Abstract
Concentrations of total mercury ([THg]) and selenium ([TSe]) were measured in several tissue compartments in Steller sea lion (Eumetopias jubatus) pups; in addition we determined specific compartment and body burdens of THg. Compartmental and body burdens were calculated by multiplying specific compartment fresh weight by the [THg] (summing compartment burdens equals body burden). In all 6 pup tissue sets 1) highest [THg] was in hair, 2) lowest [THg] was in bone, and 3) pelt, muscle and liver burdens contributed the top three highest percentages of THg body burden. In 5 of 6 pups the Se:Hg molar ratios among compartments ranged from 0.9 to 43.0. The pup with the highest hair [THg] had Se:Hg molar ratios in 9 of 14 compartments that were ≤ 0.7 potentially indicating an inadequate [TSe] relative to [THg].
Keywords: mercury, selenium, burden, molar ratio, Steller sea lions
Introduction
Mercury (Hg) is a naturally occurring element with known toxic effects in humans and terrestrial mammals. Recently, there has been increasing concern over total Hg concentrations ([THg]) found in some Steller sea lions (Eumetopias jubatus) in the western Aleutian Islands (Fig. 1) [1]. Steller sea lions are piscivorous marine mammals that biomagnify relatively high Hg concentrations through the diet and easily transfer Hg through the placenta to the developing fetus [2] similar to other piscivorous mammals [3,4]. Steller sea lions from the Aleutian Islands are part of the western distinct population segment (DPS) which are genetically different from the Steller sea lions found in Southeast Alaska. The Steller sea lion populations in the western DPS, particularly those in the western Aleutian Islands, have been slow to recover from dramatic population declines that occurred during the 1970s and 1980s [5-8]. Several causes have been hypothesized, such as nutritional stress, fisheries competition, and chemical pollution among others, but no studies have produced conclusive evidence [5,9-11]. Chemical pollution, including exposure to contaminants such as polychlorinated biphenyls (PCB) and Hg, has been under study for several years as a potential cause for the lack of recovery of Steller sea lions in the western DPS [1,2,9,12]. Determining Hg distribution in Steller sea lion tissues (and in piscivorous marine mammal tissues in general) is an important step in understanding Hg toxicity in this species, especially in the fetus and neonate as the main cohorts of concern (e.g., exposure of the dam during gestation).
Figure 1.
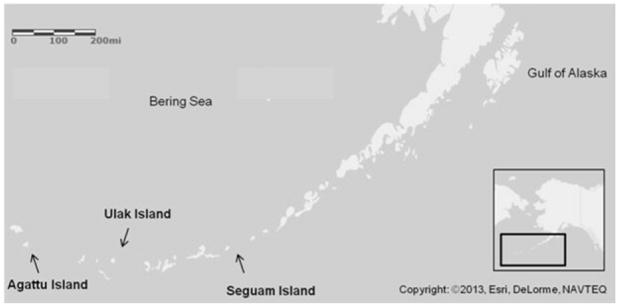
Steller sea lion (Eumetopias jubatus) pup collection site map. Collection sites of dead Steller sea lion pups from the Aleutian Islands include Agattu Island (52°26’07”N, 173°34’32”E), Ulak Island (51°21’54”N, 178°56’50”W), and Seguam Island (52°19’24”N, 172°27’58”W). The arrow tips point to the approximate sample locations.
Toxicity of Hg is dependent on the bioavailability and chemical form of Hg which dictates distribution among tissues. Monomethyl mercury (MeHg+) has been known to have adverse effects on reproductive, immunological, and neurological functions in humans and rats [13-16]. MeHg+ can cross the blood-brain barrier as well as other organs such as the placenta and gastrointestinal tract, and > 90% of ingested MeHg+ can be absorbed into blood [3,4,17]. Thus, MeHg+ distribution is systemic, reaching all vital organs including the brain, and accumulates in several tissues including erythrocytes, muscle, and hair [16]. On the other hand, inorganic mercury (Hg2+) is generally found in greater concentrations in two target organs, the liver and kidney. Several studies have found that both liver and kidney have demethylating mechanisms that convert MeHg+ to Hg2+ [16,18,19]. Demethylating mechanisms develop with increasing age [18,20] leaving fetal and very young mammals more vulnerable to MeHg+ toxic effects when compared to adult mammals.
