Abstract
The variability and predictors of urinary concentrations of phthalate metabolites in preschool-aged children have not been thoroughly examined. Additionally, the impact of temporal changes in the use and restriction of phthalates in children’s products has not been assessed. Our objective was to identify demographic, behavioral, and temporal predictors of urinary phthalate metabolite concentrations in young children. Between 2004 and 2011, we collected up to five urine samples from each of 296 children participating in a prospective birth cohort during annual study visits at ages 1–5 years. We used linear mixed models to calculate intraclass correlation coefficients (ICCs), a measure of within-individual reproducibility, and identify demographic predictors of urinary phthalate metabolites. We used multivariable linear regression to examine cross-sectional relationships between food packaging or personal care product use and phthalate metabolites measured at age 5 years. Across annual measurements, monoethyl phthalate exhibited the least variation (ICC = 0.38), while di-2-ethylhexyl phthalate (ΣDEHP) metabolites exhibited the most variation (ICC = 0.09). Concentrations changed with age, suggesting age-related changes in phthalate exposure and perhaps metabolism. Our findings suggest that fast food consumption may be a source of butylbenzyl phthalate and di-isononyl phthalate (DiNP) exposure, and some personal care products may be sources of diethyl phthalate exposure. Concentrations of ΣDEHP metabolites decreased over the study period; however, concentrations of DiNP metabolites increased. This finding suggests that manufacturer practices and regulations, like the Consumer Product Safety Improvement Act of 2008, may decrease DEHP exposure, but additional work characterizing the nature and toxicity of replacements is critically needed.
Introduction
Phthalates are used in a range of commercial products, resulting in ubiquitous human exposure.1,2 Low molecular weight (LMW) phthalates, such as diethyl phthalate (DEP), di-n-butyl phthalate (DnBP), and di-isobutyl phthalate (DiBP), are commonly used as solvents in personal care products, including perfumes, lotions, and cosmetics,3,4 and as excipients in medications.5 High molecular weight (HMW) phthalates, such as di-2-ethylhexyl phthalate (DEHP), butyl benzyl phthalate (BBzP), di-n-octyl phthalate (DnOP), di-isononyl phthalate (DiNP), and di-isodecyl phthalate (DiDP), are used to add flexibility to plastics.6,7 Adults, infants, and children are routinely exposed to both LMW and HMW phthalates from personal care products, food packaging, and vinyl flooring.8−12
Early life exposure to phthalates may be associated with adverse health outcomes in childhood, including obesity,13 altered neurodevelopment,14−16 and asthma or allergic symptoms.17 However, previous epidemiological studies have typically measured phthalate metabolites in one spot urine sample. Because phthalate exposure is likely episodic and phthalate metabolites have short biological half-lives, urine concentrations can vary considerably within individuals.2,18−20 Sustained exposure could result from contact with phthalates present in household dust, although concentrations in indoor environments can also vary.21 Thus, phthalate exposure misclassification is an important consideration in epidemiological studies.
In response to growing concern about potential health impacts of phthalate exposure, the Consumer Product Safety Improvement Act (CPSIA) of 2008 banned the use of several phthalates in children’s toys and other child care articles in the United States. Specifically, DEHP and DnBP were banned from all children’s toys, while DINP, DIDP, and DnOP were banned from children’s toys that are more likely to be placed in a child’s mouth.22 In addition, the patterns of phthalate use in consumer products have changed, likely in response to concerns over the potential toxicity of these chemicals.23
One previous study assessed temporal variability in urinary phthalate metabolite concentrations over a six month period among 6–10 year old minority children in New York City,2 while another assessed spot urinary metabolite concentrations among 5 and 6 year olds in Germany.24 However, we are not aware of any studies evaluating the variability or predictors of urinary phthalate metabolite biomarkers among toddlers or preschool aged children. In addition, information is needed to identify potential sources of phthalate exposure and evaluate whether regulations, like the 2008 CPSIA, have reduced children’s phthalate exposures. Our objective was to characterize variability in phthalate metabolite urinary concentrations and determine if demographic, behavioral, and temporal factors predicted urinary phthalate metabolite concentrations in young children using a robust, longitudinal study design.
Materials and Methods
Study Participants
Our study sample comprised mothers and their children participating in the Health Outcomes and Measures of the Environment (HOME) Study, an ongoing prospective birth cohort in Cincinnati, Ohio. Participant recruitment and eligibility criteria have been previously described.25 Of 1263 eligible pregnant women, 468 enrolled in our study (37%) between March 2003 and January 2006. The current analysis was restricted to 327 children for whom we had at least one urinary phthalate metabolite measurement from one of five annual visits conducted between 1 and 5 years of age. Institutional review boards at Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention (CDC) approved this study, and all mothers provided written informed consent for themselves and their children prior to participation.
Urinary Phthalate Metabolite Concentrations
Children provided urine samples during annual study visits between 2004 and 2011, which included an annual clinic visit at ages 1–5 years and an annual home visit at ages 1–3 years. A subset of 61 participants provided urine samples at both the home and clinic visit at the 1, 2, or 3 year study visit (n = 16, 25, and 26 respectively). In our primary analyses, we gave priority to samples collected at the clinic visit if a child provided a urine sample at both clinic and home visits.
Prior to sample collection, each child’s genital area was wiped with a phthalate-free Wet Nap by their caregiver. For children who were not toilet trained, we collected urine samples by placing a surgical insert into a clean diaper at the beginning of the study visit. We checked the diapers for urine at the end of the study visit. If urine was present and the diaper was free of stool, the insert was placed into a polyethylene urine collection cup, and urine was later expressed from the insert with a syringe in the laboratory. For children who were in the process of being toilet trained, a training potty was lined with inserts to maximize urine collection. For children who were toilet trained, urine samples were collected directly into a urine collection cup with the aid of the child’s caregiver. All samples were refrigerated for <24 h prior to processing and then stored at −20 °C until shipment to the CDC, where they were stored at ≤−20 °C until analysis.
We measured 11 phthalate metabolites, including mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), monocarboxyoctyl phthalate (MCOP), monocarboxynonyl phthalate (MCNP), monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), and monoisobutyl phthalate (MiBP), in urine (μg/L) using previously described methods26 described in detail in the Supporting Information (SI) (Text S1). We created a summary DEHP metabolite measure (∑DEHP) by calculating the molar sum of MEHP, MEHHP, MEOHP, and MECPP. The molar sum was calculated by dividing each metabolite concentration by its molar mass and then summing the individual metabolite concentrations (μmol/L). We were not able to measure MEHP, MnBP, or MiBP in urine samples collected at 1, 2, and 3 year visits due to phthalate contamination from the diaper inserts. Because MEHP contributed relatively little to ∑DEHP metabolite concentrations at 4 and 5 years of age, (Table S1, SI) the missing MEHP values are not likely to substantively affect the ΣDEHP metabolite summary measure from 1, 2, and 3 years of age.
