Abstract
Objective
To evaluate the role of bilirubin UDP-glucuronosyltransferase family 1, polypeptide A1 (UGT1A1) gene variations on prolonged unconjugated hyperbilirubinemia associated with breast milk feeding (breast milk jaundice [BMJ]).
Study design
UGT1A1 gene allelic variation was analyzed in 170 Japanese infants with BMJ with polymerase chain reaction-direct sequencing, and their genotypes compared with serum bilirubin concentrations. In 62 of 170 infants, serum bilirubin concentration was followed after 4 months of life. Genotypes were examined in 55 infants without BMJ.
Results
Of 170 infants with BMJ, 88 (51.8%) were homozygous UGT1A1*6. Serum bilirubin concentrations (21.8 ± 3.65 mg/dL) were significantly greater than in infants with other genotypes (P < .0001). The Gilbert UGT1A1*28 allele was not detected in infants with BMJ, except in an infant who was compound heterozygous with UGT1A1*6. At 4 months of age, serum bilirubin concentration improved to >1 mg/dL, except in 2 infants who were homozygous UGT1A1*7. Homozygous UGT1A1*6 was not detected in the control group.
Conclusion
One-half of the infants with BMJ were homozygous UGT1A1*6 and exhibited a serum bilirubin concentration significantly greater than other genotypes. This finding indicates that UGT1A1*6 is a major cause of BMJ in infants in East Asia. Previous finding have demonstrated that 5β-pregnane-3α,20β-diol present in breast milk inhibits p.G71R-UGT1A1 bilirubin glucuronidation activity. Thus, prolonged unconjugated hyperbilirubinemia may develop in infants with UGT1A1*6 who are fed breast milk.
Prolonged unconjugated hyperbilirubinemia associated with breast milk feeding (breast milk jaundice [BMJ]) is a phenomenon in infants fed with mother’s breast milk.1 This phenomenon is observed from the late neonatal period to 4 months of age. Hyperbilirubinemia decreases when breast milk is replaced with infant formula, and even if breast milk feeding continues, prolonged unconjugated hyperbilirubinemia improves over time. BMJ causes anxiety in infants, their parents, and pediatricians. In addition, kernicterus (bilirubin encephalopathy) is an occasional risk in severe unconjugated hyperbilirubinemia induced by BMJ.2 Arias et al1 andNewman and Gross3 reported in 1963 that breast milk was a factor in the development of neonatal jaundice. Many substances in breast milk were subsequently suspected to cause BMJ, including pregnane-3α,20β-diol, nonesterified fatty acid, and β-glucuronidase.4–6 However, a reliable causative agent of BMJ has not yet been conclusively elucidated.7–9
In a previous study of BMJ, we showed an association between BMJ and variants of the bilirubin UDP-glucuronosyltransferase (UDP-glucuronosyltransferase family 1, polypeptide A1 [UGT1A1]) gene.10 Our preliminary study on 17 infants with BMJ showed the c.211G>A (p.G71R) in the coding region of UGT1A1 (UGT1A1*6) might cause BMJ. From 17 infants with BMJ, 8 with BMJ were homozygous for UGT1A1*6, 7 were heterozygous UGT1A1*6, and 2 infants did not express the UGT1A1*6 allele.
UGT1A1 (EC 2.4.1.17) belongs to the UDP-glucuronosyltransferase type 1 (UGT1) family and plays a role in phase II drug metabolism.11 UGT1A1 catalyzes glucuronidation of many endobiotics and xenobiotics, converting hydrophobic substances to hydrophilic substances as a detoxification.12 Bilirubin is selectively catalyzed by UGT1A1.13 Defects in the UGT1A1 gene generate hereditary unconjugated hyperbilirubinemia, specifically Crigler-Najjar syndrome type I (MIM #21880), type II (MIM #606785), and Gilbert syndrome (MIM #143500).13–17 Crigler-Najjar syndrome type I and type II are severe and moderate phenotypes of hereditary unconjugated hyperbilirubinemia, respectively, in which the hyperbilirubinemia is life long.18,19 The clinical diagnosis of Gilbert syndrome occurs in approximately 3%–8.6% of the population.20–22 Two frequent polymorphisms associated with Gilbert syndrome are a missense mutation in exon 1 c.211G>A generating a p.G71R change (UGT1A1*6) and a c.-3279T>G in the promoter region that is linked to the A(TA)7TAA in the TATA box (UGT1A1*28).23
Among white, black, and west Asian subjects, homozygous UGT1A1*28 is associated with the clinical diagnosis of Gilbert syndrome.16,17 However, in east Asian patients (Japanese, Koreans, and Chinese), UGT1A1*6 is an important cause of adult hyperbilirubinemia, which is described clinically as Gilbert syndrome.24 In this study, we demonstrate that during neonatal development, the role of the UGT1A1*6 allele predominates over the other polymorphisms in its contribution towards the onset of breast milk induced neonatal hyperbilirubinemia.25 Thus, the UGT1A1*6 allele is predicted to be a risk factor for breast milk–induced jaundice during neonatal development.