Hair has been commonly used as an indicator tissue for MeHg+ exposure for piscivores. Hair total Hg concentration ([THg]) is highly correlated with [THg] in blood and is thought to be a good indicator of [THg] in circulation [1,2,21]. Keratin is the main structural protein found in hair, nails and epidermis and contains multiple disulfide cross linkages as well as cysteine residues. Cysteine is a sulfur-containing amino acid and is likely the binding site for MeHg+ in hair. It is estimated that approximately 80% of total mercury (THg) in hair is in the form of MeHg+ [22]. In recent studies [THg] in hair of Steller sea lion pups have been found to be higher in the Aleutian Islands when compared to Southeast Alaska [1,2] and in some cases have exceeded numerous human and wildlife thresholds for Hg adverse effects [23,24]. Steller sea lion pups are born with a natal pelage (lanugo) that is molted when they are 4 to 6 months old. Therefore, Hg in the hair of pups under 4 months of age represents Hg exposure via placental transfer from the mother during gestation. Since it is more difficult to sample tissues such as muscle, liver, kidney and brain in live pups, this study will determine how representative hair [THg] is of other tissue compartments from pups found dead on rookeries.
Some investigators have hypothesized that Hg toxicosis in humans and marine mammals can be diminished through the association of the essential trace element selenium (Se) and Hg particularly when the Se:Hg molar ratio is greater than 1 in the kidney, liver and possibly other tissues [25-28]. The antioxidant role of Se in the mammalian diet is under homeostatic control [29,30] but we also likely need to consider direct interactions with toxic elements such as Hg (covalent linkages, Hg-Se). Selenium can increase Hg half-life in the blood and liver, make it less reactive, and have a significant effect in organ distribution and excretion of Hg [28,31]. Selenium tends to be higher in marine mammals when compared to terrestrial mammals likely due to a greater intake of Se in the marine fish diet. High Se intake is also an advantage for marine mammals in that it can play a physiological role as an antioxidant for diving mammals [32,33] as well as a role in ameliorating adverse effects of Hg [28].
Evaluating THg body burden along with individual tissue compartment burdens and concentrations in pinnipeds such as Steller sea lion pups will provide understanding about Hg distribution and storage in various biological tissues. In particular, this will put [THg] measured in traditionally sampled tissues (e.g., hair, liver and skeletal muscle) into better perspective (e.g., % of total body burden). We compare [THg] from all tissues collected from pup carcasses, taking into account mass of tissue, to determine rank order of tissues from the highest to lowest [THg] and THg burden. We evaluate the [TSe] in order to determine Se:Hg molar ratio among tissues of the body to provide insight on the possible protective role of Se within the whole body as well as specific tissue compartments.
Materials and Methods
Sample collection
Steller sea lion pups (n = 6) found dead on their natal rookeries in the Aleutian Islands, Alaska (Fig. 1) in 2011, 2012 and 2013 were collected by the Alaska Department of Fish and Game (ADF&G) (MMPA/ESA Permit No. 14325). Pup age was estimated by using collection dates (Jun23-Jul2) and assumed birthing dates (May15-Jul15) as determined by Pitcher et al. [34]. Necropsies of thawed animals were performed under an educational outreach setting with university student volunteers assisting in total body measurements (weight, length, girth, etc.), external examination, sex determination, tissue collection, and dissection under professional guidance and instruction. All tissues were processed and analyzed whole except pelt, muscle and bone. All subsamples were collected using stainless steel disposable scalpels and cutting knives. A subsample of hair was removed from the pelt using battery operated grooming clippers (Wahl ® Super Pocket Pro ® Clippers). Hair samples were washed in 1% Triton X-100 following a previously published hair washing protocol for metals analysis [1,2,21]. A 5g subsample of the pelt (hair, epidermis, and dermis) was collected to represent whole pelt [THg]. Muscle subsamples for THg analysis were collected in equal proportion by mass from the scapular region and the pelvic region of each pup. All remaining skeletal muscle was removed from bone and weighed. Total skeletal muscle mass was a combination of the scapular region subsample, pelvic region subsample, and skeletal muscle removed from bones.