Predictors of Phthalate Concentrations
A computer-assisted questionnaire was administered by trained research staff to collect demographic information from participating mothers. During pregnancy, we collected information on maternal race, maternal education, and other sociodemographic variables. We collected data on the sex of the child from hospital medical charts, and on the race of the child and household income using questionnaires administered at annual visits in early childhood. We measured serum cotinine in samples collected at the 1, 2, and 3 year visits, which were averaged together for each participant as a summary measure of second-hand smoke exposure over the entire study period.
At the 5 year follow-up visit, we asked mothers questions about their child’s use of plastic food-packaging and personal care products. These included how often their child ate or drank food stored or heated in plastic, fast food, or prepackaged beverages, and if they had done so in the 48 h prior to the study visit. We also asked if their child had used various personal care products, including shampoo, conditioner, soap, hand sanitizer, hairspray or gel, sunscreen, makeup, or nail polish in the 48 h prior to the study visit.
Statistical Analysis
We did not normalize phthalate metabolite measurements by creatinine values (i.e., standardizing each individual phthalate value for the creatinine concentration of that specific sample) because changes in kidney function, muscle mass, and other physiological factors that occur during childhood influence urinary creatinine excretion. Thus, comparing urinary creatinine or creatinine-normalized biomarker concentrations between a one and a five year old may be inappropriate.27 As an alternative, we compared methods of adjustment for urine dilution (i.e., including creatinine as a separate covariate in regression models) by calculating intraclass correlation coefficients (ICC) for phthalate metabolites using three models: unadjusted for urine creatinine; adjusted for urine creatinine; and adjusted for age-specific urine creatinine z-score. We calculated urine creatinine z-scores separately for each annual study visit to avoid comparing urine creatinine levels in children of different ages.
We calculated ICCs using random intercept linear mixed models with an unstructured covariance matrix to estimate between- and within-subject variability of log10-transformed urinary phthalate metabolite concentrations. ICCs are a measure of the reproducibility of a measurement within an individual, where a value of zero indicates no reproducibility and a value of one indicates perfect reproducibility.
We calculated ICCs for annual phthalate metabolite measurements over the entire study period (i.e., annual ICC), using all participants who had phthalate measurements from at least two annual visits. We also calculated ICCs using the subset of participants who provided urine samples at both the home and clinic portion of the 1, 2, and 3 year visits to quantify variability over a shorter time frame (i.e., short-term ICC). On average, these samples were collected 13 days apart (range 1–42 days).
We used linear mixed models to examine associations between urinary phthalate metabolite concentrations and demographic and temporal variables. Demographic variables such as sex and race of the child, maternal education, and household income, as well as mean serum cotinine were entered as fixed effects, while child age, and creatinine or creatinine z-scores were entered as time-varying effects. We selected covariates based on prior knowledge and biological plausibility that they might be associated with both phthalate exposure and the predictors under investigation. The outcome variables were log10-transformed phthalate metabolite concentrations in μmol/L for the ∑DEHP summary measure and μg/L for individual phthalate metabolites. Beta estimates were exponentiated to obtain the multiplicative difference between groups for categorical predictors or per unit change for continuous predictors, and are presented as percent difference with a 95% confidence interval.
We evaluated temporal changes in phthalate exposure by examining children’s urinary phthalate metabolite concentrations as a function of age and time. We examined DiNP, DiDP, and DiBP metabolites since use of these phthalates may have increased due to changes in manufacturer practices. We evaluated BBzP, DnBP, DEP, and DEHP metabolites due to more extensive restrictions on certain phthalate use in children’s products and concern over their potential toxicity.22 Specifically, the CPSIA regulations went into effect in early 2009 and could have reduced phthalate exposure in children born in 2005–2006 since urine samples provided at 3, 4, and 5 years of age would have been collected after the ban was in effect. Since participants born in 2003 would be 5 years old in 2008 and all their samples would have been collected before the ban, we created a dichotomous “year of birth” variable to distinguish between children born in 2003–2004 from those born in 2005–2006. We modeled phthalate metabolite concentrations as a function of age, year of birth, and their product interaction (age X year of birth). This model allowed us to compare age and calendar time-related changes in phthalate metabolite concentrations among children born in 2005–2006, who would have been affected by the changes in phthalate use to those born in 2003–2004, who would have been less affected by these changes. We controlled these analyses for sex and race of the child, household income, maternal education, mean serum cotinine, and creatinine z-score.
We used multivariable linear regression to examine food packaging and personal care product use as predictors of urinary phthalate metabolites measured at the 5-year visit. We assessed food packaging as a predictor of the metabolites of DEHP, DiNP (i.e., MCOP), DiDP (i.e., MCNP), and BBzP (i.e., MBzP) in urine, as these phthalates are potential food contaminants.28,29 Similarly, we assessed personal care product use as predictors of MEP in urine, as DEP is the parent phthalate found in such products.30 We included the covariates sex, race, household income, maternal education, mean serum cotinine, creatinine z-score, and year of birth in all models examining food packaging or personal care use as predictors of urinary phthalate metabolites. When modeling the use of specific personal care products as predictors of urinary phthalate metabolites, we additionally controlled for the total number of other personal care products used.
