0.004–0.125 | 0.016–0.064 | 0.012–0.032 |
| Cochliobolus lunatus | this study | [19], [48] | 0.003–0.064 | 0.38->256 | <0.002–0.5 | 0.008–1.0 | 0.003–0.19 | |
| Cochliobolus verruculosus | this study | [19] | 0.047 | 1.5 | 0.004 | 0.016 | 0.012 | |
| C. senegalensis | [43] | - | 0.06–0.50 | 2.0–16.0 | 0.06–1.0 | 0.12–4.0 | 0.03–0.50 | |
| C. lunata | [43] | - | 0.12->16.0 | >64 | 0.12->16 | 0.25–1.0 | 0.03–0.5 | |
| Curvularia spp. | [43] | - | 0.06->16.0 | 64->64 | 0.03->16 | 0.15->16 | 0.03–4.0 | |
| Exophiala | E. dermatitidis | this study | [19], [49] | 0.5 | 24.0 | 0.38 | 0.032 | 0.094 |
| E. spinifera | this study | [19], [50], [51] | 0.047 | 2.0 | 0.064 | 0.032 | 0.018 | |
| E. xenobiotica | this study | [52], [53] | 0.064 | 6.0 | <0.002 | 0.016 | 0.016 | |
| E. dermatitidis | [43] | - | 0.01–0.5 | - | 0.03–0.5 | 0.06–1.0 | 0.03–0.25 | |
| E. spinifera | [43] | - | 0.25–4.0 | 0.12->64 | 0.01–0.12 | 0.06–1.0 | 0.01–0.06 | |
| E. jeanselmei | [43] | - | 0.25–2.0 | 8.0–32.0 | 0.01–0.25 | 0.06–2.0 | 0.01–0.06 | |
| Exserohilum | E. rostratum | this study | [19], [54]–[57] | 0.047 | 32.0 | 0.125 | 0.25 | 0.012 |
| E. rostratum | [43] | - | 0.03–0.12 | - | 0.03–0.12 | 0.03–1.0 | 0.03–0.12 | |
| Neoscytalidium | N. dimidiatum | this study | [31], [58] | 0.008–0.064 | 0.125–16 | 0.003–12 | 0.016–0.064 | 0.003–0.125 |
| N. dimidiatum/N. novaehollandiae | this study | - | 0.032 | 0.19–3 | 0.032; 1 | <0.002–0.003 | 0.008–0.047 | |
| N. dimidiatum | [43] | - | 0.06–1.0 | - | 0.03->16 | 0.03–4 | 0.06–32 | |
| Ochroconis | O. constricta | this study | [59] | >32 | 0.38->256 | 0.024–0.25 | 0.19–0.75 | 0.047–0.25 |
| O. gallopava | [43] | - | 0.12–1.0 | 16.0->64.0 | 0.01–0.50 | 0.12–2.0 | 0.01–0.12 | |
| O. tshawytschae | [43] | - | 4 | >64.0 | 0.5 | 0.12 | - | |
| Phoma | Phoma multirostrata | this study | [60] | 0.75 | 8.0 | 0.094 | 0.064 | 0.19 |
| Phoma multirostrata/Epicoccum sorghi | this study | [19] | 0.016–0.023 | 0.19->256 | 0.19–0.25 | 0.064–0.125 | 0.125–0.19 | |
| Phoma spp. | [43] | - | 0.5-1.0 | - | 0.25–8.0 | 0.25–8.0 | - | |
| Pyrenochaeta | P. unguis-hominis | this study | [19], [40] | 1.5 | >256 | >32 | >32 | >32 |
| P. romeroi | [43] | - | 4 | >64 | 0.5 | 4 | 0.5 | |
| Rhinocladiella | R. atrovirens | this study | [20], [61], [62] | 8 | >256 | 0.19–0.38 | 0.5–0.75 | 0.008–0.047 |
| R. aquaspersa | [43] | - | 1.0–2.0 | 32–64 | 0.06–0.12 | 2 | 0.06–0.12 | |
| R. mackenziei | [43] | - | 1.0->16.0 | 16.0->64.0 | 0.01–0.25 | 0.01–2.0 | 0.01–0.25 | |
The cases reported are from de Hoog et al. 2000 [19], Ellis et al. 2007 [20], Khan et al. 2009 [31], Hubka et al. 2011 [44], Mikosz et al. 2014 [45], Da Cunha et al. 2012 [46], Guarro et al. 1999 [47], Carter and Boudreaux 2004 [48], Oztas et al. 2009 [49], Rajendran et al. 2003 [50], Wang et al. 2013 [51], Aoyama et al. 2009 [52], Morio et al. 2012 [53], Hsu and Lee 1993 [54], Andes and Casadevall 2013 [55], Pappas et al. 2013 [56], Saint-jean et al. 2007 [57], Mani et al. 2008 [58], Malani et al. 2001 [59], Singh and Barde 1990 [60], English 1980 [40], Del Palacio-Hernanz et al. 1989 [61], and Rajput et al. 2011 [62].
