Abstract
Cadmium (Cd) and arsenic (As) are toxic to all living organisms, including plants and humans. In plants, Cd and As are detoxified by phytochelatins (PCs) and metal(loid)-chelating peptides and by sequestering PC–metal(loid) complexes in vacuoles. Consistent differences have been observed between As and Cd detoxification. Whereas chelation of Cd by PCs is largely sufficient to detoxify Cd, As–PC complexes must be sequestered into vacuoles to be fully detoxified. It is not clear whether this difference in detoxification pathways is ubiquitous among plants or varies across species. Here, we have conducted a PC transport study using vacuoles isolated from Arabidopsis and barley. Arabidopsis vacuoles accumulated low levels of PC2–Cd, and vesicles from yeast cells expressing either AtABCC1 or AtABCC2 exhibited negligible PC2–Cd transport activity compared with PC2–As. In contrast, barley vacuoles readily accumulated comparable levels of PC2–Cd and PC2–As. PC transport in barley vacuoles was inhibited by vanadate, but not by ammonium, suggesting the involvement of ABC-type transporters. Interestingly, barley vacuoles exhibited enhanced PC2 transport activity when essential metal ions, such as Zn(II), Cu(II) and Mn(II), were added to the transport assay, suggesting that PCs might contribute to the homeostasis of essential metals and detoxification of non-essential toxic metal(loid)s.
Keywords: ABC transporter, heavy metal, phytochelatin transporter, trace metals, vacuole
Introduction
Metal(loid)s are important raw materials for industry but crucial nutrients for living organisms. Because of their high reactivity, excess metal(liod)s can damage living organisms. Non-essential metal(loid)s, such as As, Cd, Hg and Pb, can displace essential metals with similar properties; interact with functional groups of catalytic or transport proteins, thereby modifying protein structures; and disturb the cellular redox status by generating reactive oxygen species (Sandalio et al. 2001; Sharma & Dietz 2009; Yadav 2010). Many mechanisms to detoxify toxic levels of metal ions in plants have been described, including inhibition of toxic metal uptake via secretion of chelators, efflux of the metals at the root epidermis, dilution via metal translocation to the shoot, metal chelation in the cytosol and metal sequestration into vacuoles (Cobbett 2000; Hall 2002; Mendoza-Cózatl et al. 2011). Among the molecules that promote heavy metal resistance, phytochelatins (PCs) are the most important in plant systems (Cobbett 2000; Clemens 2006). PCs are glutathione-derived peptides that tightly bind to heavy metals. First identified as a cadmium-binding peptide (CBP) in Schizosaccharomyces pombe (fission yeast; Murasugi et al. 1981), these peptides were later also found in plants, algae and worms (Grill et al. 1987; Ahner & Morel 1995). The importance of PCs in Cd detoxification was elucidated through phenotypic analyses of an Sc. pombe mutant unable to form high molecular mass Cd–phytochelatin–sulphide complexes. Growth of this mutant was severely inhibited compared with its isogenic wild type (WT) (Ortiz et al. 1992; Speiser et al. 1992). After the discovery of PCs, genes encoding phytochelatin synthases (PCS) were identified in Arabidopsis thaliana, Triticum aestivum (wheat) and fission yeast by Ha et al. (1999), Vatamaniuk et al. (1999) and Clemens et al. (1999), respectively. The Arabidopsis PCS1 knockout mutant is highly sensitive to Cd and As (Ha et al. 1999), and heterologous expression of PCS genes from T. aestivum and Sc. pombe in budding yeast enhances Cd resistance (Ha et al. 1999). Surprisingly, overexpression of AtPCS1 in Arabidopsis does not enhance tolerance to heavy metals, but rather decreases resistance to Cd and Zn (Lee et al. 2003). This result suggests that PCs are essential, but not sufficient, for heavy metal resistance, and that the next step, that is, vacuolar sequestration of PC–metal(loid) conjugates, is required to complete the resistance mediated by PCs.
A putative PC transporter was first found in Sc. pombe, and named SpHMT1. This half-sized ABC protein transports PCs as well as PCs conjugated to Cd into the vacuole using energy from ATP hydrolysis (Ortiz et al. 1992, 1995). Salt & Rauser (1995) suggested that plants also have an ABC-type PC transporter at the tonoplast because oat vacuoles exhibit vanadate-sensitive and ammonium-insensitive PC transport. Recently, Song et al. (2010) reported that vacuolar PC–As transport is essential for As resistance, and that AtABCC1 and AtABCC2, which belong to a different ABC clade from SpHMT1, are the major vacuolar PC–As transporters in Arabidopsis (Song et al. 2010). The abcc1abcc2 double mutant is hypersensitive to As and As-containing herbicides, and contains reduced levels of PCs compared with the WT when treated with As. Furthermore, AtABCC1 or AtABCC2 enhance As resistance and PC accumulation in Saccharomyces cerevisiae heterologously expressing TaPCS1. Vesicles isolated from yeast expressing either AtABCC1 or AtABCC2 together with TaPCS1 exhibit strong ATP-dependent PC–As transport activity that is sensitive to vanadate and insensitive to ammonium. The same vesicles, however, exhibit low rates of apo–PC2 transport. Furthermore, atabcc1 atabcc2 double knockout mutants exhibit negligible vacuolar PC–As transport compared with the high level measured in the WT.
AtABCC1 and AtABCC2 confer resistance to other heavy metals as well. The atabcc1 single and atabcc1 atabcc2 double knockout mutants are hypersensitive to Cd and Hg and overexpression of AtABCC1 enhances Cd tolerance and accumulation in Arabidopsis (Park et al. 2012). However, the Cd-sensitive phenotype of the abcc1 abcc2 double knockout mutant was mild compared with the As or Cd sensitivity of the PC-deficient cad1-3. These results suggest that vacuolar sequestration of PC–Cd might not be strictly required for Cd detoxification in Arabidopsis, but that PC–As sequestration in vacuoles is a key step in As detoxification. Here, we addressed the question of whether the difference in the detoxification pathways for Cd and As can be generalized to other plants. For this purpose, we used barley vacuoles because they represent an ideal system to study vacuolar transport in monocotyledonous species and are derived from a crop plant. Our results show significant differences in the vacuolar PC–metal(loid) uptake properties between Arabidopsis and barley, suggesting that the dynamics of PC–metal(loid) accumulation in vacuoles varies between plant species.
Materials and Methods
Plant culture conditions
Barley (Hordeum vulgare L. cv. Baraka) was grown in soil in a controlled artificial growth chamber [16/8 h light (300 μE m−2 s−1)/dark cycles at 25 °C] for 8 d. For experiments using barley vacuoles treated with Cd, barley plants were grown for 5 d in pots containing normal soil and then treated with 50 μm CdCl2 solution for 3 additional days. To isolate vacuoles from Arabidopsis, plants were cultured in soil under short day conditions [8/16 h light (40 μE m−2 s−1)/dark cycles at 22 °C] for 7–8 weeks.