Methods
We studied 170 Japanese infants (95 male and 75 female) with prolonged unconjugated hyperbilirubinemia associated with breast milk feeding. Infants had a gestational age greater than 35 weeks (range, 35 weeks, 1 day to 41 weeks, 3 days; mean, 38 weeks, 5 days ± 10.6 days). Birth weights were greater than 2300 g (2305–3902 g; mean, 3003 ± 385 g). All infants showed apparent prolonged unconjugated hyperbilirubinemia beyond 3 weeks of life. Total and indirect bilirubin concentrations at diagnosis ranged from 7.3 mg/dL to 32.5 mg/dL (124.8–555.7 µmol/L), and more than 95% of bilirubin was indirect. Except for jaundice, the infants were healthy and did not show signs of hemolytic anemia, liver dysfunction, or hypothyroidism. Serum bilirubin concentrations were within the normal range (<1mg/dL) or jaundice disappeared visually for all infants at 4 months of age even if breast milk feeding continued, except for infants with a particular genotype [homozygous p.Y486D (UGT1A1*7)].26 We followed serum bilirubin concentrations after 4 month of age in 62 cases and checked the association between genotypes and final serum bilirubin concentrations. The project was approved by the ethics committee of Shiga University of Medical Science.
The control group comprised 55 term infants (21 male and 34 female). All infants were fed breast milk. At an obligatory neonatal health check at 1 month of age, they showed no visible evidence of prolonged jaundice. Mean gestational age was 39 weeks 4 days ± 7 days (range, 37 weeks, 4 days to 41 weeks), and birth weights were greater than 2300 g (mean, 3050 ± 337 g; range, 2386–3900 g). After informed consent fromthe parents was received, genomic DNA was extracted from lymphocytes in stored cord blood.
Sequence Analysis of UGT1A1
For sequence analysis of UGT1A1 polymorphisms, genomic DNA was isolated from the leucocytes of infants, with parental informed consent. We amplified exons, the promoter region, and phenobarbital responsive enhancer module (gtPBREM) of UGT1A1 from genomic DNA by using polymerase chain reaction (PCR). In brief, approximately 100 ng of total genomic DNA was amplified with pairs of oligonucleotide primers. Exons 2, 3, and 4 and their intervening introns were simultaneously amplified as a single DNA fragment using a primer pair of 5′-CTCTATCT CAAACACGCATGCC-3′/ 5′-TTTTATCATGAATGCCATG ACC-3′. The 5′ region of UGT1A1, including the TATA box to exon 1, exon 5, and gtPBREM, was amplified separately with primer pairs of 5′-AAGTGAACTCCCTGC TACCTT-3′/5′-GCTTGCTCAGCATATATCTGGG-3′ (5′-region to exon 1), 5′-GAGGATTGTTCATACCACAGG-3′/ 5′-GCACTCTGGGGCTGATTAAT-3′ (exon 5), and 5′-CTGG GGATAAACATGGGATG-3′/5′-CACCACCACTTCTGGAAC CT-3′ (gtPBREM), respectively. Conditions for PCR were as follows: initial denaturation for 2 minutes at 94°C, followed by 1 minute at 94°C, 1 minute at 60°C, and 2 minutes at 72°C for 30 cycles with aMinicycler (MJ Research, Inc,Watertown, Massachusetts). A final extension for 10minutes at 72°C was performed to ensure complete extension of PCR products.
The sequences of the amplified DNA fragments were determined directly using the following sequencing primers. Sequence primers used for the determination of gtPBREM, TATA box, and coding region are as follows: for sequencing of gtPBREM: 5′-TGAGTTTATATAACCTC-3′; for the TATA box and exon 1: 5′-CTATTTCATGTCCCCTCTGC-3′, 5′-GT CTTTTGTTAGTCTCGGGC-3′, 5′-TTGTTGTGCAGTAAG TGGGA-3′, 5′-CCATTCTCCTACGTGCCCAG-3′, and5 ′ -AA GGGTTGCATACGGGGAATA-3′; for exon 2: 5′-GGAAGCT GGAAGTCTGGG-3′; for exon 3: 5′-CTAGTTAGTATAGCA GAT-3′; for exon 4: 5′-CAGCTGTGAAACTCAGAG-3′; and for exon 5: 5′-TGCTGACAGTGGCCTTCATC-3′ and 5′-GG TAGCCATAAGCACAACAT-3′. The sequences of the amplified DNA fragments were determined directly using a BigDye Terminator v1.1 Cycle Sequencing Kit and Genetic analyzer ABI Prism 3130×l (Applied Biosystems, Carlsbad, California).
Statistical Analyses
Serum bilirubin concentrations for the different genotypes detected were analyzed by ANOVA and the Scheffé test for pairwise comparisons using JMP9 (SAS Institute Inc, Cary, North Carolina) and Statview 4.5 (Abacus Corporation, Baltimore, Maryland). The analysis was performed among 5 frequently observed groups. Homozygous UGT1A1*6 (encodes the p.G71R variant), compound heterozygous for UGT1A1*6 and UGT1A1*60 (promoter c.-3279T>G in the gtPBREM region), heterozygous UGT1A1*6, and homozygous UGT1A1*1 (the normal common allele). All DNA samples were screened for mutations in the gtPBREM region, promoter and TATA box, exons, and exon-intron boundaries of the UGT1A1 gene.