Results
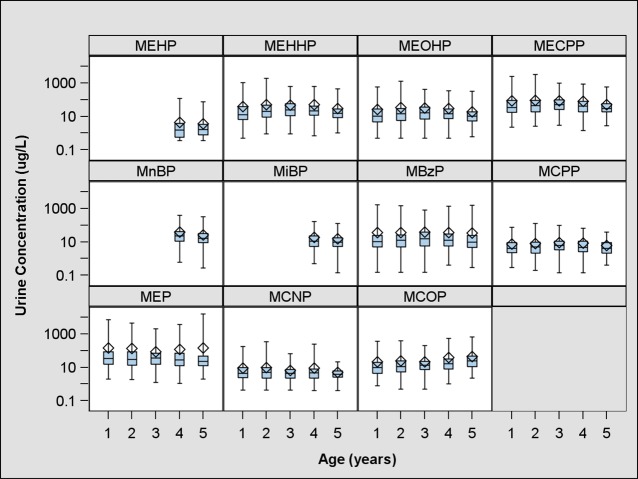
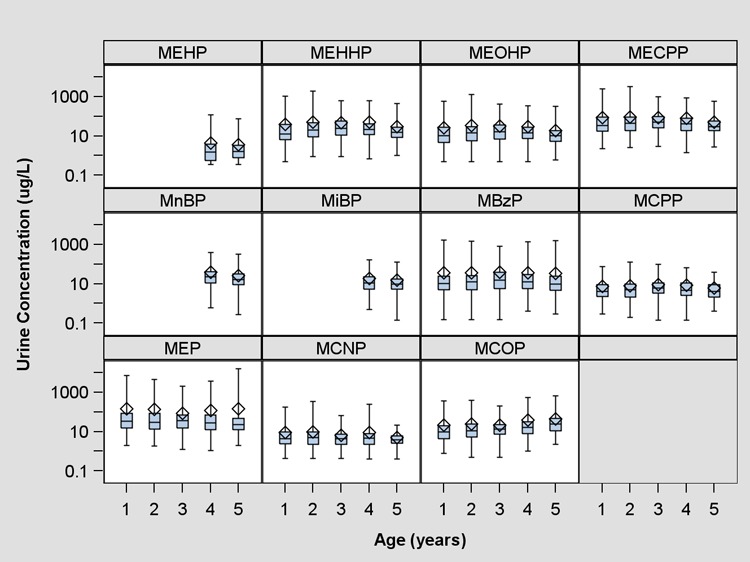
A total of 327 children provided at least one urine sample during their first 5 years of life. Of these children, 296 (n = 1050 samples) had complete covariate information on race, sex, household income, maternal education, serum cotinine, and urine creatinine (Table S2, SI). At age five, 190 children provided a urine sample and had complete covariate information. All measured phthalate metabolites were detected in greater than 99% of urine samples with the exception of MEHP, which was detected in 79% of urine samples. Unadjusted measures of urinary ∑DEHP metabolite concentrations did not change with age (Table S3, Figure S1, SI). We did, however, observe age-related trends with some individual phthalate metabolites (Figure 1, Table S3, SI). Specifically, as child age increased, MEP concentrations decreased slightly, while MCOP concentrations increased.
Figure 1.
Unadjusted urinary phthalate metabolite concentrations in HOME Study children (μg/L). Diamond indicates arithmetic mean, whiskers indicate minimum and maximum, edges of box indicate 25th and 75th percentile, and middle line indicates median. One year n = 277, 2 year n = 232, 3 year n = 234, 4 year n = 170, 5 year n = 201 (All subjects with at least 1 phthalate measurement).
Variability
The reproducibility of urinary phthalate concentrations within individuals was low for most metabolites, with annual ICCs ranging from 0.09 for ∑DEHP metabolites to 0.39 for MEP over the entire study period (adjusted for creatinine z-score) (Table 1). In general, phthalate metabolite concentrations in samples collected approximately 2 weeks apart varied less than in annually collected samples (e.g., ∑DEHP metabolites: short-term ICC = 0.20, annual ICC = 0.09). Among the subset of participants with urine samples collected at both the clinic and home portion, short-term variability was generally lower when we looked at duplicate sample pairs separately at 1, 2, and 3 years, rather than duplicate sample pairs from all visit years together. However, short-term ICCs fluctuated across visits for many phthalate metabolites (e.g., MEP: visit 1 ICC = 0.50, n = 16; visit 2 ICC = 0.33, n = 25; visit 3 ICC = 0.76, n = 27), possibly because of the relatively small number of participants who contributed two urine samples at more than one study visit, or they were not consistently the same individuals from year to year. Methods for urine dilution adjustment (unadjusted, creatinine adjusted, creatinine z-score adjusted) did not meaningfully change our reported measures of variability (Table 1).
Table 1. Intraclass Correlation Coefficient of HOME Study Children’s Urinary Phthalate Metabolite Concentrations Collected (A) Annually from 1–5 Years of Age and (B) Twice ∼2 Weeks Apart at 1–3 Years of Agea.
| A: annual ICCs | B: short-term
ICCs |
||||
|---|---|---|---|---|---|
| metabolite | allb | 1 year | 2 years | 3 yrs | allb |
| unadjusted | N = 283 subjects; 1,070 samplesc | N = 16 subjects | N = 25 subjects | N = 27 subjects | N = 61 subjects; 136 samplesd |
| ∑DEHPe | 0.18 (0.10, 0.27) | 0.35 (0.00, 0.61) | 0.27 (0.00, 0.67) | 0.44 (0.00, 0.73) | 0.29 (0.05, 0.49) |
| MBzP | 0.26 (0.17, 0.35) | 0.51 (0.30, 0.63) | 0.14 (0.00, 0.42) | 0.56 (0.29, 0.74) | 0.34 (0.09, 0.54) |
| MCPP | 0.20 (0.12, 0.29) | 0.57 (0.18, 0.85) | 0.29 (0.00, 0.67) | 0.45 (0.00, 0.75) | 0.31 (0.09, 0.49) |
| MCOP | 0.17 (0.09, 0.26) | 0.54 (0.13, 0.85) | 0.00 (0.00, 0.37) | 0.16 (0.00, 0.50) | 0.15 (0.00, 0.33) |
| MCNP | 0.21 (0.13, 0.29) | 0.71 (0.37, 0.86) | 0.20 (0.00, 0.59) | 0.25 (0.00, 0.70) | 0.25 (0.09, 0.41) |
| MEP | 0.36 (0.26, 0.45) | 0.30 (0.08, 0.52) | 0.39 (0.15, 0.61) | 0.53 (0.13, 0.79) | 0.32 (0.08, 0.53) |
| MnBPf | 0.22 (0.02, 0.43) | N/Af | N/Af | N/Af | N/Af |
| MiBPf | 0.30 (0.10, 0.49) | N/Af | N/Af | N/Af | N/Af |
| Creatinine Adjusted | |||||
| ∑DEHPe | 0.11 (0.03, 0.19) | 0.41 (0.00, 0.85) | 0.24 (0.00, 0.56) | 0.52 (0.16, 0.81) | 0.20 (0.00, 0.41) |
| MBzP | 0.25 (0.18, 0.35) | 0.26 (0.08, 0.42) | 0.25 (0.03, 0.56) | 0.61 (0.35, 0.75) | 0.39 (0.19, 0.57) |
| MCPP | 0.19 (0.11, 0.27) | 0.60 (0.19, 0.91) | 0.60 (0.21, 0.83) | 0.34 (0.00, 0.63) | 0.25 (0.07, 0.43) |
| MCOP | 0.15 (0.06, 0.24) | 0.50 (0.00, 0.86) | 0.00 (0.00, 0.26) | 0.22 (0.00, 0.59) | 0.03 (0.00, 0.20) |
| MCNP | 0.19 (0.09, 0.27) | 0.72 (0.45, 0.91) | 0.28 (0.00, 0.69) | 0.17 (0.00, 0.79 | 0.19 (0.00, 0.38) |
| MEP | 0.35 (0.22, 0.44) | 0.46 (0.29, 0.61) | 0.28 (0.00, 0.72) | 0.66 (0.38, 0.85) | 0.29 (0.00, 0.52) |
| MnBPf | 0.20 (0.01, 0.39) | N/Af | N/Af | N/Af | N/Af |
| MiBPf | 0.31 (0.06, 0.50) | N/Af | N/Af | N/Af | N/Af |
| Creatinine z-Score Adjusted | |||||
| ∑DEHPe | 0.09 (0.02, 0.16) | 0.46 (0.07, 0.75) | 0.28 (0.00, 0.53) | 0.48 (0.26, 0.67) | 0.20 (0.00, 0.40) |