minimum inhibitory concentration.
Amphotericin B.
Fluconazole.
Itraconazole.
Voriconazole.
Posaconazole.
The development of antifungal susceptibility testing is relatively recent. Various methods are now available to assess antifungal properties [63] but these have been used mostly on common fungal pathogens such as Candida and Aspergillus spp. [3]. The Clinical and Laboratory Standards Institute (CLSI) has guidelines for both broth microdilution [64] and disk diffusion [65] testing of filamentous fungi that cause cutaneous and invasive fungal infections. However, the interpretation of results is still problematic as no reliable breakpoints have been published for mold MICs. In the general guidelines recently proposed for a limited number of antifungals [66], molds are considered susceptible to AMB, ITC, PSC, VRC, and CAS when the MIC is ≤1 µg/mL, intermediate when the MIC is 2 µg/mL, and resistant when the MIC is ≥4 µg/mL. It is not known whether these guidelines can be applied to dematiaceous fungi. In this study, we used the Etest which is a commercial gradient diffusion test that has been introduced to facilitate antifungal testing in diagnostic laboratories. This test has been shown to correlate well with the CLSI methods in the testing of yeasts [67] and Aspergillus spp. [68], [69] against azoles and echinocandins. In the absence of established MIC breakpoints for dematiaceous fungi, we categorized the MICs obtained for our isolates arbitrarily, to indicate potential susceptibility (MIC ≤1 µg/mL) and two levels of potential resistance (>1–32 µg/mL or >1–256 µg/mL, and >32 or >256 µg/mL). The results showed ITC, PSC and VRC to have the best in vitro activity against all the dematiaceous fungi tested. In contrast, more than a third of the isolates had high MICs (>256 µg/mL) with FLC. These observations are consistent with previous reports on the enhanced antifungal activity of the later generation triazoles [70], [71] and the limited activity of FLC on molds [72]. Similarly, CAS which had been previously reported to be less active against filamentous fungi other than Aspergillus spp. [73] was shown in our study to be less active on dematiaceous fungi than ANID and the azoles other than FLC. Our results support the recommendation by the ESCMID and ECMM to use ITC, VRC, and PSC as the treatment of choice for phaeohyphomycosis [43]. However, it is also evident that there is considerable variation in the pattern of potential resistance among the diverse species of dematiaceous fungi; hence, the choice of antifungal agents for therapy should, as far as possible, be based on the results of antifungal susceptibility testing in individual cases.
Conclusions
Many dematiaceous fungi are associated with invasive human infections. In this study, we have successfully used ITS-based phylogenetic analysis in conjunction with morphological characteristics to resolve and classify the dematiaceous fungi isolates from a Malaysian hospital. We have also demonstrated a congruence of results from the ITS-based technique with those by morphological traits. A combination of both approaches, together with antifungal drug susceptibility testing, would greatly aid in the rapid identification of these fungal species as well as in determining the appropriate therapeutic options for human infections caused by dematiaceous fungi.
Supporting Information
Isolation strain, clinical source, morphological identity, molecular identity based on phylogenetic analysis, accession number in GenBank, and minimum inhibitory concentration (MIC) data of 75 UM isolates of dematiaceous fungi.