Results
Genotype in the BMJ Group
Homozygous UGT1A1*6 was the most frequent genotype detected in the BMJ group (Table I). Approximately one-half the patients had this genotype (88 cases, 51.7%). Other frequent genotypes were heterozygous UGT1A1*6 (26 cases, 15.2%) and compound heterozygous for UGT1A1*6 and UGT1A1*60 (23 cases, 13.5%). Four cases were homozygous UGT1A1*60. The other groups included less common genotypes such as heterozygous UGT1A1*60 (5 cases), compound heterozygous for UGT1A1*6 and UGT1A1*28 (1 case), homozygous polymorphisms that encode the p.Y486D variant in exon 5 (UGT1A1*7) (3 cases), compound heterozygous for UGT1A1*6 and UGT1A1*7 (3 cases), compound heterozygous for UGT1A1*60 and c.-3279T>G+p.P364L (1091C>T in exon 4: UGT1A1*63) (2 cases), compound heterozygous for UGT1A1*6 and UGT1A1*63 (1 case), heterozygous for p.A471V (c.1412C>T) (1 case), homozygous for UGT1A1*63 (1 case), UGT1A1*6 /p.[G71R;Y486D] (2 cases), and UGT1A1*7/p.[G71R;Y486D] (1 case). In 8 cases, no mutation was detected in the gtPBREM, promoter, exons, and exon-intron boundaries of UGT1A1 (UGT1A1*1). In this study, no infants were homozygous for the UGT1A1 allele encoding the p.[G71R;Y486D]-UGT1A1 protein, the typical genotype of the Japanese patient with Crigler-Najjar type II.15
Table I.
Differences in UGT1A1 genotypes between infants with and without BMJ
| Genotype | Infants with BMJ, n = 170 |
% | Infants without BMJ, n = 55 |
% | |
|---|---|---|---|---|---|
| Allele 1 | Allele 2 | ||||
| UGT1A1*6 | UGT1A1*6 | 88 | 51.7 | 0 | 0 |
| UGT1A1*6 | UGT1A1*60 | 23 | 13.5 | 2 | 3.6 |
| UGT1A1*6 | UGT1A1*1 | 26 | 15.2 | 15 | 27.2 |
| UGT1A1*6 | UGT1A1*28 | 1 | 0.58 | 3 | 5.4 |
| UGT1A1*28 | UGT1A1*28 | 0 | 0 | 1 | 1.8 |
| UGT1A1*28 | UGT1A1*60 | 0 | 0 | 2 | 3.6 |
| UGT1A1*28 | UGT1A1*1 | 0 | 0 | 7 | 12.7 |
| UGT1A1*60 | UGT1A1*60 | 4 | 2.3 | 1 | 1.8 |
| UGT1A1*60 | UGT1A1*1 | 5 | 2.9 | 7 | 12.7 |
| UGT1A1*1 | UGT1A1*1 | 8 | 4.7 | 17 | 30.9 |
| Other | Other* | 15 | 9.4 | 0 | 0 |
UGT1A1*1, wild-type allele; UGT1A1*6, pG71R; UGT1A1*28, c.-3279T>G+A(TA)7TAA; UGT1A1*60, c.-3279T>G.
Other/other genotype in the BMJ group includes homozygous for p.Y486D (UGT1A1*7) (3 cases), compound heterozygous for UGT1A1*6 and UGT1A1*7 (4 cases), compound heterozygous for UGT1A1*60 and c.-3279T>G+p.P364L (UGT1A1*63) (2 cases), compound heterozygous for UGT1A1*6 and UGT1A1*63 (1 cases), heterozygous for p.A471V (c.1412C>T) (1 case), homozygous UGT1A1*63 (1 case), UGT1A1*6/p.[G71R;Y486D] (2 cases), and UGT1A1*7/p.[G71R;Y486D] (1 case).
Difference in Serum Bilirubin Concentration among the Genotypes in BMJ Group
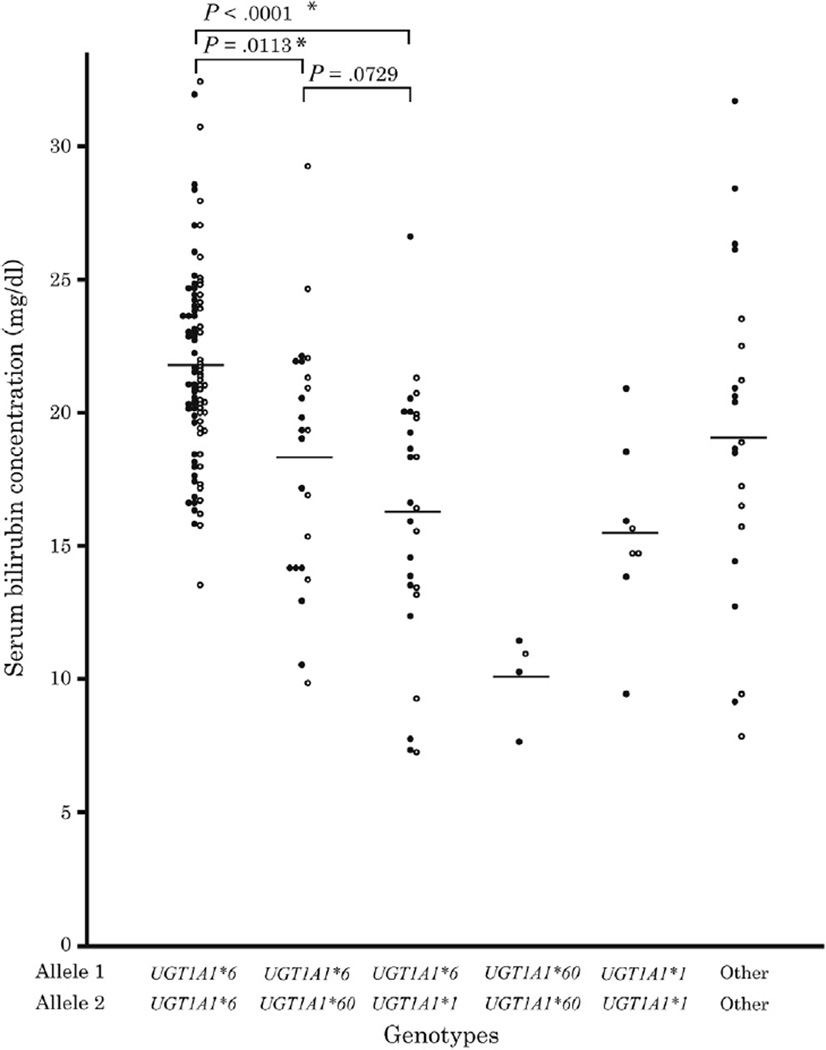