PC transport assay in intact vacuoles from barley and Arabidopsis
To isolate vacuoles from barley, protoplasts were released from primary leaves by treatment with MCP buffer [0.5 m sorbitol, 10 mm MES–KOH (pH 5.6) and 1 mm CaCl2] containing 0.6% (w/v) cellulose YC (Seishin Pharmaceuticals, Tokyo, Japan) and 0.06% (w/v) pectolyase Y-23 (Seishin Pharmaceuticals) for 1 h at 30 °C Protoplasts were collected by centrifugation at 100 g for 7 min on a cushion of Percoll (GE-Healthcare, Pittsburgh, PA, USA) solution A [Percoll containing 0.5 m sorbitol, 20 mm MES (pH 5.6) and 1 mm CaCl2]. To purify mesophyll protoplasts, they were resuspended in Percoll solution A, and overlaid on 30% (v/v) Percoll solution A diluted with MCP buffer [0.5 m sorbitol, 20 mm MES (pH 5.6) and 1 mm CaCl2], overlaid with MCP buffer at the top of the tube and centrifuged at 100 g for 7 min. The intact protoplasts were collected at the interface between the layers of MCP and 30% Percoll solution, mixed with two volumes of lysis buffer [200 mm sorbitol, 10% Ficoll, 20 mm ethylenediaminetetraacetic acid (EDTA), 20 mM HEPES–KOH (pH 8), 1.5 mg mL−1 bovine serum albumin (BSA) and 1 mM dithiothreitol (DTT)] and lysed by incubation in a hot water bath (37–40 °C) or mechanical stimulus using a syringe. To purify vacuoles released from the protoplasts, the lysate was overlaid with lysis buffer (without EDTA and DTT) and betaine buffer [400 mm glycine betaine, 30 mm KCl, 20 mm HEPES–KOH (pH 7.2) and 1.5 mg mL−1 BSA] and centrifuged at 100 g for 7 min. The vacuoles from the top layer were again overlaid with betain buffer and centrifuged at 100 g for 7 min to remove EDTA and DTT from the vacuoles. The purified vacuoles were resuspended in Percoll solution [to a final concentration of 10% (v/v) Percoll, 500 mm sorbitol and 20 mm HEPES–KOH (pH 7.2)] and directly used for the PC transport assay.
Two hundred fifty picomoles of PC2 containing 50 nCi (920 Bq) of 35S-PC2 per 1 reaction tube was used in the uptake assays. To form PC2 complexes with As(III), Cd(II), Cu(II), Fe(II), Mn(II) or Zn(II), the metal(loid), PC2 containing 35S-PC2 and DTT were mixed at a molar ratio of 1:1:1 and incubated at room temperature for 40 min. Uptake of PCs was assayed using the method described by Martinoia et al. (1993). For each condition and time point, four 400 μL polyethylene microcentrifuge tubes were prepared as follows: 70 μL of transport buffer [22% (v/v) Percoll (pH 7.2), 500 mm sorbitol, 30 mm KCl, 20 mm HEPES–KOH (pH 7.2) and 0.1% (w/v) BSA] containing 0.05 μCi (920 Bq) 3H2O, 2.5 μm PC2–metal was combined with 30 μL of vacuole suspension, and then 200 μL silicone oil (AR 200) and 60 μL water were rapidly layered on the mixture. After incubation, vacuoles were floated at the water phase by centrifugation at 10 000 g for 20 s. Radioactivity from the vacuoles was measured using a liquid scintillation counter (PerkinElmer, Wellesley, MA, USA). To identify the ratio of Cd/PC2 uptake in vacuoles, Cd containing 109Cd and PC2 containing 35S-PC2 was incubated at room temperature. Furthermore, 50 nCi (1840 Bq) of 35S-labelled PC2, 50 nCi (1840 Bq) 109Cd and 50 nCi (1840 Bq) 3H2O were used as tracers, and 2.5 μm PC2–Cd was used as substrate in each experiment. After the PC2–Cd transport assay, vacuoles were collected and 109Cd radioactivity was detected using a gamma counter (PerkinElmer). 35S-PC2 and 3H2O were detected using a liquid scintillation counter. The volume of vacuoles was determined using the value of 3H2O.
Cd resistance test in yeast cells
Yeast strains SM4 (ycf1::His3, yhl035c::HIS3-MX6, yll015w:: Kan-MX6,yll048c::TRP1-MX6) and SM7 (ycf1::His3, yhl035c:: HIS3-MX6,yll015w::Kan-MX6,yll048c::TRP1-MX6, TaPCS1::cup1-1) carrying an empty vector (EV), pNEV-AtABCC1 or pYES3-AtABCC2 were grown in medium at 30 °C. To analyse Cd resistance in the yeast lines, yeast cells were cultured in SD Ura-medium at 30°C until the mid-log phase (OD600 = 2), harvested by centrifugation at 140 g for 30 s and then adjusted with water to OD600 = 10.0−0.01. Three microliters of cells diluted with water was taken from each strain, spotted on the medium with or without CdCl2 and cultured at 30 °C in an incubator for 3d.
Isolation of membrane vesicles from yeast and transport assays
Vesicles were prepared from SM7 yeast cells carrying empty vector (pNEV-Ura), pNEV-AtABCC1 or pYES3-AtABCC2 grown in SD Ura-liquid medium. Before cell wall digestion, cells were incubated in YPD medium for 30 min, collected by centrifugation for 5 min at 2100 g and digested with digest buffer [500 mm sorbitol, 25 mm Tris–MES (pH 7.4), 0.5 mM EDTA and 15 mM DTT] containing lyticase (1000 units g−1 fresh weight cells; Sigma, St Louis, MO, USA) for 0.5∼1 h, and then spheroplasts were harvested by centrifugation for 10 min at 2100 g. The cells were washed with 1 m sorbitol, resuspended in homogenization buffer [1.1 m glycerol, 50 mm Tris-ascorbate (pH 7.4), 5 mm EDTA, 1.5% (w/v) polyvinylpyrrolidone, 1 mm DTT, 2% (w/v) BSA, 1 mM phenylmethanesulfonyl fluoride, and one proteinase inhibitor cocktail tablet (Roche, Basel, Switzerland)], and then microsomal vesicles were isolated as described previously (Tommasini et al., 1996). For the PC2 transport assay, 50 nCi (920 Bq) of 35S-PCs was used as a tracer of PCs in the PC transport assay. Transport experiments were carried out using transport buffer [4 mm ATP, 5 mm MgCl2, 10 mm creatine phosphate, 16 units mL−1 creatine kinase, 1 mg mL−1 BSA, 100 mm KCl and 25 mm Tris–MES (pH 7.4)] containing 2.5 μm PC2 for the indicated times. Before starting the PC transport experiments, 10 μL of vesicles (100 μg of protein) was mixed with 90 μL of transport buffer containing substrate and kept on ice for 5 min. The reactions were performed at 25 °C, stopped by adding 1 mL of ice-cold washing buffer [100 mm KCl and 25 mm Tris–MES (pH 7.4)], filtered immediately under vacuum through a 0.45-μm-diameter pore size nitrocellulose filter (Millipore, Billerica, MA, USA) and washed two times with 2 mL of washing buffer. Vanadate (1 mm) and NH4Cl (5 mm) were used as an inhibitor of ATPase and pH gradient, respectively.