(DOCX)
Acknowledgments
We would like to thank Tuck Soon Soo-Hoo for his guidance and useful advice in the fungal microscopy and morphological identification work.
Funding Statement
The study was supported by High Impact Research Chancellory Grant UM.C/625/1/HIR/004 (Account no. J-00000-73551) from the University of Malaya, High Impact Research MoE Grant UM.C/625/1/HIR/MOHE/MED/31 (Account no. H-20001-00-E000070) from the Ministry of Education Malaysia and Postgraduate Research Grant (PPP) PV051/2012A (awarded to Ms. Su Mei Yew) from the University of Malaya, Malaysia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kumar KK, Hallikeri K (2008) Phaeohyphomycosis. Indian J Pathol Microbiol 51: 556–558. [DOI] [PubMed] [Google Scholar]
- 2. Rossmann SN, Cernoch PL, Davis JR (1996) Dematiaceous fungi are an increasing cause of human disease. Clin Infect Dis 22: 73–80. [DOI] [PubMed] [Google Scholar]
- 3. Revankar SG, Sutton DA (2010) Melanized fungi in human disease. Clin Microbiol Rev 23: 884–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kubak BM, Huprikar SS (2009) Emerging & rare fungal infections in solid organ transplant recipients. Am J Transplant 9 Suppl 4: S208–S226. [DOI] [PubMed] [Google Scholar]
- 5. Vermeire SEM, de Jonge H, Lagrou K, Kuypers DRJ (2010) Cutaneous phaeohyphomycosis in renal allograft recipients: report of 2 cases and review of the literature. Diagn Microbiol Infect Dis 68: 177–180. [DOI] [PubMed] [Google Scholar]
- 6. Brandt ME, Warnock DW (2003) Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother 15 Suppl 2: 36–47. [DOI] [PubMed] [Google Scholar]
- 7. Levin TP, Baty DE, Fekete T, Truant AL, Suh B (2004) Cladophialophora bantiana Brain Abscess in a Solid-Organ Transplant Recipient: Case Report and Review of the Literature. J Clin Microbiol 42: 4374–4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López Martínez R, Méndez Tovar LJ (2007) Chromoblastomycosis. Clin Dermatol 25: 188–194. [DOI] [PubMed] [Google Scholar]
- 9. Afroz N, Khan N, Siddiqui FA, Rizvi M (2010) Eumycetoma versus actinomycetoma: Diagnosis on cytology. J Cytol 27: 133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Tawfiq JA, Amr SS (2009) Madura leg due to Exophiala jeanselmei successfully treated with surgery and itraconazole therapy. Med Mycol 47: 648–652. [DOI] [PubMed] [Google Scholar]
- 11. Revankar SG, Sutton DA, Rinaldi MG (2004) Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis 38: 206–216. [DOI] [PubMed] [Google Scholar]
- 12. Thomas PA (2003) Current perspectives on ophthalmic mycoses. Clin Microbiol Rev 16: 730–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karsten E, Watson SL, Foster LJR (2012) Diversity of microbial species implicated in keratitis: a review. Open Ophthalmol J 6: 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Derber C, Elam K, Bearman G (2010) Invasive sinonasal disease due to dematiaceous fungi in immunocompromised individuals: case report and review of the literature. Int J Infect Dis 14 Suppl 3: e329–e332. [DOI] [PubMed] [Google Scholar]
- 15. Schubert MS (2009) Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol 47: S324–S330. [DOI] [PubMed] [Google Scholar]
- 16. Viola GM, Sutton R (2010) Allergic fungal sinusitis complicated by fungal brain mass. Int J Infect Dis 14 Suppl 3: e299–e301. [DOI] [PubMed] [Google Scholar]
- 17. Shahid SK (2012) Rhinosinusitis in children. ISRN Otolaryngol 2012: 851831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Hoog GS, Vicente VA, Gorbushina AA (2013) The bright future of darkness-the rising power of black fungi: black yeasts, microcolonial fungi, and their relatives. Mycopathologia 175: 365–368. [DOI] [PubMed] [Google Scholar]
- 19.De Hoog GS, Guarro J, Gene J, Figueras MJ (2000) Atlas of clinical fungi. 2nd edi. The Netherlands: Centraalbureau voor Schimmekulture.