| MBzP | 0.25 (0.16, 0.34) | 0.39 (0.22, 0.53) | 0.42 (0.20, 0.68) | 0.59 (0.35, 0.79) | 0.36 (0.16, 0.55) |
| MCPP | 0.19 (0.11, 0.26) | 0.64 (0.43, 0.86) | 0.59 (0.32, 0.78) | 0.33 (0.07, 0.53) | 0.25 (0.08, 0.43) |
| MCOP | 0.13 (0.03, 0.22) | 0.47 (0.22, 0.63) | 0.00 (0.00, 0.11) | 0.22 (0.00, 0.40) | 0.03 (0.00, 0.20) |
| MCNP | 0.20 (0.09, 0.28) | 0.61 (0.51, 0.68) | 0.24 (0.00, 0.53) | 0.18 (0.00, 0.66) | 0.19 (0.00, 0.38) |
| MEP | 0.39 (0.26, 0.48) | 0.50 (0.36, 0.67) | 0.33 (0.02, 0.69) | 0.76 (0.30, 0.88) | 0.33 (0.04, 0.56) |
| MnBPf | 0.16 (0.00, 0.38) | N/Af | N/Af | N/Af | N/Af |
| MiBPf | 0.31 (0.07, 0.50) | N/Af | N/Af | N/Af | N/Af |
| creatinine | 0.18 (0.08, 0.28) | 0.45 (0.21, 0.65) | 0.03 (0.00, 0.40) | 0.30 (0.00, 0.54) | 0.17 (0.00, 0.34) |
ICC = intraclass correlation coefficient; N/A = not available.
Models adjusted for visit number.
All participants with at least 2 phthalate measurements, but not necessarily complete covariate information.
Some participants provided 2 urine samples in multiple years but are counted only once in the final column.
∑DEHP= Sum of MEHP, MEHHP, MEOHP, and MECPP.
MnBP and MiBP measured only at 4 and 5 years of age.
Demographic Predictors
Race predicted select urinary phthalate concentrations, with black children having 91% (95% CI: 64, 123) higher MEP concentrations, but 26% (95% CI: 34, 17) lower MCPP concentrations, compared to white children after adjustment for confounders (Table 2). Children in the “Other” race category (Asian, Pacific Islander, or American Indian) had higher concentrations of MEP, MnBP, and MiBP compared to white children (Table 2), although this analysis was limited by the relatively small number of children in the “Other” category (n = 19). Higher maternal education predicted lower concentrations of all measured phthalates, with children of mothers who were college graduates having 10%, 33%, and 36% lower concentrations of ∑DEHP metabolites, MBzP, and MEP, respectively, compared to children of mothers who did not graduate from high school. Boys had 12% (95% CI: −23, 0.3) lower urinary concentrations of MEP compared to girls. Children’s average serum cotinine levels did not predict urinary phthalate metabolite concentrations after adjustment for other covariates.
Table 2. Adjusted Difference in HOME Study Children’s Urinary Phthalate Concentrations at 1-5 Years of Age According to Sociodemographic Factors or Serum Cotinine Levels (N = 296 With a Total of 1050 Repeated Measures)a.
| ∑DEHPb | MBzP | MCPP | MCOP | MCNP | MEP | MnBPc | MiBPc | ||
|---|---|---|---|---|---|---|---|---|---|
| variable | N (%) or mean (SD) | % differenced (95% CI) | % differenced (95% CI) |
% differenced (95% CI) |
% differenced (95% CI) |
% differenced (95% CI) | % differenced (95% CI) |
% differenced (95% CI) |
% differenced (95% CI) |
| Child Race | |||||||||
| non-hispanic white | 194 (66) | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| non-hispanic black | 83 (28) | –6 (−17, 7) | –6 (−19, 10) | –26 (−34, −17) | –19 (−29, 8) | –9 (−19, 1) | 91 (64, 123) | –18 (−33, 1) | 2 (−17, 25) |
| othere | 19 (6) | 6 (−13, 30) | –1 (−23, 26) | –2 (−18, 16) | 1 (−18, 24) | 12 (−6, 34) | 97 (55, 152) | 41 (1, 96) | 53 (10, 113) |
| Sex | |||||||||
| female | 161 (54) | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| male | 135 (46) | –2 (−12, 10) | 12 (−3, 28) | 5 (−4, 16) | –2 (−13, 10) | –4 (−13, 6) | –12 (−23, 0.3) | –18 (−32, −1) | –15 (−30, 3) |
| Age (% change/year) | 2.94 (1.47) | –2 (−5, 1) | –2 (−6, 1) | –0.2 (−3, 3) | 21 (18, 26) | –6 (−9, −4) | –11 (−14, −7) | –24 (−34, −13) | –13 (−23, −1) |
| Year of Birth | |||||||||
| born 2003–2004 | 142 (48) | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| born 2005–2006 | 154 (52) | –15 (−24, −6) | –18 (−27, −6) | –3 (−11, 6) | 1 (−9, 13) | –4 (−12, 5) | –18 (−28, −7) | –19 (−32, 4) | –3 (−18, 15) |
|
Household Income (% difference/$10,000) |
62,276 (40,570) | 0.5 (−1, 2) | –4 (−6, −1) | 0 (−2, 2) | –0.4 (−2, 2) | –1 (−2, 1) | –2 (−4, 1) | 0.3 (−3, 3) | –1 (−3, 2) |
| Maternal Education | |||||||||
| less than grade 12 | 25 (8) | ref. | ref. | ref. | ref. | ref. | ref. | ref. | ref. |
| high school graduate | 32 (11) | 4 (−12, 23) | –21 (−35, −3) | –11 (−24, 3) | 2 (−14, 22) | –17 (−28, −4) | 13 (−8, 38) | –15 (−35, 12) | –26 (−44, −3) |
| some college | 77 (26) | 7 (−5, 21) | –12 (−24, 2) | –1 (−11, 10) | –0.1 (−12, 13) | –11 (−20, −1) | –14 (−26, −1) | 17 (−3, 42) | –2 (−19, 19) |
| college graduate | 162 (55) | –10 (−19, 0.1) | –33 (−41, −24) | –17 (−25, −10) | –12 (−21, −2) | –20 (−27, −12) | –36 (−44, −28) | –9 (−23, 8) | –8 (−23, 9) |
|
Mean Cotinine (% difference/ng/mL) |
0.66 (1.97) | 1 (−2, 5) | 5 (−0.1, 10) | 1 (−3, 4) | –2 (−5, 2) | –1 (−5, 2) | 0 (−5, 5) | 3 (−2, 9) | 0.5 (−5, 6) |
Results adjusted for all other variables listed in Table 2 and urinary creatinine z-score. CI= confidence interval.