35S-phytochelatin-2 synthesis
35S-PC2 was synthesized using recombinant AtPCS1-6xHis protein and 35S-GSH high-performance liquid chromatography (HPLC), as previously described (Song et al. 2010). 35S-GSH was purchased from PerkinElmer and the specific activities ranged from 660 to 840 Ci mmol−1 between batches. PCs were separated by HPLC, monitored at 220 nm and confirmed by mass spectrometry, as previously described (Mendoza-Cózatl et al., 2008). 35S-PCs were finally re-suspended in 0.1 m Tris (pH 8.0), 0.5 mm DTT and 0.5 mm of non-radioactive PC2 (AnaSpec, San Jose, CA, USA). After purification, the specific activity of 35S-PC2 varied between 2 and 5.9 nCi mmol−1.
Phylogenetic analysis of the plant ABCC1 family
All ABCC protein sequences of Arabidopsis, rice, Brachypodium, maize and grape were collected from the ARAMEMNON website (http://aramemnon.botanik.unikoeln.de/), and a barley AtABCC1 orthologue was identified through the blastp program in the Barley BLAST Server (http://webblast.ipk-gatersleben.de/barley/viroblast.php).
All members of the plant ABCC protein family were aligned using ClustralW programs with default values in mega and BioEdit software, and phylogenetic analyses were conducted using mega version 4 (Tamura et al. 2007) using amino acid sequence homologies. A phylogenetic tree was constructed using the neighbour-joining method (Saitou & Nei 1987). The bootstrap values (percentage) of 1000 replicates are shown at the branching points.
Results
PC2–Cd transport in A. thaliana
In two former studies, it was shown that AtABCC1 and AtABCC2 play an important role in arsenic (As) and cadmium (Cd) resistance (Song et al. 2010; Park et al. 2012). To compare the detoxification efficiency of As and Cd through vacuolar sequestration of PC–Cd and PC–As mediated by AtABCC1 and AtABCC2 in Arabidopsis, we compared the sensitivities of the atabcc1 atabcc2 double mutant and the PC-deficient mutant cad1-3 to As and Cd. As summarized in Fig. 1, atabcc1 atabcc2 double knockout plants were not as sensitive to Cd as was cad1-3. Increasing the Cd concentration from 20 to 40 μm severely limited the growth of the atabcc1 atabcc2 mutant, while growth was almost completely abolished in the cad1-3 mutant at 30 μm (Fig. 1a,c). In contrast, atabcc1 atabcc2 and cad1-3 mutants exhibited similar and almost complete root growth inhibition (90%) in medium containing 20 μm As(V). In both mutants, growth was completely arrested on plates containing 30 μm As(V) (Fig. 1b,d). These results indicate that chelation of Cd by PCs is sufficient to detoxify the majority of cytosolic Cd, but that vacuolar sequestration by AtABCC1 or AtABCC2 is necessary for full detoxification of As.
Figure 1.
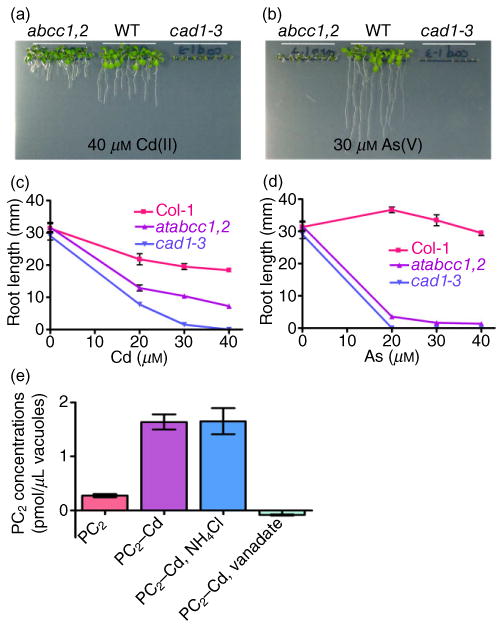
Phytochelatin (PC)-deficient and PC transporter mutants show differential sensitivity to As and Cd.
(a–d) Phenotypic analysis of Cd and As toxicity in Arabidopsis ABCC1 and 2 double knockout (abcc1 abcc2) and phytochelatin synthase (cad1-3) mutants. Seeds of atabcc1,2, cad1-3 and Col-0 (WT) were sown on half-strength MS agar medium supplemented with or without CdCl2 (a) or NaHAsO47H2O (b) and grown for 12 d before the root lengths were measured (c, d) (n = 10, N = 2).
(e) PC2–Cd transport activity in vacuoles isolated from Arabidopsis mesophyll protoplasts. The transport activity of apo–PC2 and PC2–Cd (final concentrations of 2.5 μm in the buffer) was assayed in the presence or absence of 5 mm NH4Cl or 1 mm vanadate. The PC2 content was measured after an incubation time of 15 min at room temperature. The values indicate the averages and standard errors of values obtained in the absence of Mg–ATP subtracted from those obtained in the presence of Mg–ATP (n = 4, N = 2).
As vacuolar sequestration of PC-Cd might not be strictly required for efficient detoxification of Cd in abcc1 abcc2 (Fig. 1a,d), we tested whether the major PC2–As transporters, AtABCC1 and AtABCC2, can also efficiently sequester Cd conjugated with PCs (PC2–Cd) in vitro. The transport activity of PC2–Cd was 1.7 pmol/1 μL vacuole/15 min (Fig. 1e). Transport was abolished by vanadate, an inhibitor of P-type ATPases and ABC transporters, but not by NH4Cl, which dissipates the proton gradient (Fig. 1e). Similar results were described for PC2–As transport mediated by AtABCC1 and AtABCC2, as reported by Song et al. (2010).
To test whether AtABCC1 and AtABCC2 confer cadmium resistance by compartmentalizing a GS–Cd or PCs–Cd complex into the vacuole, we expressed the transporters in SM4, a quadruple mutant of four vacuolar ABCC-type ABC transporters, and SM7, a yeast mutant expressing the wheat PC synthase1 in the SM4 background. Surprisingly, neither AtABCC1 nor AtABCC2 expression increased Cd tolerance in yeast, and AtABCC2 expression increased Cd sensitivity in both yeast strains, regardless of whether or not the transporters were co-expressed with a wheat PC synthase1 gene (Fig. 2a). Nonetheless, vesicles from yeast expressing AtABCC1 and AtABCC2 exhibited PC2–Cd transport activity. This activity was much lower than that observed for PC2–As (Fig. 2b). For instance, PC2–Cd transport activities mediated by AtABCC1 and AtABCC2 were only 1.52 and 1.47 times higher than those of the empty vector control, which is significantly lower than the >500-fold increase in PC2–As transport activity observed in vesicles expressing either AtABCC1 or AtABCC2 (Fig. 2b). These results suggest that AtABCC1 and AtABCC2 can transport PC2–Cd, but have a strong preference for PC2–As as their substrate.
Figure 2.