- 20.Ellis D, Davis S, Alexiou H, Handke R, Bartley R (2007) Descriptions of medical fungi. 2nd edi. Australia: Adelaide Medical Centre for Women and Children. [Google Scholar]
- 21. Hinrikson HP, Hurst SF, Lott TJ, Warnock DW, Morrison CJ (2005) Assessment of ribosomal large-subunit D1-D2, internal transcribed spacer 1, and internal transcribed spacer 2 regions as targets for molecular identification of medically important Aspergillus species. J Clin Microbiol 43: 2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rakeman JL, Bui U, LaFe K, Chen Y-C, Honeycutt RJ, et al. (2005) Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J Clin Microbiol 43: 3324–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desnos-Ollivier M, Bretagne S, Dromer F, Lortholary O, Dannaoui E (2006) Molecular identification of black-grain mycetoma agents. J Clin Microbiol 44: 3517–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jang J, Lee JH, Ki C, Lee NY (2012) Identification of clinical mold isolates by sequence analysis of the internal transcribed spacer region, ribosomal large-subunit D1/D2, and β-tubulin. Ann Lab Med 32: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White TJ, Burns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. Academic Press. pp. 315–322. [Google Scholar]
- 27.Denisov GA, Arehart AB, Curtin MD (2004) System and method for improving the accuracy of DNA sequencing and error probability estimation through application of a mathematical model to the analysis of electropherograms. US Patent 6681186.
- 28. Chou H-H, Holmes MH (2001) DNA sequence quality trimming and vector removal. Bioinformatics 17: 1093–1104. [DOI] [PubMed] [Google Scholar]
- 29. Moretti S, Armougom F, Wallace IM, Higgins DG, Jongeneel CV, et al. (2007) The M-Coffee web server: a meta-method for computing multiple sequence alignments by combining alternative alignment methods. Nucleic Acids Res 35: W645–W648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 31. Khan ZU, Ahmad S, Joseph L, Chandy R (2009) Cutaneous phaeohyphomycosis due to Neoscytalidium dimidiatum: First case report from Kuwait. J Mycol Médicale/J Med Mycol 19: 138–142. [Google Scholar]
- 32. Ju YM, Rogers JD, San Martin F (1997) A revision of the genus Daldinia . Mycotaxon 61: 243–293. [Google Scholar]
- 33. De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, et al. (2010) Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma . Mycologia 102: 1066–1081. [DOI] [PubMed] [Google Scholar]
- 34. De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, et al. (2013) Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, et al. (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Stud Mycol 58: 57–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Hoog GS, Vicente VA, Najafzadeh MJ, Harrak MJ, Badali H, et al. (2011) Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27: 46–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD (2011) Cochliobolus: an overview and current status of species. Fungal Divers 51: 3–42. [Google Scholar]
- 38. Manamgoda DS, Cai L, McKenzie EHC, Crous PW, Madrid H, et al. (2012) A phylogenetic and taxonomic re-evaluation of the Bipolaris - Cochliobolus - Curvularia Complex. Fungal Divers 56: 131–144. [Google Scholar]
- 39. Ryberg M, Kristiansson E, Sjökvist E, Nilsson RH (2009) An outlook on the fungal internal transcribed spacer sequences in GenBank and the introduction of a web-based tool for the exploration of fungal diversity. New Phytol 181: 471–477. [DOI] [PubMed] [Google Scholar]
- 40. English MP (1980) Infection of the finger-nail by Pyrenochaeta unguis-hominis . Br J Dermatol 103: 91–94. [DOI] [PubMed] [Google Scholar]