∑DEHP= MEHP, MEHHP, MEOHP, and MECPP.
MnBP and MiBP measured only at visits 4 and 5.
% Difference = percent difference in geometric means compared to the reference group for categorical variables or for a one unit increase for continuous variables.
Other race = Asian, Pacific Islander, or American Indian.
Food Packaging and Personal Care Products
Eating fast food at least once per week was associated with 35% higher MCOP (95% CI: −2, 85) and 55% higher MBzP (95% CI: 9, 123) urine concentrations compared to eating fast food less than once per week after adjustment for covariates (Table 3). Five year-old children who ate foods that had been stored in plastic at least once per day had urinary MCOP concentrations 38% higher (95% CI: −2, 96) than those who ate such foods less than once per week, while children who ate foods that had been heated in plastic within the past 48 h had MCOP concentrations 34% lower than those who did not (95% CI: −56, −0.4). Urinary concentrations of the ∑DEHP metabolites, MCNP, MCPP (a nonspecific metabolite of HMW phthalates and a minor metabolite of DnBP), and MBzP were not associated with eating food stored or heated in plastic. Drinking prepackaged beverages within the past 48 h was associated with 29% higher (95% CI: 4, 62) urinary MCPP concentrations, but was not associated with ΣDEHP metabolite, MCOP, MCNP, or MBzP concentrations.
Table 3. Adjusted Difference in HOME Study Children’s Urinary Phthalate Metabolite Concentrations at 5 Years of Age According to Parent-Reported Child Food Packaging Use and Diet (N = 190)a.
| ∑DEHPb (μmol/L) | MBzP (μg/L) | MCPP (μg/L) | MCOP (μg/L) | MCNP (μg/L) | ||
|---|---|---|---|---|---|---|
| variable | N (%) | % differencec (95% CI) | % differencec (95% CI) | % differencec (95% CI) | % differencec (95% CI) | % differencec (95% CI) |
| Food Stored in Plastic | ||||||
| <1/week | 15 (7.9) | ref. | ref. | ref. | ref. | ref. |
| 1–6/week | 80 (42.1) | 25 (1, 56) | 32 (−7, 86) | 11 (−12, 40) | –4 (−28, 30) | 9 (−12, 35) |
| ≥1/day | 62 (32.6) | –3 (−25, 25) | 28 (−14, 91) | 5 (−19, 38) | 38 (−2, 96) | 20 (−6, 53) |
| Food Stored in Plastic in Past 48 h | ||||||
| no | 39 (20.5) | ref. | ref. | ref. | ref. | ref. |
| yes | 113 (59.5) | –9 (−27, 13) | 15 (−19, 63) | 0 (−21, 26) | –7 (−32, 26) | 16 (−6, 44) |
| Food Heated in Plastic | ||||||
| <1/week | 93 (48.9) | ref. | ref. | ref. | ref. | ref. |
| ≥1/week | 64 (33.7) | 3 (−19, 30) | –3 (−32, 40) | –4 (−24, 23) | –3 (−30, 33) | 11 (−11, 39) |
| Food Heated in Plastic in Past 48 h | ||||||
| No | 127 (66.8) | ref. | ref. | ref. | ref. | ref. |
| Yes | 25 (13.2) | –14 (−36, 14) | –25 (−53, 19) | –28 (−47, −3) | –34 (−56, −0.4) | –20 (−40, 5) |
| Fast Food | ||||||
| <1/week | 85 (44.7) | ref. | ref. | ref. | ref. | ref. |
| ≥1/week | 72 (37.9) | –1 (−22, 25) | 55 (9, 123) | 19 (−7, 51) | 35 (−2, 85) | 11 (−11, 39) |
| Prepackaged Beverages | ||||||
| <1/week | 53 (27.9) | ref. | ref. | ref. | ref. | ref. |
| 1–6/week | 65 (34.2) | 1 (−20, 28) | 15 (−20, 67) | 22 (−5, 57) | 15 (−17, 60) | 17 (−6, 47) |
| ≥1/day | 37 (19.5) | –17 (−36, 7) | –29 (−53, 5) | 14 (−12, 48) | 16 (−19, 64) | 1 (−21, 29) |
| Prepackaged Beverages in Past 48 h | ||||||
| no | 68 (35.8) | ref. | ref. | ref. | ref. | ref. |
| yes | 83 (43.7) | 16 (−7, 44) | –8 (−35, 29) | 29 (4, 62) | 18 (−12, 57) | 1 (−17, 24) |
Adjusting for sex, race of child, household income, maternal education, mean serum cotinine, urinary creatinine z-score and year of birth. CI = confidence interval.
∑DEHP= MEHP, MEHHP, MEOHP, and MECPP.
% Difference = percent difference in geometric means compared to the reference category.
Most participants used shampoo and various types of soap within 48 h of urine collection, but less than 15% used hairspray or hair gel, makeup, or nail polish. Recent use of hairspray or hair gel was associated with 63% higher urinary MEP concentrations (95% CI: 7, 148) among 5 year-old participants after adjustment for covariates, while the use of other personal care products was not (Table S4, SI).