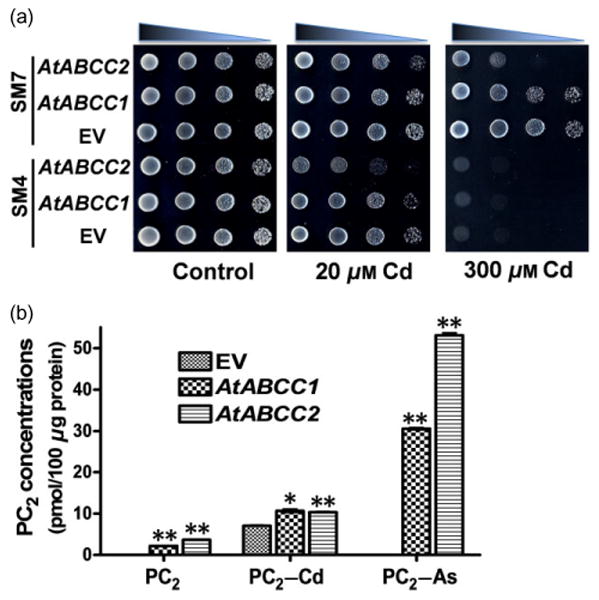
Vacuolar sequestration of PC2–Cd mediated by AtABCC1 and AtABCC2 in yeast mutant lines. (a) Phenotypic analysis of yeast lines SM4 and SM7 expressing AtABCC1 or AtABCC2 in the presence or absence of various concentrations of Cd. Yeast cells harbouring empty vector, AtABCC1, or AtABCC2 were subcultured in SD Ura-liquid medium, spotted on SD Ura-agar plates supplemented with or without CdCl2, and cultured in a 30 °C incubator for 3–4 d. (b) Transport activities for apo–PC2, PC2–Cd and PC2–As in yeast vesicles isolated from yeast cells expressing AtABCC1, AtABCC2 or empty vector (EV). Apo–PC2, PC2–Cd and PC2–As (final concentrations of 2.5 μm in the assay buffer) were added to transport assay buffer supplemented with or without Mg–ATP, and the reactions were incubated at room temperature for 40 min. The values indicate the average and standard error obtained in the absence of Mg–ATP subtracted from those obtained in the presence of Mg–ATP (n = 4, N = 2). Significant differences were calculated relative to the corresponding EV control value. *P< 0.05, **P< 0.01 (Student's t-test).
PC uptake in barley vacuoles is activated by cadmium
To obtain more biochemical information about PC–Cd transport activity in other plant species, and to establish whether PC transport is similar in two different plant systems, we carried out PC–Cd transport assays using barley vacuoles isolated from barley mesophyll protoplasts. Firstly, we examined the transport activities of barley vacuoles for PC2–Cd and PC3–Cd complexes. In the absence of Mg-ATP, there was no transport of PC2–Cd or PC3–Cd, and the transport activities of apo–PC2 and apo–PC3 were extremely low, even in the presence of Mg–ATP (Fig. 3a). However, the transport activities for PC2 and PC3 were enhanced in the presence of Cd. PC2 and PC3 transport into vacuoles was 53 and 17 times greater when Cd was present in the uptake assay. Uptake of PC2–Cd into vacuoles was time and ATP dependent (Fig. 3b). To determine whether Cd acts only as an activator of PC transport or whether Cd conjugated with PC2 is actually transported into vacuoles, we analysed PC2 and Cd concentrations in vacuoles incubated in the presence of PC2–Cd with or without Mg–ATP for 15 min. To produce the PC2–Cd complex, we mixed PC2 and CdCl2 at a one-to-one molar ratio, and performed a double-tracer experiment using 35S-PC2 and 109Cd as tracers for PC2 and Cd, respectively. In the presence of Mg–ATP, uptake of PC2 and Cd increased by 80 and 100% compared to assays in which no Mg–ATP was added (Fig. 3c). Therefore, the molar ratio of PC2 to Cd transported by Mg–ATP was close to 1. A similar ratio was reported for PC transport assays performed by Salt & Rauser (1995). These authors found that PC3, which contains three thiols per molecule, and Cd were transported at ratios of 1:1.7 and 1:1.5 (PC3:Cd), respectively, when measured as vanadate-sensitive or NH4Cl-insensitive transport.
Figure 3.
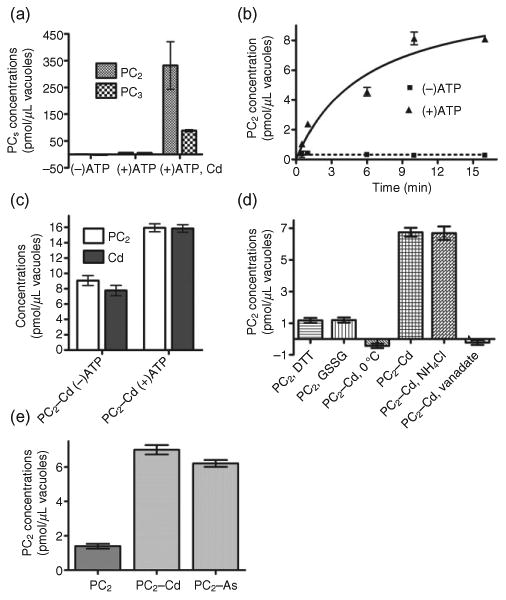
PC2–Cd transport assay in vacuoles isolated from barley mesophyll cells. (a) ATP-dependent PC2 and PC3 transport assay. Apo–PC2, apo–PC3, PC2–Cd and PC3–Cd (final concentrations of 150 μm in the assay buffer) were added to transport buffer containing purified barley vacuoles, and the reactions were incubated at room temperature for 2.5 and 17.5 min, respectively. The values were calculated by deducing the 2.5 min from the 17.5 min values to remove the non-specific adsorption. Values represent averages and standard errors (n = 5).
(b) Time-dependent PC2–Cd transport assay. The final concentration of PC2–Cd was 2.5 μm in this transport assay. The values represent averages and standard errors of values obtained at the indicated time points minus those obtained after 2.5 min of incubation (n = 4, N = 2). (c) PC2 and Cd concentrations in barley vacuoles after an incubation time of 15 min in the absence (−) or presence (+) of Mg–ATP. PC2–Cd was added (final concentration of 2.5 μm in the assay buffer). 35S-PC2 and 109Cd were used as tracers for PC2 and Cd in this study (n = 4, N = 2). (d) Effects of inhibitors of PC2–Cd uptake into barley vacuoles. Barley vacuoles were incubated in transport buffer supplemented with 1 mm DTT, 1 mm GSSG, 5 mm NH4Cl or 1 mm vanadate, respectively for 15 min. Values indicate averages and standard errors (n = 4, N = 2).
(e) Comparison of PC2–Cd and PC2–As transport activities. Barley vacuoles were incubated in transport buffer supplemented with or without Mg–ATP for 15 min. The values indicate averages and standard errors (n = 4, N = 2). The values in (d) and (e) represent values obtained in the absence of Mg–ATP subtracted from those obtained in the presence of Mg–ATP.