One femur and a cartilaginous rib (4th rib) were used to represent bone [35]. Cartilage was separated from bone and its weight was added to the final bone mass. Bone was placed in distilled water for 3 weeks to soften the remaining fascial tissue for removal. Bone was then air dried under a fume hood and dry weight was recorded. Freeze drying of samples was performed using a Freezone 4.5 Freeze Dry System (Labconco, Kansas City, MO). Percent moisture of most tissues was calculated: [(wet weight - dry weight) / wet weight] × 100. Homogenization of complete tissue compartments and subsampled tissues was performed using a Retsch Cryomill (Retsch Inc, Newton, PA). All samples were stored in polyethylene Whirlpaks ® and 1 gallon Ziploc ® bags at -20°F and -80°F prior to freeze drying. Dry homogenized samples were stored short-term at room temperature and returned to ADF&G for archiving after chemical analysis.
Mercury analysis
Approximately 0.010-0.020g of homogenized powdered tissues were analyzed for [THg] using the Direct Mercury Analyzer (Milestone, Inc, Shelton, CT; EPA Method 7473) [1,2,21,36]. Approximately 6.0mg of hair was analyzed separately from the total pelt. All samples were analyzed in triplicate and were considered acceptable with a 15% error from the mean. Each run included one blank, a liquid standard (0.001μg/g HgCl2 or 1μg/g HgCl2 standard; Perkin Elmer, Waltham, Massachusetts) and two certified reference materials (DORM 3 = 0.382μg/g and DOLT 4 = 2.58μg/g; National Research Council Canada, Institute for National Measurement Standards, Ottawa, Canada). The detection limits were 0.075 μg/g (0.374 μM) for 0.010g of tissue and 0.038μg/g (0.189 μM), for 0.020g of tissue. Recovery range of standard and certified reference materials were 87-101% (0.010μg/g HgCl2 standard), 94-104% (1 μg/g HgCl2 standard), 91-118% (DORM 3) and 102-114% (DOLT 4).
Selenium analysis
Approximately 0.030-0.050g of each homogenized powdered tissue was digested by microwave using nitric acid (HNO3) and hydrogen peroxide (H2O2) and analyzed for total selenium concentration ([TSe]) following previous methods [37-39] . For each set of digestions, quality control samples included a blank spike (Perkin Elmer, Waltham, Massachusetts), duplicate, sample spike, internal standard (DORM 3) and certified reference material (DOLT 4 = 8.30μg/g; National Research Council Canada, Institute for National Measurement Standards, Ottawa, Canada). The detection limit range was 0.39-0.66μg/g (4.99-8.32μM). Recovery range of quality control samples and certified reference materials were 80-111% (1.01 μg/g blank spike), 80-122% (sample spike), and 78-108% (DOLT 4).
Data analysis and calculations
Due to the small sample size (n = 6 individual pups) significant differences were not assessed. A cumulative rank was applied to [THg] and THg tissue burden data. The cumulative rank was determined by assigning each tissue compartment with a value of 1 to 14 with 1 being the tissue with the highest [THg] or THg tissue burden and 14 being the tissue with the lowest [THg] or THg tissue burden. Each pup had an independent set of values by tissue type depending on the [THg] distribution and THg tissue burden. Because pups are numbered 1-6 based on THg concentrations reported in Table 1, tissue ranking for pup no. 1 was used as a standard tissue list prior to sorting tissues by cumulative rank. The sum of these rank values for each tissue from the 6 pups was determined to be the cumulative rank number for that specific tissue (i.e., brain [THg] cumulative rank: 7 + 13 + 6 + 11 + 11 + 13 = 61). Spearman correlation (rs) was used to assess the association between [THg] and THg tissue burdens for the group of pups.
Table 1.