Temporal Trends vs Age
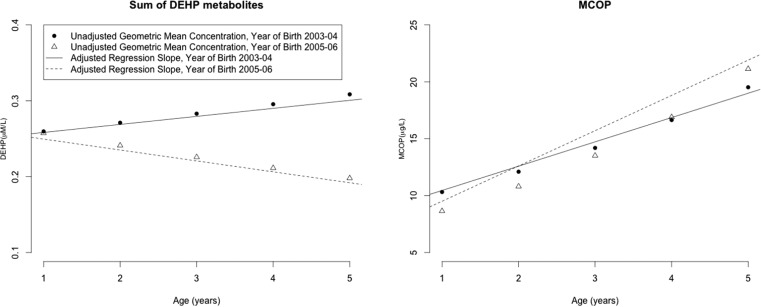
Increasing age and later year of birth (2005–2006 compared to 2003–2004) were both associated with lower concentrations of several phthalate metabolites after adjusting for race, sex, household income, maternal education, serum cotinine, and creatinine z-score (Table 2). The association between birth year and ∑DEHP metabolites changed with age, where those born in 2005–2006 had increasingly lower relative concentrations compared to those born in 2003–2004 as they got older, although this interaction was not statistically significant after adjustment for creatinine z-score (p = 0.24) (Figure 2, Table S5, SI). In contrast, age was positively associated with urinary MCOP concentrations and the association between birth year and MCOP or MCNP concentrations changed with age, such that MCOP and MCNP concentrations were higher among children born in 2005–2006 compared to those born in 2003–2004 (p-value for interaction <0.0001 for both metabolites) (Figure 2). For instance, compared to children born in 2003–2004, those born in 2005–2006 had 22% lower MCOP concentrations at one year of age, but 33% higher concentrations at five years of age (Table S5, SI).
Figure 2.
Geometric mean concentrations and smoothed regression of urinary ∑DEHP metabolites and MCOP between 1 and 5 years of age among those born between 2003 and 2004 or 2005–2006. ∑DEHP = sum of MEHP, MEHHP, MEOHP, and MECPP. Children born in 2003–2004 provided urine samples before the CPSIA went into effect, while children born in 2005–2006 provided their 3, 4, and 5 year urine samples after the CPSIA went into effect.
Discussion
Our longitudinal study design allowed us to evaluate variability of urinary phthalate metabolite concentrations during early childhood over both short and long-term exposure periods, as well as the effect of policy and manufacturer related changes in phthalate use during the study period. In addition, our cross-sectional analysis allowed us to examine specific consumer products as sources of phthalate exposure in preschool aged children. Our findings suggest that exposure to certain phthalates varies during early childhood by race, maternal education, use of personal care products such as hairspray or gel, consumption of fast food, and possibly consumption of food stored in specific types of packaging. Our findings also suggest that changes in manufacturer practices due to market-forces and the CPSIA of 2008, which restricted DEHP use in children’s products, may have resulted in decreased exposure to DEHP among study participants. However, urinary concentrations of MCOP, a metabolite of DiNP, increased during this same time period.
We found that phthalate metabolite concentrations in spot urine samples collected approximately 2 weeks apart were weakly to moderately correlated, while concentrations measured a year or more apart were less correlated. Since phthalates have biological half-lives of less than 24 h,31,32 urinary metabolite concentrations reflect only recent exposure. However, because some phthalate exposures are linked to routine behaviors that may vary little over short periods of time, a single spot urine sample may be a reasonable measure of short-term exposure to these phthalates. This may be especially true during infancy, when dietary variation tends to be limited and personal care product use is a routine part of child care. For example, in a study examining phthalate exposure among infants, the significant association between recent use of baby lotion and urinary MEP concentrations was strongest among infants less than 8 months old.9 Our results, which indicate that urinary MCOP, MCNP, and MCPP concentrations measured 2 weeks apart varied less among one year-olds than older children (Table 1), are consistent with the hypothesis that exposures become more varied as children mature. While a single spot sample may be sufficient to characterize exposure over a relatively narrow time window (e.g., weeks), one spot urine sample may not adequately capture exposure over months or years, particularly for toddlers and preschool aged children, since diet and personal care product use, as well as physiology, change considerably over the first five years of life. Consistent with this hypothesis, the annual ICCs reported in the present study are generally lower, suggesting more variability, compared to ICCs reported in a number of studies in adult populations.18−20,33
We observed less variability (e.g., higher reproducibility) in urinary MEP concentrations compared to other phthalate metabolites, consistent with previous studies.18−20,33 This may be a result of the pathways by which most young children are exposed to the parent compound DEP. DEP is found mainly in personal care products, which are often used regularly, but their use varies substantially between people. In contrast, ∑DEHP metabolites show substantial within-person variation, likely due to diet being the primary source of exposure.
It was difficult to separate the effects of physiological and behavioral patterns during child development from temporal changes in phthalates used in consumer products over the study period on measured urinary phthalate metabolite concentrations. Because age-related physiological changes in kidney function and muscle mass during early childhood affect creatinine excretion,27 the use of creatinine to adjust for urine dilution is complicated. We observed increasing urinary creatinine concentrations from 1 to 5 years of age (SI Figure S2) and substantially lower ICCs when using creatinine normalization (data not shown). As children grow older and more independent, age related changes in behavior may also affect phthalate exposures during this time. In addition, changes in manufacturer practices and the implementation of the CPSIA in February of 2009 further complicated this issue. Indeed, after controlling for urine dilution using age-specific creatinine z-scores, we detected differences in urinary ∑DEHP metabolite concentrations between children born in 2003–2004 and those born in 2005–2006. For example, at five years of age, children born later had lower ∑DEHP metabolite concentrations, a phthalate targeted by CPSIA, but higher MCOP concentrations compared to those born earlier.
Our data are consistent with possible increasing use of DiNP in consumer products in recent years, although the CPSIA also placed some restrictions on the use of this phthalate in specific children’s products.22 It is estimated that DiNP and DiDP make up about 33% of the U.S. plasticizer market.34 Our results suggest that changes in manufacturer practices and the CPSIA may have played a role in reducing exposure to some key phthalates among the target population (children), as the later born children had lower concentrations of ∑DEHP metabolites and MCPP (a DnOP and DnBP metabolite) at all ages. Similar decreases in urinary phthalate metabolite concentrations, specifically DEHP metabolites, can be seen in Europe as well.35 However, HOME study children who provided urine samples after the CPSIA went into effect had higher MCOP and MCNP concentrations than children providing urine samples before the CPSIA. These are metabolites of DiNP and DiDP, respectively, which may have increased in use as DEHP was phased out. Similar trends were recently reported among children, adolescents, and adults in data from the National Health and Nutrition Examination Survey (NHANES) collected from 2001 to 2010, where urinary DEHP metabolite, MnBP, and MBzP concentrations decreased over time, while urinary MCOP, MCNP, and MiBP concentrations increased.23 The CPSIA, which limited phthalate usage in children’s products, would not be expected to lead to the observed decreases in phthalate exposure among older children and adults, suggesting that other factors, such as additional regulatory pressures and consumer-driven market forces, may have also played a role in decreasing exposure to specific phthalates among children, as well as adults.