The relationship between genotype and serum bilirubin concentration was compared between 5 genotypes (Figure) of 149 infants in the BMJ group (homozygous UGT1A1*6, compound heterozygous UGT1A1*6 and UGT1A1*60, heterozygous UGT1A1*6, homozygous UGT1A1*60, and homozygous UGT1A1*1). Results of ANOVA revealed significant differences among the genotypes (degrees of freedom 4/144, F = 19.456, P < .0001; Table II). The severity of hyperbilirubinemia was as follows: homozygous UGT1A1*6 > compound heterozygous for UGT1A1*6 and UGT1A1*60 > heterozygous UGT1A1*6 > homozygous UGT1A1*1 > homozygous UGT1A1*60 (Table II). Comparison of the 5 groups showed that the serum bilirubin concentration in homozygous UGT1A1*6 was significantly greater than in compound heterozygous UGT1A1*6 and UGT1A1*60 and heterozygous UGT1A1*6 (P < .0001 and P < .0113, respectively; Figure).
Figure.
Difference in serum bilirubin concentration among the genotypes in BMJ groups. Closed circles and open circles represent male and female infants, respectively. The relationship between genotypes and serum bilirubin concentration was compared between the 5 genotypes. Results of ANOVA revealed significant differences among the genotypes (degrees of freedom: 4/144, F = 19.456, P < .0001).
Table II.
Genotypic profile of infants with BMJ
| Genotype | No. (total = 170) |
Male | Female | Birth weight, g* |
Mean ± SD | Gestational age, (weeks, days)* |
Mean ± SD | Serum bilirubin concentration | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Allele 1 | Allele 2 | mg/dL | Mean ± SD | |||||||
| UGT1A1*6 | UGT1A1*6 | 88 | 47 | 41 | 2305–3902 | 3041 ± 385 | 35 w, 4 d-41 w, 3 d | 38 w, 5 d ± 9.4 d | 13.6–32.5 | 21.8 ± 3.65 |
| UGT1A1*6 | UGT1A1*60 | 23 | 13 | 10 | 2374–3408 | 2944 ± 316 | 35 w, 1 d-41 w, 2 d | 39 w, 1 d ± 12.2 d | 9.9–29.3 | 18.3 ± 4.70 |
| UGT1A1*6 | UGT1A1*1 | 26 | 15 | 11 | 2320–3818 | 3016 ± 390 | 35 w, 1 d-41 w, 1 d | 38 w, 4 d ± 11.3 d | 7.3–26.7 | 16.3 ± 4.69 |
| UGT1A1*60 | UGT1A1*60 | 4 | 3 | 1 | 3066–3430 | 3220 ± 188 | 38 w, 4 d-39 w, 4 d | 39 w, 0 d ± 3.5 d | 7.7–11.5 | 10.1 ± 1.68 |
| UGT1A1*1 | UGT1A1*1 | 8 | 5 | 3 | 2315–3400 | 3031 ± 452 | 37 w, 0 d-41 w, 3 d | 38 w, 6 d ± 12.6 d | 9.5–21.0 | 15.5 ± 3.37 |
| Other | Other† | 21 | 12 | 9 | 2306–3375 | 2889 ± 390 | 36 w, 4 d-40 w, 5 d | 39 w, 2 d ± 7.7 d | 7.9–31.8 | 19.3 ± 6.19 |
Birth weight and gestational age are not statistically different among the each group.
Other/other genotype group in infants with BMJ include the following genotypes: heterozygous UGT1A1*60 (5 cases), compound heterozygous for UGT1A1*6 and UGT1A1*28 (1 cases), homozygous for UGT1A1*7 (3 cases), compound heterozygous for UGT1A1*6 and UGT1A1*7 (4 cases), compound heterozygous for UGT1A1*60 and UGT1A1*63 (2 cases), compound heterozygous for UGT1A1*6 and UGT1A1*63 (1 cases), heterozygous for p.A471V (1 case), homozygous UGT1A1*63 (1 case), UGT1A1*6/p.[G71R;Y486D] (2 cases), and UGT1A1*7/p.[G71R;Y486D] (1 case).
In the other groups, the severity of elevation in serum bilirubin concentration differed considerably depending on genotype. Infants with the UGT1A1*7 or p.[G71R:Y486D] allele showed serum bilirubin concentrations >25 mg/dL.