- 41. Kantarcioglu AS, de Hoog GS (2004) Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 47: 4–13. [DOI] [PubMed] [Google Scholar]
- 42. Muralidhar S, Sulthana M (1997) Nigrospora causing corneal ulcer—a case report. Indian J Pathol Microbiol 40: 549–551. [PubMed] [Google Scholar]
- 43. Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, et al. (2014) ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect 20 Suppl 3: 47–75. [DOI] [PubMed] [Google Scholar]
- 44. Hubka V, Mencl K, Skorepova M, Lyskova P, Zalabska E (2011) Phaeohyphomycosis and onychomycosis due to Chaetomium spp., including the first report of Chaetomium brasiliense infection. Med Mycol 49: 724–733. [DOI] [PubMed] [Google Scholar]
- 45. Mikosz CA, Smith RM, Kim M, Tyson C, Lee EH, et al. (2014) Fungal endophthalmitis associated with compounded products. Emerg Infect Dis 20: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Da Cunha KC, Sutton DA, Fothergill AW, Cano J, Gené J, et al. (2012) Diversity of Bipolaris species in clinical samples in the United States and their antifungal susceptibility profiles. J Clin Microbiol 50: 4061–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guarro J, Akiti T, Horta RA, Morizot Leite-Filho LA, Gené J, et al. (1999) Mycotic keratitis due to Curvularia senegalensis and in vitro antifungal susceptibilities of Curvularia spp. J Clin Microbiol 37: 4170–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carter E, Boudreaux C (2004) Fatal cerebral phaeohyphomycosis due to Curvularia lunata in an immunocompetent patient. J Clin Microbiol 42: 5419–5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oztas E, Odemis B, Kekilli M, Kurt M, Dinc BM, et al. (2009) Systemic phaeohyphomycosis resembling primary sclerosing cholangitis caused by Exophiala dermatitidis . J Med Microbiol 58: 1243–1246. [DOI] [PubMed] [Google Scholar]
- 50. Rajendran C, Khaitan BK, Mittal R, Ramam M, Bhardwaj M, et al. (2003) Phaeohyphomycosis caused by Exophiala spinifera in India. Med Mycol 41: 437–441. [DOI] [PubMed] [Google Scholar]
- 51. Wang L, She X, Lv G, Shen Y, Cai Q, et al. (2013) Cutaneous and mucosal phaeohyphomycosis caused by Exophiala spinifera in a pregnant patient: case report and literature review. Mycopathologia 175: 331–338. [DOI] [PubMed] [Google Scholar]
- 52. Aoyama Y, Nomura M, Yamanaka S, Ogawa Y, Kitajima Y (2009) Subcutaneous phaeohyphomycosis caused by Exophiala xenobiotica in a non-Hodgkin lymphoma patient. Med Mycol 47: 95–99. [DOI] [PubMed] [Google Scholar]
- 53. Morio F, Berre J-Y Le, Garcia-Hermoso D, Najafzadeh MJ, de Hoog S, et al. (2012) Phaeohyphomycosis due to Exophiala xenobiotica as a cause of fungal arthritis in an HIV-infected patient. Med Mycol 50: 513–517. [DOI] [PubMed] [Google Scholar]
- 54. Hsu MM, Lee JY (1993) Cutaneous and subcutaneous phaeohyphomycosis caused by Exserohilum rostratum . J Am Acad Dermatol 28: 340–344. [DOI] [PubMed] [Google Scholar]
- 55. Andes D, Casadevall A (2013) Insights into fungal pathogenesis from the iatrogenic epidemic of Exserohilum rostratum fungal meningitis. Fungal Genet Biol 61: 143–145. [DOI] [PubMed] [Google Scholar]
- 56. Pappas PG, Kontoyiannis DP, Perfect JR, Chiller TM (2013) Real-time treatment guidelines: considerations during the Exserohilum rostratum outbreak in the United States. Antimicrob Agents Chemother 57: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saint-jean M, St-germain G, Laferrière C, Tapiero B (2007) Hospital-acquired phaeohyphomycosis due to Exserohilum rostratum in child with leukemia. Can J Infect Dis Med Microbiol 18: 200–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mani RS, Chickabasaviah YT, Nagarathna S, Chandramuki A, Shivprakash MR, et al. (2008) Cerebral phaeohyphomycosis caused by Scytalidium dimidiatum: a case report from India. Med Mycol 46: 705–711. [DOI] [PubMed] [Google Scholar]