To further characterize the vacuolar PC2 transport characteristics in barley vacuoles, we performed PC2 transport assays under different conditions (Fig. 3d). As expected, when the transport reaction was performed on ice, no PC2–Cd transport activity was observed. Apo–PC2 transport activities were not influenced by reducing and oxidizing agents (1 mm DTT and 1 mm GSSG, respectively; Fig. 3d). As observed for Arabidopsis vacuoles, PC2–Cd transport activity was strongly inhibited by vanadate, but not affected by NH4Cl treatment. These results indicate that ATP hydrolysis is required for vacuolar PC–Cd transport, but that the proton gradient is not. Finally, we compared PC2–Cd and PC2–As transport activities in vacuoles isolated from barley leaves. In contrast to what was observed in Arabidopsis, PC2–Cd transport activity was as high as PC2–As uptake (Fig. 3e). This result indicates that, although both plants can efficiently transport PCs, the affinities for the different complexes may vary between plant species.
Phytochelatins may contribute to sequester essential metals in barley vacuoles
Plants often take up excess amounts of essential heavy metals. To test whether these metals can also be transported into vacuoles as PC conjugates, we produced PC2 complexes with several metal ions by incubating PC2 and the ions (at one-to-one ratios) for 1 h at room temperature. We added the PC2 complexes into the transport buffer containing Mg–ATP. The transport activities observed for PC2 complexes with As(III), Mn(II), Cu(II) and Zn(II) were much higher than those detected for apo–PC2 (Fig. 4a), and similar to those observed for the PC2–Cd complex. In contrast, the uptake of the PC2–Fe complex was not significantly higher than that of apo–PC2. Furthermore, the transport activities of PC2–Mn, PC2–Cu and PC2–Cd were consistently higher in vacuoles isolated from barley treated with 100 μm Cd(II) for 3 d compared with vacuoles isolated from untreated control plants (Fig. 4b). This suggests that the transport activity of PC2– metal complexes was induced by Cd treatment. These results also indicate that essential metal ions can be transported into vacuoles via the PC-dependent pathway, and that this pathway can be activated by exposure to non-essential metals such as Cd.
Figure 4.
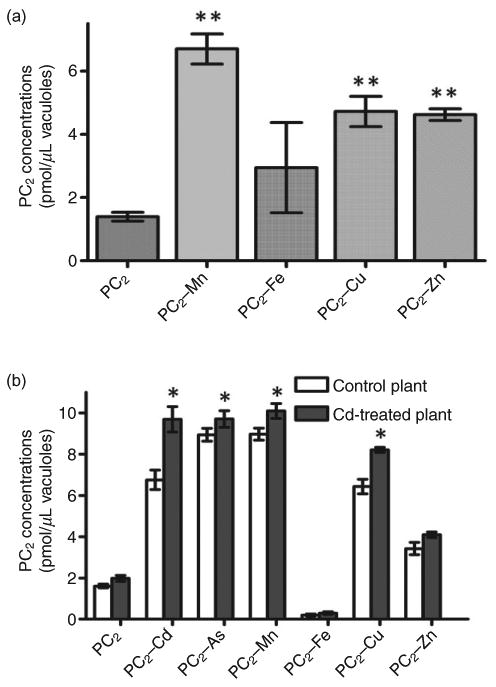
Essential metal ions increase PC transport activity in barley vacuoles. (a) Transport activity of PC2 complexes in the presence of essential metal ions. (b) Comparison of PC2 transport activity in barley vacuoles isolated from plants grown on soil with or without 50 μm CdCl2. Apo–PC2 and PC2 complexes (one-to-one ratio of PC2 and metal ion) with the indicated metal ions were added to transport buffer containing vacuoles. The final concentrations of apo–PC2 and PC2 complexes in the transport medium were 2.5 μm, and the reactions were incubated at room temperature for 15 min. The values indicate averages and standard errors from values obtained in the absence of Mg–ATP subtracted from those obtained in the presence of MgATP (n = 4, N = 2). *P < 0.05, **P < 0.01 (Student's t-test).
Putative phytochelatin transporters in monocots
To explore whether monocotyledonous plants have AtABCC1 and AtABCC2 orthologues encoding putative PC2–Cd transporters, we developed a phylogenetic tree of ABCC-type transporters in monocot and eudicot plants based on amino acid sequence alignments (Fig. 5). In this analysis, we included H. vulgare and Sorghum bicolor putative orthologues of ABCC1-, ABCC2- and ABCC-type transporters from A. thaliana, Populus trichocarpa, Vitis vinifera, Oryza sativa, Zea mays and Brachypodium distachyon. Using the blastp program in the Barley BLAST Server, we identified only two partial sequences of an AtABCC1 barley orthologue, that is, MLOC_15955 and MLOC_56261. Whereas MLOC_15955 covers approximately 583 amino acids of the N-terminus, MLOC_56261 covers 965 amino acids at the C-terminus. However, a segment covering approximately 90 amino acids between the two termini is not represented in the publicly available genome sequence, as the recently published barley genome sequence (International Barley Genome Sequencing Consortium, 2012) is incomplete. The protein sequence of the barley putative orthologue (Hvul_ABCC1) is provided in Supporting Information Appendix S1.
Figure 5.
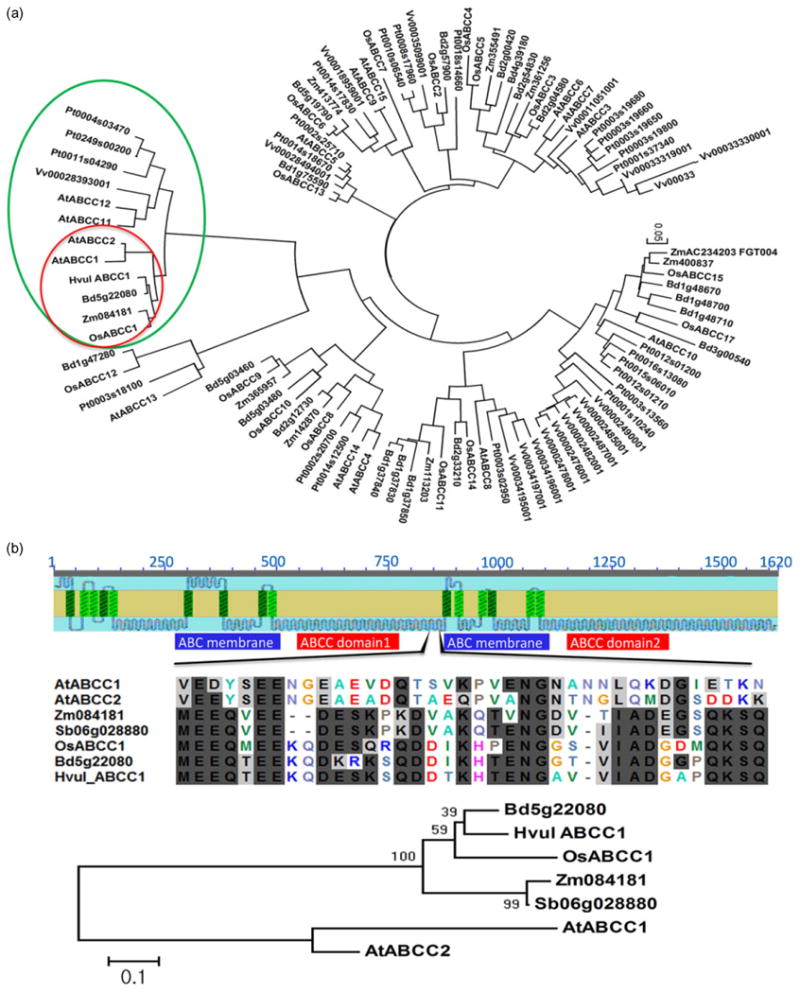
Phylogenetic analysis of genes similar to AtABCC1 in plants. (a) Phylogenetic tree of genes related to AtABCC1 in plants. Green circle: subgroup of AtABCC1 and 2; red circle: AtABCC1 and 2 orthologues). The phylogenetic tree was generated from multiple alignments of ABCC protein sequences. (b) Comparison of amino acid sequences of AtABCC1 and 2 and their putative monocot orthologues. The upper figure indicates a distinct region (amino acid positions 842 to 889) of AtABCC1, 2, and their monocot orthologues. The middle figure compares the amino acid sequences from a distinct region of AtABCC1, 2 and their monocot orthologues. The bottom figures show the phylogenetic tree generated by multiple alignments of amino acid sequences from the distinct region of AtABCC1, 2 and their monocot orthologues indicated in the middle figure. Bioedit software was used for this alignment. Identical amino acid residues are in black boxes. Phylogenetic analyses were performed using mega version 4.0 and were based on multiple alignments of ABCC protein sequences generated by the neighbour-joining method. The bootstrap values (percentage) of 1000 replicates are shown at the branching points. The scale bars indicate unit of branch length.