Follow-Up of Serum Bilirubin Concentration in 62 Patients with BMJ
In all, serum bilirubin concentrations of 62 patients with BMJ were followed up after 4–6 months for life (Table III). In most infants, serum bilirubin concentration was reduced <1 mg/dL (17.1 µmol/L), except for infants with 1 genotype (homozygous UGT1A1*7). Serum bilirubin concentration of 32 infants with homozygous UGT1A1*6 was reduced from 21.1 ± 3.8 mg/dL to 0.81 ± 0.31 mg/dL. Two infants with homozygous UGT1A1*7 showed prolonged unconjugated hyperbilirubinemia of Gilbert syndrome, (3.3 and 3.4 mg/dL, respectively) after 6 months of age.
Table III.
Differences in serum bilirubin concentration depending on the type of mutations in infants with BMJ
| Genotype | Mean serum bilirubin concentration, mg/dL | |||
|---|---|---|---|---|
| Allele 1 | Allele 2 | No. (total = 62) | At diagnosis | 4 months later |
| UGT1A1*6 | UGT1A1*6 | 33 | 21.2 ± 3.8 | 0.81 ± 0.31 |
| UGT1A1*6 | UGT1A1*60 | 8 | 16.3 ± 2.9 | 0.85 ± 0.42 |
| UGT1A1*6 | UGT1A1*1 | 9 | 14.9 ± 4.5 | 0.82 ± 0.73 |
| UGT1A1*60 | UGT1A1*60 | 1 | 11.5 | 0.58 |
| UGT1A1*7 | UGT1A1*7 | 2 | 15.1 | 3.35 |
| Other | Other* | 6 | 19.3 ± 7.8 | 0.83 ± 0.34 |
| UGT1A1*1 | UGT1A1*1 | 3 | 17.6 ± 2.9 | 0.61 ± 0.22 |
Other/other genotype group includes a rare combination of UGT1A1 mutations; compound heterozygous for UGT1A1*6 and p.[G71R:Y486D] (1 case), homozygous UGT1A1*63 (1 case), compound heterozygous for UGT1A1*6 and UGT1A1*7 (2 cases), and heterozygous for UGT1A1*60 (2 cases).
Genotypes in the Nonhyperbilirubinemic Group with Breast Milk Feeding
Genotype analysis of infants in the control group showed the normal common allele, homozygous UGT1A1*1 (17 cases, 30.9%), heterozygous UGT1A1*6 (15 cases, 27.2%), heterozygous UGT1A1*28 (7 cases, 12.7%), heterozygous UGT1A1*60 (7 cases, 12.7%), compound heterozygous for UGT1A1*6 and UGT1A1*28 (3 cases), compound heterozygous for UGT1A1*6/UGT1A1*60 (2 cases), UGT1A1*28/UGT1A1*60 (2 cases), homozygous UGT1A1*60 (1 case), and homozygous UGT1A1*28 (1 case). No infant was homozygous UGT1A1*6 (Table I).
Discussion
UGT1A1 mutations are a known cause of unconjugated hyperbilirubinemia in patients with type I and type II Crigler-Najjar syndrome and in Gilbert syndrome.14–17,23 In the neonatal period, infants with Crigler-Najjar syndrome show severe-to-moderate unconjugated hyperbilirubinemia, which continues throughout life. In contrast, mutations in the coding region, but not the regulatory region of UGT1A1 in Gilbert syndrome, are a risk factor for neonatal hyperbilirubinemia and BMJ.10,24 We show that mutations in UGT1A1 in infants also were associated with BMJ. The causes of BMJ can be classified into 2 categories: agents in the breast milk and infant diathesis. The causative agents in breast milk have not yet been determined. However, in an in vitro expression study, we recently showed that 5α-pregnane-3α, 20β-diol inhibits p.G71R UGT1A1, which is encoded by UGT1A1*6.27 Although genetic factors in neonatal hyperbilirubinemia have been reported,28,29 no significant association between genetic factors and BMJ has yet been demonstrated. In this study, we show a causal relationship between UGT1A1*6 polymorphism and BMJ.
These studies selected infants from across Japan who showed prolonged neonatal hyperbilirubinemia while nursing on breast milk. The most frequent genotype in the BMJ group was homozygous UGT1A1*6, occurring in 88 of 170 infants (51.7%) (Table I). Other frequent genotypes were heterozygous UGT1A1*6 (26 cases, 15.2%) and compound heterozygous for UGT1A1*6 and UGT1A1*60 (23 cases, 13.5%). In the Japanese population, the allelic frequency of UGT1A1*6 is 0.16 (Table IV; available at www.jpeds.com), and the estimated incidence of homozygous carriers of UGT1A1*6 is 2.56%.25 The high incidence of homozygous UGT1A1*6 in Japanese infants with BMJ implicates UGT1A1*6 is a genetic cause of BMJ.
Table IV.