We observed higher urinary MEP concentrations among blacks and other nonwhite children compared to whites, but similar concentrations of most other phthalate metabolites. This finding is consistent with observations among older children in biomonitoring studies such as NHANES.1,36 Higher urinary phthalate concentrations among black and other nonwhite children could be due to differences in the frequency or type of personal care products used.
Similar to previous studies, we observed lower urinary MEP concentrations in males compared to females.1,6 Among five year-olds we also observed a positive association between the use of hairspray or gel within the past 48 h and urinary MEP concentrations and 18 of the 22 participants who reported using these products were female. We did not see associations between the use of other personal care products and MEP concentrations, possibly due to the small number of participants reporting use of most products or our reliance on maternal report of children’s exposure history. Self-reported information is often subject to misclassification, and a mother’s recall of their child’s experience may result in additional misclassification. For comparison, a study of phthalate exposure among infants found that use of baby lotion was positively associated with urinary MEP concentrations,9 while a recent study of 8–13 year olds reported sex-specific positive associations between urinary MEP concentrations and deodorant, cologne or perfume, and hair conditioner use, but not use of hair spray or gel.12
We found that consumption of fast food more than once a week was associated with higher urinary MBzP and MCOP concentrations, although it is uncertain if fast food packaging, fast food itself, or both are sources of phthalate exposure. We also observed a positive association between urinary MCOP concentrations and eating food stored in plastic containers, but a negative association between MCOP and eating food that had been heated in plastic within the past 48 h. Although these associations did not reach statistical significance, these contrasting findings make it difficult to reach a conclusion about exposure via plastic food packaging among this population. Our 48 h window may have been too long to detect meaningful differences in phthalate metabolite concentrations, and we did not collect potentially important information such as food type and food storage time and temperature.37 In addition, we were not able to identify the type or brand of plastic food containers used and different plastic formulations may contain different amounts of phthalate residues. A study evaluating predictors of phthalates among older children also did not observe an association between maternal report of exposure to food packaging in the previous 48 h and urinary phthalate metabolites.12 However, researchers evaluating food packaging as a source of phthalate exposure using dietary intervention observed a significant decrease in urinary phthalate metabolite concentrations when participants were provided catered meals with minimal plastic packaging.10
A major limitation of this analysis was that we used creatinine to adjust for urine dilution. Because kidney function and muscle mass are changing rapidly across the age range studied here, creatinine normalization is not an ideal method for adjustment because it could over- or underestimate exposure at different ages. As an alternative, we included age-specific creatinine z-scores in regression models to adjust for urine dilution. In addition, we did not ask participants how often their children played with toys made of plastic, the age of these toys, mouthing behaviors, or collect other information that would have helped us more directly examine whether the temporal trends in certain urinary phthalate metabolites were a result of the CPSIA. We were also not able to report concentrations of the hydrolytic monoester metabolites MEHP, MnBP, and MiBP in urine samples collected during visits 1, 2, or 3 due to potential contamination from diaper inserts.9 Another limitation is our modest sample size, which limits our ability to detect associations, especially for behavioral factors that are less accurately reported. We also do not have measures of phthalates in participant’s indoor environments, a potential source of exposure particularly in this age group.11,34 Phthalate concentrations in household dust can be quite high,21 and exposure through this pathway may substantially contribute to participant’s urinary phthalate metabolite levels.38 We also did not investigate maternal use of personal care products as a potential pathway of child phthalate exposure, although this could be pursued in a future analysis.
In conclusion, urinary phthalate metabolite concentrations in young children vary over time, likely due to a combination of age related changes in exposure and perhaps metabolism, as well as changes in the use of phthalates in commercial products. Consistent with previous reports in adults,29 our findings suggest that personal care products are a source of exposure to DEP, and that fast food consumption may be a source of exposure to DiNP and BBzP among young children. Finally, we found that DEHP exposure may have decreased over the course of our study, possibly in part as a result of market forces and the CPSIA, suggesting that the regulation of chemicals used in consumer products may be an effective method for decreasing exposure among sensitive populations, particularly with compounds that have a short biological half-life.
Acknowledgments
This work was supported by NIEHS grants R00 ES020346, PO1 ES11261, R01 ES014575, and R01 ES020349. We like to acknowledge the Centers for Disease Control and Prevention (CDC) laboratory staff who performed the analyses. Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supporting Information Available
Additional tables and figures are available as Supporting Information as mentioned in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): Two coauthors have potential conflicts of interest. Dr. Lanphear has served as an expert witness and as a consultant to the California Attorney Generals Office (with no compensation) and as a consultant on a US Environmental Protection Agency research study (with compensation). Dr. Braun has provided statistical consulting to plaintiffs in a lead poisoning trial. The other authors declare that they have no actual or potential competing financial interests..
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Silva M. J.; Barr D. B.; Reidy J. A.; Malek N. A.; Hodge C. C.; Caudill S. P.; Brock J. W.; Needham L. L.; Calafat A. M. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004, 1123331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S. L.; Britton J. A.; Calafat A. M.; Ye X.; Silva M. J.; Reidy J. A.; Galvez M. P.; Brenner B. L.; Wolff M. S. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 2008, 1062257–269. [DOI] [PubMed] [Google Scholar]
- Koniecki D.; Wang R.; Moody R. P.; Zhu J. P. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 1113329–336. [DOI] [PubMed] [Google Scholar]
- Koo H. J.; Lee B. M. Estimated exposure to phthalates in cosmetics and risk assessment. J. Toxicol. Environ. Health, Part A 2004, 6723–241901–1914. [DOI] [PubMed] [Google Scholar]