Phylogenetic analysis of the plant ABCC family revealed that the PC2–As transporters AtABCC1 (At1g30400) and AtABCC2 (At2g34660) form a subgroup distinct from other ABCCs (Fig. 5a). Included in the same subgroup with AtABCC1 and AtABCC2 were ABCC12 (At1g30410) and ABCC11 (At1g30420) of Arabidopsis, ABCC1 (Os04g52900) of rice, three members of poplar ABCCs, one member of Brachypodium and one member each of grape, maize, sorghum and barley (Fig. 5a, green circle). ABCC-type transporters OsABCC1 (Os04g52900), Bd5g22080, Zm084181, Hvul_ABCC1 and Sb06g028880 from rice, Brachypodium, maize, barley and sorghum, respectively, were closely related to the major Arabidopsis PC2–As transporters AtABCC1 and AtABCC2 (Fig. 5a, red circle). However, three ABCC homologues in poplar (Pt0004s03470, Pt0249s00200 and Pt0011s04290) and grape (Vv0028393001) were more similar to Arabidopsis ABCC12 (At1g30410) and ABCC11 (At1g30420), whose knockout mutants did not exhibit any arsenical pesticide disodium methanearsonate phenotype (Song et al. 2010). Interestingly, phylogenetic analysis showed that the four closest grass homologues cluster together in an order that reflects the phylogenetic relationships of the four species (i.e. barley, rice, sorghum and Brachypodium) and that each grass contains only one member of this clade. Arabidopsis ABCC1 and 2 exhibited a high level of amino acid sequence identity with the monocot AtABCC1 and 2 putative orthologues; 71.2% with OsABCC1 of O. sativa, 72.4% with Zm084181 of Z. mays and 70.6% with Bd5g22080 of B. distachyon. AtABCC1 and AtABCC2 orthologues in monocots had a high level of amino acid sequence identity, although O. sativa, S. bicolor and B. distachyon belonged to different subfamilies. OsABCC1 and Sb06g028880, OsABCC1 and Bd5g22080, and Sb06g028880 and BdABCC1 shared 91.2, 92.1 and 90.8% amino acid identity, respectively.
Although the ABCC1 and ABCC2 orthologues in monocots and dicots have high levels of amino acid sequence identity, there is a distinct sequence proximal to amino acid position 842–879 in AtABCC1 between ABCC domain and the second ABC membrane domain, where AtABCC1 and 2 and their monocot orthologues exhibited only 22∼35% amino acid identity. However, ABCC1 monocot orthologues had a high amino acid identity of 59∼78% at this position (Fig. 5b). Therefore, ABCCs of monocots and Arabidopsis cluster to distinct groups in this phylogenetic analysis (Fig. 5b).
Discussion
Cd resistance mediated by ABCC transporters
PCs were identified as being responsible for the major detoxification mechanism of a large number of metal(liod)s in plants, Sc. pombe, an Caenorhabditis elegans (Murasugi et al. 1981; Grill et al. 1987; Clemens et al. 2001). When PCS1 was deleted or mutated, all these organisms exhibited severe growth inhibition when grown on media containing Cd, As or Hg (Clemens et al., 1999; Ha et al. 1999; Vatamanuik et al. 2001). PC synthase is constitutively expressed, but PC synthesis is only induced in the presence of non-essential metals, such as Cd(II), As(III) and Hg(I), or an elevated concentration of essential transition metals, such as Zn(II) and Cu(II) (Grill et al. 1989; Ahner & Morel 1995; Vatamanuik et al. 2001). It is widely accepted that PCs form complexes with metal(loid)s and that these complexes are transported into the vacuole for final detoxification. However, because of the different stability constants of metal(loid)–PC complexes, which are further affected by pH, the requirements for the efficient transport of PC conjugates into vacuoles may differ between metal(loids) and thus be reflected by different transport rates (Fig. 4).