Allelic frequencies of UGT1A1 polymorphism in the BMJ group, non-BMJ group, and Japanese population
| BMJ group | Non-BMJ group | Previous report* | |||
|---|---|---|---|---|---|
| n = 340 | Frequency | n = 110 | Frequency | Frequency | |
| UGT1A1*1 | 48 | 0.141 | 63 | 0.573 | |
| UGT1A1*6 | 236 | 0.694 | 20 | 0.182 | 0.151–0.16 |
| UGT1A1*28 | 1 | 0.003 | 14 | 0.127 | 0.121–0.15 |
| UGT1A1*60 | 34 | 0.100 | 13 | 0.118 | 0.115 |
| Other alleles | 21 | 0.062 | 0 | 0 | |
Allelic frequencies in Japanese population as determined in previous reports.25
In contrast, homozygous UGT1A1*1 was the most prevalent (30.9%) genotype in the randomized control group (Table I). Other frequent genotypes in this group were heterozygous UGT1A1*6 (27.2%), heterozygous UGT1A1*28 (12.7%), and heterozygous UGT1A1*60 (12.7%). One neonate was homozygous for UGT1A1*28. No infants were homozygous UGT1A1*6. The incidence of UGT1A1*6 and UGT1A1*28 in the control group was similar to that previously reported in the Japanese population (0.16 and 0.15, respectively).24 The UGT1A1*28 allele was not detected in the BMJ group, except in infants who were compound heterozygous. All results confirm our previous finding that the UGT1A1*28 allele does not induce hyperbilirubinemia in the neonatal period.25 Conversely, it might have a protective effect against the development of hyperbilirubinemia in the neonatal period, because the frequency of UGT1A1*28 in the BMJ group was significantly lower compared with the reported frequency in the normal Japanese population and control group (Table IV).
In our studies, serum bilirubin concentration in infants with BMJ differed among the genotypes. The serum bilirubin concentration in the infants with homozygous UGT1A1*6 was significantly greater than in other genotypes (Figure). The serum bilirubin concentration in compound heterozygous for UGT1A1*6 and UGT1A1*60 was greater than that in heterozygous UGT1A1*6, but the difference was not significant. Homozygous UGT1A1*60 showed the lowest concentration, but in combination with UGT1A1*6, it contributed significantly to hyperbilirubinemia.
In 62 cases that were followed beyond 4 months of age, serum bilirubin concentration in most cases decreased below 1 mg/dL (Table III). Concentration in the homozygous carriers of UGT1A1*6 was 0.81 ± 0.31 mg/dL. A previous report has suggested that homozygous UGT1A1*6 is not characteristic of Gilbert syndrome but mimics Crigler-Najjar syndrome type II.30 However, the present study confirms that homozygous UGT1A1*6 carriers do not develop Crigler-Najjar syndrome type II. UGT1A1 concentrations at birth are >1% of the concentration at adulthood, which appears to be reached by 3–4 months of age.31 BMJ is usually observed before 3 months of age and disappears even if breastfeeding is continued. Serum bilirubin concentrations of infants who are homozygous for UGT1A1*6 is reduced to <1 mg/dL. Those infants may develop Gilbert syndrome after puberty.10,32 BMJ with UGT1A1*6 should be a phenotype of Gilbert syndrome in infants. Two infants with homozygous p.Y486D (UGT1A1*7) showed mild elevation of serum bilirubin concentration after 4 months of life (3.3 and 3.1 mg/dL, respectively). Infants with UGT1A1*7 (10% of wild type) mutations showed a larger reduction in UGT1A1 activity than UGT1A1*6 (30%–80% of wild type) mutations.27,33 Therefore, carriers of homozygous UGT1A1*7 may continue to show mild hyperbilirubinemia even after 4 months of age.
Approximately 50% of the infants with BMJ were UGT1A1 genotypes other than homozygous UGT1A1*6. This finding implicates other potential mechanisms may contribute towards BMJ. A recent study using a humanized UGT1 mouse model clarified that expression of intestinal UGT1A1, but not hepatic UGT1A1, correlated with glucuronidation of bilirubin in the neonatal period.34 Inhibition of hepatic UGT1A1 expression was mediated in part by pregnane X receptor suppression of the UGT1A1 gene.35 Although the lack of hepatic UGT1A1 would be expected to produce dramatic hyperbilirubinemia, delayed expression of intestinal UGT1A1 prevented the onset of bilirubin induced neurological defects. Even though the UGT1A1*6 genotype was not evaluated in the UGT1A1*1 and UGT1A1*28 mouse models, breast milk was shown to play an important role in suppression of intestinal UGT1A1 expression. These findings implicated an important regulatory role for the I-κB kinase/nuclear factor kappa B inflammatory pathway in controlling expression of intestinal UGT1A1.36
The components of breast milk clearly contribute to the onset of neonatal jaundice.37 Our in vitro investigation showed the inhibitory effect of 5α-pregnane-3α, 20β-diol on UGT1A1 activity.26 5α-pregnane-3α, 20β-diol does not inhibit transcriptional activity of either the UGT1A1*1 wild-type enhancer-promoter or the c.-3279T>G+A(TA)7TAA enhancer-promoter (UGT1A1*28). 5α-pregnane-3α, 20β-diol also does not inhibit UGT1A1 that is encoded by the UGT1A1*1 allele. However, glucuronidation activity of p.G71R UGT1A1, encoded by the UGT1A1*6 allele, is inhibited by 5α-pregnane-3α, 20β-diol. Although our analysis of UGT1A1 expression using HepG2 cells showed no inhibitory effect on expression of RNA, recent reports using humanized UGT1 mice demonstrate that breast milk reduces expression of intestinal UGT1A1.36 Thus, breast milk may not only inhibit glucuronidation activity of p.G71R-UGT1A1 directly by 5α-pregnane-3α, 20β-diol but also decrease activity by inhibiting expression of intestinal UGT1A1.
In conclusion, we report that a homozygous UGT1A1*6 mutation is an important cause of BMJ in infants in East Asia, although the role of UGT1A1 polymorphisms for developing BMJ is in other ethnic groups is unknown. In infants with homozygous UGT1A1*6 fed with breast milk, 5β-pregnane-3α, 20β-diol may inhibit p.G71R-enzyme activity. In addition to the inhibitory effect of breast milk on intestinal induction of UGT1A1, these infants may exhibit prolonged BMJ up to 4 months of age.