- Kelley K. E.; Hernandez-Diaz S.; Chaplin E. L.; Hauser R.; Mitchell A. A. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ. Health Perspect 2012, 1203379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R.; Calafat A. M. Phthalates and human health. J. Occup. Environ. Med. 2005, 6211806–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. M.; Sathyanarayana S.; Hauser R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 252247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley J. P.; Palmieri R. T.; Matuszewski J. M.; Herring A. H.; Baird D. D.; Hartmann K. E.; Hoppin J. A. Consumer product exposures associated with urinary phthalate levels in pregnant women. J. Exposure Sci. Environ. Epidemiol. 2012, 225468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S.; Karr C. J.; Lozano P.; Brown E.; Calafat A. M.; Liu F.; Swan S. H. Baby care products: Possible sources of infant phthalate exposure. Pediatrics 2008, 1212E260–E268. [DOI] [PubMed] [Google Scholar]
- Rudel R. A.; Gray J. M.; Engel C. L.; Rawsthorne T. W.; Dodson R. E.; Ackerman J. M.; Rizzo J.; Nudelman J. L.; Brody J. G. Food packaging and bisphenol A and bis(2-Ethyhexyl) phthalate exposure: Findings from a dietary intervention. Environ. Health Perspect. 2011, 1197914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt F.; Jonsson B. A. G.; Bornehag C. G. PVC flooring is related to human uptake of phthalates in infants. Indoor Air 2013, 23132–39. [DOI] [PubMed] [Google Scholar]
- Lewis R. C.; Meeker J. D.; Peterson K. E.; Lee J. M.; Pace G. G.; Cantoral A.; Tellez-Rojo M. M. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere 2013, 93, 2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum S. L.; Mervish N.; Moshier E. L.; Vangeepuram N.; Galvez M. P.; Calafat A. M.; Silva M. J.; Brenner B. L.; Wolff M. S. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ. Res. 2012, 112, 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt R. M.; Liu X. H.; Rauh V. A.; Calafat A. M.; Just A. C.; Hoepner L.; Diaz D.; Quinn J.; Adibi J.; Perera F. P.; Factor-Litvak P. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ. Health Perspect. 2012, 1202290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Ha E. H.; Kim E. J.; Park H.; Ha M.; Kim J. H.; Hong Y. C.; Chang N.; Kim B. N. Prenatal exposure to phthalates and infant development at 6 months: Prospective mothers and children’s environmental health (MOCEH) study. Environ. Health Perspect. 2011, 119101495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S. M.; Miodovnik A.; Canfield R. L.; Zhu C. B.; Silva M. J.; Calafat A. M.; Wolff M. S. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect. 2010, 1184565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornehag C. G.; Sundell J.; Weschler C. J.; Sigsgaard T.; Lundgren B.; Hasselgren M.; Hagerhed-Engman L. The association between asthma and allergic symptoms in children and phthalates in house dust: A nested case-control study. Environ. Health Perspect 2004, 112141393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. M.; Smith K. W.; Williams P. L.; Calafat A. M.; Berry K.; Ehrlich S.; Hauser R. Variability of urinary phthalate metabolite and bisphenol A Concentrations before and during Pregnancy. Environ. Health Perspect. 2012, 1205739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker J. D.; Calafat A. M.; Hauser R. Urinary phthalate metabolites and their biotransformation products: Predictors and temporal variability among men and women. J. Exposure Sci. Environ. Epidemiol. 2012, 224376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine D. E.; Cordero J. F.; Rivera-Gonzalez L. O.; Anzalota Del Toro L. V.; Ferguson K. K.; Mukherjee B.; Calafat A. M.; Crespo N.; Jimenez-Velez B.; Padilla I. Y.; Alshawabkeh A. N.; Meeker J. D. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: Distribution, temporal variability, and predictors. Environ. Int. 2013, 62C, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubwabo C.; Rasmussen P. E.; Fan X.; Kosarac I.; Wu F.; Zidek A.; Kuchta S. L. Analysis of selected phthalates in Canadian indoor dust collected using household vacuum and standardized sampling techniques. Indoor Air 2013, 23, 506–514. [DOI] [PubMed] [Google Scholar]
- CPSIA The Consumer Product Safety Improvement Act of 2008–Phthalates. http://www.cpsc.gov/phthalates (accessed June 14).
- Zota A. R.; Calafat A. M.; Woodruff T. J. Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environ. Health Perspect 2014, 1223235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. M.; Wittassek M.; Bruning T.; Angerer J.; Heudorf U. Exposure to phthalates in 5–6 years old primary school starters in Germany–A human biomonitoring study and a cumulative risk assessment. Int. J. Hyg. Environ. Health 2011, 2143188–95. [DOI] [PubMed] [Google Scholar]
- Geraghty S. R.; Khoury J. C.; Morrow A. L.; Lanphear B. P. Reporting individual test results of environmental chemicals in breastmilk: Potential for premature weaning. Breastfeed Med. 2008, 34207–U12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. J.; Samandar E.; Preau J. L.; Reidy J. A.; Needham L. L.; Calafat A. M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2007, 8601106–112. [DOI] [PubMed] [Google Scholar]
- Mage D. T.; Allen R. H.; Kodali A. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Exposure Sci. Environ. Epidemiol. 2008, 184360–8. [DOI] [PubMed] [Google Scholar]
- Bradley E. L.; Burden R. A.; Leon I.; Mortimer D. N.; Speck D. R.; Castle L. Determination of phthalate diesters in foods. Food Addit. Contam., Part A 2013, 304722–34. [DOI] [PubMed] [Google Scholar]
- Koch H. M.; Lorber M.; Christensen K. L.; Palmke C.; Koslitz S.; Bruning T. Identifying sources of phthalate exposure with human biomonitoring: Results of a 48h fasting study with urine collection and personal activity patterns. Int. J. Hyg Environ. Health 2013, 2166672–81. [DOI] [PubMed] [Google Scholar]
- Koniecki D.; Wang R.; Moody R. P.; Zhu J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 1113329–36. [DOI] [PubMed] [Google Scholar]
- Koch H. M.; Angerer J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int. J. Hyg. Environ. Health 2007, 21019–19. [DOI] [PubMed] [Google Scholar]
- Koch H. M.; Preuss R.; Angerer J. Di(2-ethylhexyl)phthalate (DEHP): Human metabolism and internal exposure–An update and latest results. Int. J. Androl. 2006, 291155–65discussion 181–5. [DOI] [PubMed] [Google Scholar]
- Frederiksen H.; Kranich S. K.; Jorgensen N.; Taboureau O.; Petersen J. H.; Andersson A. M. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: Considerations for epidemiological studies. Environ. Sci. Technol. 2013, 472958–967. [DOI] [PubMed] [Google Scholar]
- Evaluation of New Scientific Evidence Concerning DiNP and DiDP in Relation to Entry 52 of Annex XVII to Regulation (EC) No 1907/2006 (REACH); European Chemicals Agency, 2012
- Langer S.; Beko G.; Weschler C. J.; Brive L. M.; Toftum J.; Callesen M.; Clausen G. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int. J. Hyg. Environ. Health 2013, 217, 78–87. [DOI] [PubMed] [Google Scholar]
- Trasande L.; Attina T. M.; Sathyanarayana S.; Spanier A. J.; Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ. Health Perspect. 2013, 1214501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo T.; Fasano E.; Esposito F.; Del Prete E.; Cocchieri R. A. Study on the influence of temperature, storage time and packaging type on di-n-butylphthalate and di(2-ethylhexyl)phthalate release into packed meals. Food Addit. Contam., Part A 2013, 302403–11. [DOI] [PubMed] [Google Scholar]
- Beko G.; Weschler C. J.; Langer S.; Callesen M.; Toftum J.; Clausen G. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PloS one 2013, 84e62442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.