Steller sea lion (Eumetopius jubatus) pup (n=6) collection information: Location (rookery site of collection), year collected, body weight, sex, total mercury concentration in hair and total mercury body burden (calculated from the sum of compartment burdens reported in Table 2).
| Pup No. | Location | Year | Body Weight (kg) | Sex | Hair THg (μg/g) | THg body burden (mg) |
|---|---|---|---|---|---|---|
| 1 | Ulak Island | 2012 | 19.5 | M | 5.99 | 3.14 |
| 2 | Agattu Island | 2011 | 19.1* | M | 6.75 | 4.16 |
| 3 | Ulak Island | 2012 | 14.6 | M | 7.36 | 5.45 |
| 4 | Ulak Island | 2013 | 26.7 | M | 10.67 | 12.21 |
| 5 | Seguam Island | 2012 | 26.4 | M | 11.27 | 14.24 |
| 6 | Agattu Island | 2011 | 19.1* | F | 59.17 | 30.25 |
THg = total mercury
Pups were emaciated with no grossly visible subcutaneous blubber
Total Hg tissue compartment burdens (mg) were calculated as the product of tissue mean [THg] (mg/g, wet weight) and wet tissue mass (g). Total Hg body burden for each pup was calculated as the sum of all Hg tissue compartment burdens. Molar concentrations (μM) of THg and TSe were calculated as the product of THg and TSe mass based concentrations (μg/g) and the molecular weight of each element (Hg = 200.59 μg/mole; Se = 78.96 μg/mole).
Percentages (%) of body mass, of [THg], and of THg body burden were calculated as follows:
Results
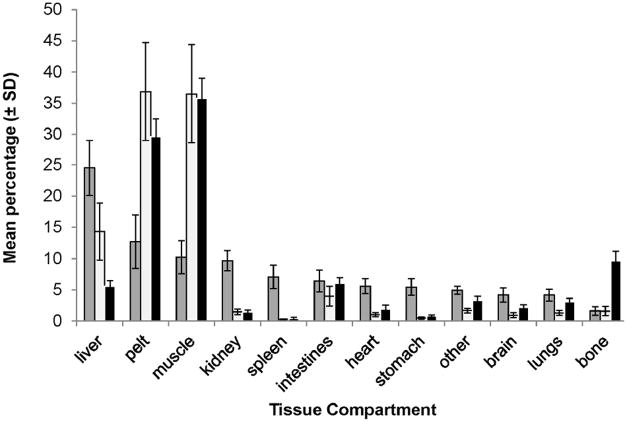
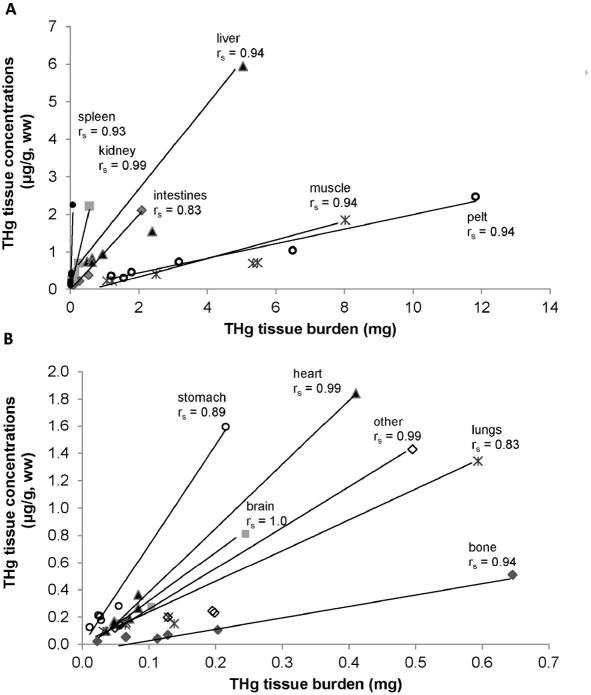
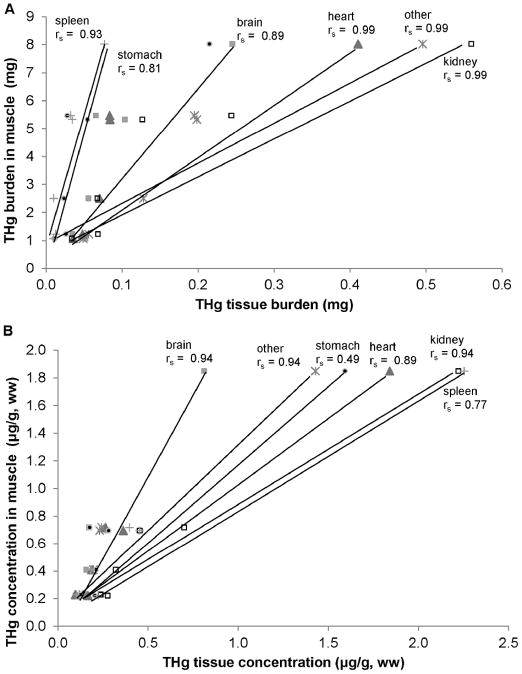
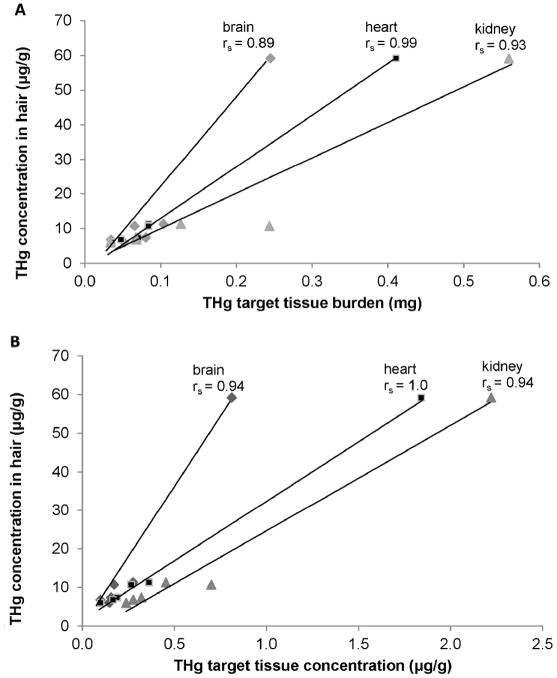