Acknowledgments
Supported by the Ministry of Education, Science, and Culture of Japan (14704032, 19591248, 24659495), the Mother and Child Health Foundation in Japan, and the US Public Health Service (ES100337 and GM086713 to R.T.).
We thank Masashi Suzaki (Central Research Laboratory, Shiga University of Medical Science) for technical assistance, and Akira Asiaka (Department of Pediatrics, Hino Memorial Hospital) for clinical assistance. We thank the pediatricians who referred the infants with BMJ.
Glossary
- BMJ
Breast milk jaundice
- gtPBREM
Phenobarbital responsive enhancer module
- PCR
Polymerase chain reaction
- UGT1
UDP-glucuronosyltransferase type 1
- UGT1A1
Bilirubin UDP-glucuronosyltransferase 1 family, polypeptide A1
Footnotes
The authors declare no conflicts of interest.
References
- 1.Arias IM, Gartner LM, Seifter S. Neonatal unconjugated hyperbilirubinemia associated with breast-feeding and a factor in milk that inhibits glucuronide formation in vitro. J Clin Invest. 1963;42:913. doi: 10.1172/JCI105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maisels MJ, Newman TB. Kernicterus in otherwise healthy, breast-fed term newborns. Pediatrics. 1995;96:730–733. [PubMed] [Google Scholar]
- 3.Newman AJ, Gross S. Hyperbilirubinemia in breast-fed infants. Pediatrics. 1963;32:995–1001. [PubMed] [Google Scholar]
- 4.Arias IM, Gartner LM, Seifter S, Furman M. Prolonged neonatal uncounjugated hyperbilirubinemia associated with breast feeding and a steroid, pregnane-3(alpha), 20(beta)-diol, in maternal milk that inhibits glucuronide formation in vitro. J Clin Invest. 1964;43:2037–2047. doi: 10.1172/JCI105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan BR, Holton JB. Inhibition of bilirubin conjugation in rat liver slices by free fatty acids, with relevance to the problem of breast milk jaundice. Clin Chim Acta. 1972;41:101–107. doi: 10.1016/0009-8981(72)90501-3. [DOI] [PubMed] [Google Scholar]
- 6.Gourley GR, Arend RA. β-Glucuronidase and hyperbilirubinemia in breast-fed and formula-fed babies. Lancet. 1986;1:644–646. doi: 10.1016/s0140-6736(86)91724-1. [DOI] [PubMed] [Google Scholar]
- 7.Ramos A, Silverberg M, Stern M. Pregnanediol and neonatal hyperbilirubinemia. Am J Dis Child. 1966;111:353–356. doi: 10.1001/archpedi.1966.02090070051003. [DOI] [PubMed] [Google Scholar]
- 8.Constantopoulos A, Messaritakis J, Matsaniotis N. Breast milk jaundice; the role of lipoprotein lipase and the free fatty acids. Eur J Pediatr. 1980;134:35–38. doi: 10.1007/BF00442400. [DOI] [PubMed] [Google Scholar]
- 9.Gaffney PT, Buttenshaw RL, Ward M, Diplock RD. Breast milk β-glucuronidase and neonatal jaundice. Lancet. 1986;1:1161–1162. doi: 10.1016/s0140-6736(86)91879-9. [DOI] [PubMed] [Google Scholar]
- 10.Maruo Y, Nishizawa K, Sato H, Sawa H, Shimada M. Prolonged unconjugated hyperbilirubinemia associated with breast milk and mutations of the bilirubin uridine diphosphate-glucuronosyltransferase gene. Pediatrics. 2000;106:E59. doi: 10.1542/peds.106.5.e59. [DOI] [PubMed] [Google Scholar]
- 11.Maruo Y, Iwai M, Mori A, Sato H, Takeuchi Y. Polymorphism of UDP-glucuronosyltransferase and drug metabolism. Curr Drug Metab. 2005;6:91–99. doi: 10.2174/1389200053586064. [DOI] [PubMed] [Google Scholar]
- 12.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 13.Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, et al. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem. 1994;269:17960–17964. [PubMed] [Google Scholar]
- 14.Bosma PJ, Chowdhury NR, Goldhoorn BG, Hofker MH, Oude Elferink RP, Jansen PL, et al. Sequence of exons and the flanking regions of human bilirubin-UDP-glucuronosyltransferase gene complex and identification of a genetic mutation in a patient with Crigler-Najjar syndrome, type I. Hepatology. 1992;15:941–947. doi: 10.1002/hep.1840150531. [DOI] [PubMed] [Google Scholar]
- 15.Aono S, Yamada Y, Keino H, Hanada N, Nakagawa T, Sasaoka Y, et al. Identification of defect in the genes for bilirubin UDP-glucuronosyltransferase in a patient with Crigler-Najjar syndrome type II. Biochem Biophys Res Commun. 1993;197:1239–1244. doi: 10.1006/bbrc.1993.2610. [DOI] [PubMed] [Google Scholar]
- 16.Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 17.Monaghan G, Ryan M, Seddon R, Hume R, Burchell B. Genetic variation in bilirubin UDP-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet. 1996;347:578–581. doi: 10.1016/s0140-6736(96)91273-8. [DOI] [PubMed] [Google Scholar]
- 18.Crigler JF, Najjar VA. Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics. 1952;10:169–179. [PubMed] [Google Scholar]