The Sc. pombe mutant hmt1 was unable to form high molecular weight PC–Cd (HMWPC-Cd-S-2) complexes (Ortiz et al., 1992, 1995). This mutant was hypersensitive to Cd, but not to As or Hg. In contrast, the PC-deficient mutant pcs was hypersensitive to As, Hg and Cd. SpHMT1 has been reported to transport apo–PC and PC–Cd. Recent results indicate that Cd detoxification mediated by HMT1 and its orthologues may result from a mechanism other than vacuolar transport of PCs. For instance, DmHMT-1, an SpHMT1 orthologue in Drosophila melanogaster, which does not produce PCs, could not complement vacuolar PC sequestration of the hmt1 pombe mutant, although it could rescue the Cd hypersensitivity of hmt1 (Sooksa-Nguan et al. 2009). Moreover, SpHMT1 enhanced the Cd resistance of Sa. cerevisiae and Escherichia coli, which do not produce PCs (Preveral et al. 2009). These results suggest that SpHMT1 may transport Cd bound to different ligands, including but not limited to PCs. The first direct demonstration that full-length ABCC-type transporters mediate PC transport into yeast and plant vacuoles was published in 2010 (Mendoza-Cózatl et al. 2010; Song et al. 2010). While Sc. pombe ABCCs were suggested to mediate Cd detoxification, two plant ABC transporters, AtABCC1 and AtABCC2, were shown to sequester PC–As into vacuoles (Mendoza-Cózatl et al. 2010; Song et al. 2010). Later studies showed that AtABCC1 and AtABCC2 also confer Cd tolerance in Arabidopsis (Park et al. 2012). Using fluorescent dyes to report the intracellular localization of Cd, Park et al. (2012) showed that Cd predominantly localized to the vacuoles of WT plants, whereas it was almost exclusively found in the cytosol of abcc1 abcc2 double mutants. In contrast to the arsenic sensitivity of abcc1 abcc2 was comparable to plants lacking PCs, the abcc1 abcc2 had a less impact on Cd sensitivity compared with the PC-deficient mutant (Park et al. 2012; Fig. 1a). Because PC–Cd transport was not addressed in that study, we were interested in comparing the transport activities of PC–As and PC–Cd complexes. Surprisingly, AtABCC1 and 2 did not enhance Cd resistance. SM4 and SM7 cells expressingAtABCC1 were even more sensitive to Cd than was the empty vector control. The cadmium hypersensitivity mediated by AtABCC21 overexpression might be caused by decreased levels of GSH or PCs in the cytosol because AtABCC21 could transport GSSG, GS conjugates and apoPCs into the vacuole (Lu et al. 1998; Song et al. 2010). Vacuoles isolated from Arabidopsis also exhibited low levels of PC2–Cd transport activity. In contrast, we found high PC2–As transport activity in yeast and plants expressing AtABCC1 and 2 (Figs 1 & 2; Song et al. 2010). Barley vacuoles, on the contrary, exhibited similar transport rates for PC2–Cd and PC2–As (Fig. 3). The transport activity was dependent on Mg–ATP, and inhibited by vanadate, but not by ammonium (Fig. 3d). These results imply that barley has a vacuolar ABC transporter for PC complexes, which differs in its substrate specificity from those described from Arabidopsis (Song et al. 2010).
According to the amino acid sequence analysis of the ABCC subfamily members in plants, the orthologues of AtABCC1 and AtABCC2 form a distinct group of ABCC transporters (Fig. 5). This suggests an evolutionary relationship with respect to the detoxification of toxic metal(loid)s through vacuolar compartmentation of PC–metal. Interestingly, monocot ABCC proteins belonged to the same clade as Arabidopsis ABCC1 and ABCC2, whereas ABCC proteins of the tree plants V. venifera and P. trichocarpa, which have high levels of homology with AtABCC1 and AtABCC2, belonged to the ABCC12 (At1g30410) and ABCC11 (At1g30420) subclades, respectively. Arabidopsis abcc11 and abcc12 were not sensitive to As or arsenic-containing pesticides (Song et al. 2010). Monocot AtABCC1 orthologues have 70∼72% identity with AtABCC1, but the region between the first ABCC domain 1 (ATP binding site) and the second ABC membrane domain (from amino acids 842 to 889) has only 22∼35% amino acid sequence identity with AtABCC1. Monocot proteins have high levels of amino acid sequence identity (59∼78%) in this region. We suggest that the regions might be responsible for the different PC–Cd transport functions in monocot and dicot ABCCs.
The role of vacuolar PC transport and nutrient homeostasis
In addition to the detoxification of non-essential metal(loid)s, PCs have also been suggested to mediate the detoxification of essential metals when present in excess. This has been discussed based on the fact that PCS is constitutively expressed and several heavy metals may at least slightly induce PC synthesis. Furthermore, during heavy metal stress, PCs may also form complexes with essential nutrients. As these essential transition metal ions, such as Zn(II), Cu(II) and Mn(II), have similar chemical characteristics as some non-essential toxic metals ions, such as Cd(II) or Hg(I), they may bind with these thiol-containing compounds (Goyer 1997; Yadav 2010). To test whether essential metals conjugated with PCs can be sequestrated into the vacuole, we performed PC2–metal transport assays using barley vacuoles. Surprisingly, barley vacuoles transported Cu(II), Zn(II) and Mn(II) conjugated with PC2 in the presence of Mg–ATP (Fig. 4a), and the transport activities were slightly higher in vacuoles isolated from barley plants grown in soil supplemented with Cd for 5 d than in those grown in uncontaminated soil (Fig. 4b). Thus, we suggest that essential metal ions, such as Zn(II), Cu(II) and Mn(II), can be transported into vacuoles as forms of PC2–metal complexes through the putative ABC transporter(s). In accordance with this explanation, there are direct and indirect lines of evidence that suggest that PCs are involved in the regulation of transition metal ion homeostasis. For example, PC synthesis is activated by essential metal ions as well as non-essential metal ions under both in vivo (Grill et al. 1987; Ahner & Morel 1995; Maitani et al. 1996) and in vitro conditions (Vatamaniuk et al. 2000). In addition, the PC–Cu complex is present in plant cells treated with Cu (Grill et al. 1987; Maitani et al. 1996). Finally, cad1-3 and sppcs1, PCS1 defective mutants of A. thaliana and Sc. pombe, respectively, exhibited Cu hypersensitivity (Clemens et al. 1999; Ha et al. 1999). Similarly, cad1-3 and cad1-6 were more sensitive than the WT and accumulated less Zn when grown on low-strength cation medium (one-tenth Hoagland medium) supplemented with 50 μm Zn (Tennstedt et al. 2009), suggesting that PCs can regulate cytosolic zinc ion levels by forming a complex with Zn.
In conclusion, we have demonstrated that barley has a vacuolar transporter for PC–Cd, and that an ABC-type transporter may participate in the vacuolar PC–Cd transport by detoxifying excess Cd. This discovery advances our understanding of metal(loid) detoxification mechanisms in plants other than Arabidopsis. In addition, it is still unclear whether vacuolar sequestration of PC–Cd complexes is indeed the final step for Cd detoxification, although many studies have shown that PCs are the major Cd-binding peptides for Cd detoxification in plants, yeast and nematodes (Clemens et al. 1999; Ha et al. 1999; Vatamaniuk et al. 1999; Vatamanuik et al. 2001). Our phylogenetic tree indicates that some putative candidates may act as vacuolar PC transporters in barley and monocots. Verification of their transport activity and further investigation of their role in conferring essential heavy metal resistance will be a fascinating topic for future research.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Research Foundation (NRF) of Korea (NRF-2013R1A1A2008492 to W-Y.S.), by the Global Research Laboratory program of the Ministry of Science, Information and CommunicationTechnology, and Future Planning (MSIP) of Korea (2013031937 to Y.L.), by the Cooperative Research Program for Agriculture Science and Technology Development (PJ906910 to S-N.A) and by a grant from the National Institute of Environmental Health Sciences (P42ES010337 to J.I.S.).
Footnotes
Supporting Information: Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Appendix S1. Protein sequence of the barley putative orthologue (Hvul_ABCC1).