Gross necropsy results could not determine an obvious cause of death; 2 of the 6 pups were emaciated with no evident subcutaneous blubber based upon gross examination. Concentrations of THg in hair samples (a tissue traditionally sampled from live captured animals) varied more than 30-fold among the 6 pups (Table 1). For all 6 pups the highest [THg] for a tissue was in hair and the lowest [THg] was in bone (Table 2A). In 4 of the 6 pups the highest percent of THg burden was in pelt. The highest percent of THg burden in the remaining 2 pups was in muscle (these 2 pups weighed 19.1kg and 26.7kg and were not emaciated). In all 6 pups the lowest percent of THg burden was in spleen (Table 2B). Table 3 summarizes [THg], THg burden, [TSe], and Se:Hg molar ratios. A comparison of [THg], tissue mass, and THg burden demonstrated the significance of individual compartment mass to the overall burden of THg in the body (Figure 2). Together pelt, muscle and liver comprised 87% of total Hg body burden and were the top three ranked compartments. Pelt and muscle comprised 65% of total body mass, 23% of the sum of [THg] in the body, and 73% of THg body burden. THg burden for various tissue compartments was highly correlated with [THg] in some tissues (rs = 0.83 or greater, Figure 3A, B). THg burden and [THg] in various tissues were both highly correlated with THg burden in muscle and [THg] in muscle, respectively (rs = 0.77 or greater, Figure 4A, B). Hair [THg] was also correlated with target organ THg tissue burdens (rs = 0.89 or greater, Figure 5A). Hair [THg] was correlated with target organ [THg] (rs = 0.94 or greater, Figure 5B) including brain, heart and kidney.
Table 2.