- 19.Arias IM, Gartner LM, Cohen M, Ezzer JB, Levi AJ. Choronic non-hemolytic unconjugated hyperbilirubinemia with Glucuronosyl transferase deficiency. Am J Med. 1969;47:395–409. doi: 10.1016/0002-9343(69)90224-1. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert A, Lereboullet P. La choléemie simple familiale. Semaine Med. 1901;21:241–243. [Google Scholar]
- 21.Owens D, Evans J. Population study on Gilbert’s syndrome. J Med Genet. 1975;12:152–156. doi: 10.1136/jmg.12.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieg A, Arab L, Schlierf G, Stiehl A, Kommerell B. Prevalence of Gilbert’s syndrome in Germany. Dtsch Med Wochenschr. 1987;112:1206–1208. doi: 10.1055/s-2008-1068222. [DOI] [PubMed] [Google Scholar]
- 23.Maruo Y, D’Addario C, Mori A, Iwai M, Takahashi H, Sato H, et al. Two linked polymorphic mutations (A(TA)7TAA and T-3279G) of UGT1A1 as the principal cause of Gilbert syndrome. Hum Genet. 2004;115:525–526. doi: 10.1007/s00439-004-1183-x. [DOI] [PubMed] [Google Scholar]
- 24.Sato H, Adachi Y, Koiwai O. The genetic basis of Gilbert’s syndrome. Lancet. 1996;347:557–558. doi: 10.1016/s0140-6736(96)91266-0. [DOI] [PubMed] [Google Scholar]
- 25.Maruo Y, Nishizawa K, Sato H, Doida Y, Shimada M. Association of neonatal hyperbilirubinemia with bilirubin UDP-glucuronosyltransferase polymorphism. Pediatrics. 1999;103:1224–1227. doi: 10.1542/peds.103.6.1224. [DOI] [PubMed] [Google Scholar]
- 26.Maruo Y, Sato H, Yamano T, Doida Y, Shimada M. Gilbert syndrome due to a homozygous missense mutation (Tyr486Asp) of bilirubin UDP-glycosyltransferase gene. J Pediatr. 1998;132:1045–1047. doi: 10.1016/s0022-3476(98)70408-1. [DOI] [PubMed] [Google Scholar]
- 27.Ota Y, Maruo Y, Matsui K, Mimura Y, Sato H, Takeuchi Y. Inhibitory effect of 5β-pregnane-3α,20β-diol on transcriptional activity and enzyme activity of human bilirubin UDP-glucuronosyltransferase. Pediatr Res. 2011;70:453–457. doi: 10.1203/PDR.0b013e31822f242e. [DOI] [PubMed] [Google Scholar]
- 28.Bozkaya OG, Kumral A, Yesilirmak DC, Ulgenalp A, Duman N, Ercal D, et al. Prolonged unconjugated hyperbilirubinaemia associated with the haem oxygenase-1 gene promoter polymorphism. Acta Paediatr. 2010;99:679–683. doi: 10.1111/j.1651-2227.2009.01678.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang MJ, Kua KE, Teng HC, Tang KS, Weng HW, Huang CS. Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res. 2004;56:682–689. doi: 10.1203/01.PDR.0000141846.37253.AF. [DOI] [PubMed] [Google Scholar]
- 30.Kadakol A, Ghosh SS, Sappal BS, Sharma G, Chowdhury JR, Chowdhury NR. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype. Hum Mutat. 2000;16:297–306. doi: 10.1002/1098-1004(200010)16:4<297::AID-HUMU2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Onishi S, Kawade N, Itoh S, Isobe K, Sugiyama S. Postnatal development of uridine diphosphate glucuronosyltransferase activity toward bilirubin and 2-aminophenol in human liver. Biochem J. 1979;184:705–707. doi: 10.1042/bj1840705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maruo Y, Wada S, Yamamoto K, Sato H, Yamano T, Shimada M. A case of anorexia nervosa with hyperbilirubinaemia in a patient homozygous for a mutation in the bilirubin UDP-glucuronosyltransferase gene. Eur J Pediatr. 1999;158:547–549. doi: 10.1007/s004310051143. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto K, Sato H, Fujiyama Y, Doida Y, Bamba T. Contribution of two missense mutations (G71R and Y486D) of the UGT1A1 gene to phenotypes of Gilbert’s syndrome and Crigler-Najjar syndrome type II. Biochim Biophys Acta. 1998;1406:267–273. doi: 10.1016/s0925-4439(98)00013-1. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara R, Nguyen N, Chen S, Tukey RH. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci U S A. 2010;107:5024–5029. doi: 10.1073/pnas.0913290107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Yueh MF, Evans RM, Tukey RH. Pregnane-x-receptor controls hepatic glucuronidation during pregnancy and neonatal development in humanized UGT1 mice. Hepatology. 2012;56:658–667. doi: 10.1002/hep.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiwara R, Chen S, Karin M, Tukey RH. Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-κB leads to hyperbilirubinemia. Gastroenterology. 2012;142:109–118. doi: 10.1053/j.gastro.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato H, Uchida T, Toyota K, Kanno M, Hashimoto T, Watanabe M, et al. Association of breast-fed neonatal hyperbilirubinemia with UGT1A1 polymorphisms: 211G>A (G71R) mutation becomes a risk factor under inadequate feeding. J Hum Genet. 2013;58:7–10. doi: 10.1038/jhg.2012.116. [DOI] [PubMed] [Google Scholar]