- 59. Malani PN, Bleicher JJ, Kauffman CA, Davenport DS (2001) Disseminated Dactylaria constricta infection in a renal transplant recipient. Transpl Infect Dis 3: 40–43. [DOI] [PubMed] [Google Scholar]
- 60. Singh SM, Barde AK (1990) Non-dermatophytes as emerging opportunistic causal agents of superficial mycoses at Balaghat (M.P). Indian J Dermatol Venereol Leprol 56: 289–292. [Google Scholar]
- 61. Del Palacio-Hernanz A, Moore MK, Campbell CK, Del Palacio-Perez-Medel A, Del Castillo-Cantero R (1989) Infection of the central nervous system by Rhinocladiella atrovirens in a patient with acquired immunodeficiency syndrome. Med Mycol 27: 127–130. [PubMed] [Google Scholar]
- 62. Rajput DK, Mehrotra A, Srivastav AK, Kumar R, Rao RN (2011) Cerebral phaeohyphomycosis mimicking glioma. Pan Arab J Neurosurg 15: 87–90. [Google Scholar]
- 63. Johnson EM (2008) Issues in antifungal susceptibility testing. J Antimicrob Chemother 61 Suppl 1: i13–i18. [DOI] [PubMed] [Google Scholar]
- 64.Clinical and Laboratory Standards Institute, CLSI (2008) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard, 2nd edi. Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 65.Clinical and Laboratory Standards Institute, CSLI (2010) Method for Antifungal Disk Diffusion Susceptibility Testing of Nondermatophyte Filamentous Fungi; Approved Guideline. Document M51-A. Clinical and Laboratory Standards Institute, Wayne, PA.
- 66. Espinel-Ingroff A, Arthington-Skaggs B, Iqbal N, Ellis D, Pfaller MA, et al. (2007) Multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconazole, posaconazole, itraconazole, amphotericin B, and caspofungin. J Clin Microbiol 45: 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guinea J, Recio S, Escribano P, Torres-Narbona M, Peláez T, et al. (2010) Rapid antifungal susceptibility determination for yeast isolates by use of Etest performed directly on blood samples from patients with fungemia. J Clin Microbiol 48: 2205–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tokarzewski S, Ziółkowska G, Nowakiewicz A (2012) Susceptibility testing of Aspergillus niger strains isolated from poultry to antifungal drugs - a comparative study of the disk diffusion, broth microdilution (M 38-A) and Etest methods. Pol J Vet Sci 15: 125–133. [DOI] [PubMed] [Google Scholar]
- 69. Martos AI, Romero A, González MT, González A, Serrano C, et al. (2010) Evaluation of the Etest method for susceptibility testing of Aspergillus spp. and Fusarium spp. to three echinocandins. Med Mycol 48: 858–861. [DOI] [PubMed] [Google Scholar]
- 70. Johnson LB, Kauffman CA (2003) Voriconazole: A New Triazole Antifungal Agent. Clin Infect Dis 36: 630–637. [DOI] [PubMed] [Google Scholar]
- 71. Sabatelli F, Patel R, Mann PA, Mendrick CA, Norris CC, et al. (2006) In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother 50: 2009–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Revankar SG (2007) Dematiaceous fungi. Mycoses 50: 91–101. [DOI] [PubMed] [Google Scholar]
- 73. Diekema DJ, Messer SA, Hollis RJ, Jones RN, Pfaller MA (2003) Activities of Caspofungin, Itraconazole, Posaconazole, Ravuconazole, Voriconazole, and Amphotericin B against 448 Recent Clinical Isolates of Filamentous Fungi. J Clin Microbiol 41: 3623–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Isolation strain, clinical source, morphological identity, molecular identity based on phylogenetic analysis, accession number in GenBank, and minimum inhibitory concentration (MIC) data of 75 UM isolates of dematiaceous fungi.
(DOCX)