References
- Ahner BA, Morel FMM. Phytochelatin production in marine algae. 2. Induction by various metals. Limnology and Oceanography. 1995;40:658–665. [Google Scholar]
- Clemens S. Evolution and function of phytochelatin synthases. Journal of Plant Physiology. 2006;163:319–332. doi: 10.1016/j.jplph.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO Journal. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Schroeder J, Degenkolb T. Caenorhabditis elegans expresses a functional phytochelatin synthase. European Journal of Biochemistry. 2001;268:3640–3643. doi: 10.1046/j.1432-1327.2001.02293.x. [DOI] [PubMed] [Google Scholar]
- Cobbett CS. Phytochelatins and their roles in heavy metal detoxification. Plant Physiology. 2000;123:825–832. doi: 10.1104/pp.123.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer RA. Toxic and essential metal interactions. Annual Review of Nutrition. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- Grill E, Winnacker EL, Zenk MH. Phytochelatins, a class of heavy-metal-binding peptides from plants are functionally analogous to metallothioneins. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E, Loffler S, Winnacker EL, Zenk MH. Phytochelatins, the heavy-metal-binding peptides of plants are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase) Proceedings of the National Academy of Sciences of the United States of America. 1989;86:6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SB, Smith AP, Howden R, Dietrich WM, Bugg S, O′Connell J, Cobbett CS. Phytochelatin synthase genes from Arabidopsis and the yeast Schizosaccharomyces pombe. The Plant Cell. 1999;11:1153–1163. doi: 10.1105/tpc.11.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany. 2002;53:1–11. [PubMed] [Google Scholar]
- International Barley Genome Sequencing Consortium. Mayer KF, Waugh R, Brown JW, Schulman A, Langridge P, Stein N. A physical, genetic and functional sequence assembly of the barley genome. Nature. 2012;491:711–716. doi: 10.1038/nature11543. [DOI] [PubMed] [Google Scholar]
- Lee S, Moon JS, Ko TS, Petros D, Goldsbrough PB, Korban SS. Overexpression of Arabidopsis phytochelatin synthase paradoxically leads to hypersensitivity to cadmium stress. Plant Physiology. 2003;131:656–663. doi: 10.1104/pp.014118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Drozdowicz YM, Hörtensteiner S, Martinoia E, Rea PA. AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-congugates and chlorophyll catabolites: functional comparisons with AtMRP1. The Plant Cell. 1998;10:267–282. doi: 10.1105/tpc.10.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitani T, Kubota H, Sato K, Yamada T. The composition of metals bound to class III metallothionein (phytochelatin and its desglycyl peptide) induced by various metals in root cultures of Rubia tinctorum. Plant Physiology. 1996;110:1145–1150. doi: 10.1104/pp.110.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Grill E, Tommasini R, Kreuz K, Amrheim N. ATP-dependent glutathione S-conjugate ‘export’ pump in the vacuolar membrane of plants. Nature. 1993;364:247–249. [Google Scholar]
- Mendoza-Cózatl DG, Butko E, Springer F, Torpey J, Komives E, Kehr J, Schroeder J. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. The Plant Journal. 2008;54:249–259. doi: 10.1111/j.1365-313X.2008.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Zhai Z, Jobe TO, Akmakjian GZ, Song WY, Limbo O, Schroeder JI. Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. Journal of Biological Chemistry. 2010;285:40416–40426. doi: 10.1074/jbc.M110.155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Jobe TO, Hauser F, Schroeder JI. Longdistance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Current Opinion in Plant Biology. 2011;14:554–562. doi: 10.1016/j.pbi.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasugi A, Wada C, Hayashi Y. Cadmium-binding peptide induced in fission yeast, Schizosaccharomyces pombe. The Journal of Biochemistry. 1981;90:1561–1564. doi: 10.1093/oxfordjournals.jbchem.a133627. [DOI] [PubMed] [Google Scholar]
- Ortiz DF, Kreppel L, Speiser DM, Scheel G, McDonald G, Ow DW. Heavy-metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO Journal. 1992;11:3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz DF, Ruscitti T, McCue KF, Ow DW. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. The Journal of Biological Chemistry. 1995;270:4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. The Plant Journal. 2012;69:278–288. doi: 10.1111/j.1365-313X.2011.04789.x. [DOI] [PubMed] [Google Scholar]
- Preveral S, Gayet L, Moldes C, Hoffmann J, Mounicou S, Gruet A, Forestier C. A common highly conserved cadmium detoxification mechanism from bacteria to humans:heavy metal tolerance conferred by the ATP-binding cassette (ABC) transporter SpHMT1 requires glutathione but not metal-chelating phytochelatin peptides. The Journal of Biological Chemistry. 2009;284:4936–4943. doi: 10.1074/jbc.M808130200. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salt DE, Rauser WE. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiology. 1995;107:1293–1301. doi: 10.1104/pp.107.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. Journal of Experimental Botany. 2001;52:2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- Sharma SS, Dietz KJ. The relationship between metal toxicity and cellular redox imbalance. Trends in Plant Science. 2009;14:43–50. doi: 10.1016/j.tplants.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Martinoia E. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21187–21192. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooksa-Nguan T, Yakubov B, Kozlovskyy VI, Barkume CM, Howe KJ, Thannhauser TW, Rea PA. Drosophila ABC transporter, DmHMT-1, confers tolerance to cadmium: DmHMT-1 and its yeast homolog, SpHMT-1, are not essential for vacuolar phytochelatin sequestration. The Journal of Biological Chemistry. 2009;284:354–362. doi: 10.1074/jbc.M806501200. [DOI] [PubMed] [Google Scholar]
- Speiser DM, Ortiz DF, Kreppel L, Ow DW. Purine biosynthetic genes are required for cadmium tolerance in Schizosaccharomyces pombe. Molecular Cell Biology. 1992;12:5301–5310. doi: 10.1128/mcb.12.12.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tennstedt P, Peisker D, Böttcher C, Trampczynska A, Clemens S. Phytochelatin synthesis is essential for the detoxification of excess zinc and contributes significantly to the accumulation of zinc. Plant Physiology. 2009;149:938–948. doi: 10.1104/pp.108.127472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini R, Evers R, Vogt E, Mornet C, Zaman GJR, Schinkel AH, Martinoia E. The human multidrug resistance-associated protein functionally complements the yeast cadmium resistance factor 1. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6743–6748. doi: 10.1073/pnas.93.13.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamaniuk O, Mari S, Lu Y, Rea P. Mechanism of heavy metal ion activation of phytochelatin (PC) synthase: blocked thiols are sufficient for PC synthase-catalyzed transpeptidation of glutathione and related thiol peptides. The Journal of Biological Chemistry. 2000;275:31451–31459. doi: 10.1074/jbc.M002997200. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Mari S, Lu YP, Rea PA. AtPCS1, a phytochelatin synthase from Arabidopsis: isolation and in vitro reconstitu-tion. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7110–7115. doi: 10.1073/pnas.96.12.7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatamanuik OK, Bucher EA, Ward JT, Rea PA. A new pathway for heavy metal detoxification in animals. Phytochelatin synthesis is required for cadmium tolerance in Caenorhabditis elegans. The Journal of Biological Chemistry. 2001;276:20817–20820. doi: 10.1074/jbc.C100152200. [DOI] [PubMed] [Google Scholar]
- Yadav SK. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany. 2010;76:167–179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