Total mercury concentration (A) and total mercury burden (B) cumulative rank order for multiple tissues (compartments) from 6 Steller sea lion (Eumetopias jubatus) pups a,b,c
| pup1 | pup2 | pup3 | pup4 | pup5 | pup6 | Tissue | Cumulative Rank |
|---|---|---|---|---|---|---|---|
| A | |||||||
| Hair | Hair | Hair | Hair | Hair | Hair | Hair | 6 |
| Liver | Liver | Liver | Liver | Pelt | Liver | Liver | 13 |
| Muscle | Pelt | Pelt | Pelt | Liver | Pelt | Pelt | 19 |
| Kidney | Kidney | Kidney | Muscle | Muscle | Spleen | Kidney | 27 |
| Pelt | Intestines | Muscle | Kidney | Kidney | Kidney | Muscle | 31 |
| Intestines | Spleen | Brain | Spleen | Spleen | Intestines | Intestines | 43 |
| Brain | Epidermis | Epidermis | Heart | Intestines | Muscle | Spleen | 44 |
| Stomach | Muscle | Stomach | Other | Heart | Heart | Stomach | 54 |
| Other | Stomach | Other | Intestines | Epidermis | Stomach | Heart | 55 |
| Heart | Heart | Intestines | Stomach | Stomach | Epidermis | Epidermis | 59 |
| Spleen | Lungs | Spleen | Brain | Brain | Other | Brain | 61 |
| Lungs | Other | Heart | Lungs | Other | Lungs | Other | 61 |
| Epidermis | Brain | Lungs | Epidermis | Lungs | Brain | Lungs | 73 |
| Bone | Bone | Bone | Bone | Bone | Bone | Bone | 84 |
|
| |||||||
| B | |||||||
| Muscle | Pelt | Pelt | Muscle | Pelt | Pelt | Pelt | 8 |
| Pelt | Muscle | Muscle | Pelt | Muscle | Muscle | Muscle | 10 |
| Liver | Liver | Liver | Liver | Liver | Liver | Liver | 18 |
| Intestines | Intestines | Intestines | Intestines | Intestines | Intestines | Intestines | 24 |
| Other | Kidney | Bone | Kidney | Bone | Bone | Other | 39 |
| Bone | Lungs | Brain | Other | Other | Lungs | Bone | 40 |
| Brain | Other | Other | Lungs | Lungs | Kidney | Kidney | 43 |
| Heart | Heart | Heart | Bone | Kidney | Other | Lungs | 46 |
| Kidney | Brain | Kidney | Heart | Brain | Heart | Brain | 51 |
| Lungs | Stomach | Lungs | Brain | Heart | Brain | Heart | 52 |
| Stomach | Bone | Stomach | Spleen | Stomach | Stomach | Stomach | 66 |
| Spleen | Spleen | Spleen | Stomach | Spleen | Spleen | Spleen | 71 |
Pelt includes hair, dermis, epidermis
Highest total mercury concentration = hair; lowest total mercury concentration = bone
Highest mercury burden = pelt; lowest mercury burden = spleen
Table 3.
Mean (± SD) and range of total mercury concentration, total mercury tissue burden, total selenium concentration and Se:Hg molar ratio in tissues of 6 Steller sea lion (Eumetopias jubatus) pupsa
| Tissue | THg | THg burden | TSe | Se:Hg |
|---|---|---|---|---|
| hair | 16.87 ± 20.83 | ND | 3.53 ± 1.03 | 0.92 ± 0.40 |
| 5.99 - 59.17 | 2.07 - 4.75 | 0.88 - 1.29 | ||
| liver | 1.79 ± 2.06 | 1.69 ± 1.79 | 0.67 ± 0.26 | 1.62 ± 1.16 |
| 0.73 - 5.95 | 0.49 - 5.05 | 0.43 - 1.04 | 0.39 - 3.61 | |
| pelt | 0.90 ± 0.81 | 4.34 ± 4.15 | 0.38 ± 0.09 | 1.69 ± 1.12 |
| 0.31 - 2.47 | 1.20 - 11.82 | 0.22 - 0.46 | 0.39 - 3.48 | |
| kidney | 0.70 ± 0.76 | 0.18 ± 0.20 | 0.68 ± 0.29 | 3.82 ± 2.15 |
| 0.24 - 2.22 | 0.03 - 0.56 | 0.48 - 1.232 | 1.41 - 6.87 | |
| muscle | 0.69 ± 0.61 | 3.49 ± 2.78 | 0.35 ± 0.10 | 1.99 ± 1.38 |
| 0.22 - 1.85 | 1.07 - 8.03 | 0.25 - 0.54 | 0.74 - 4.39 | |
| spleen | 0.60 ± 0.82 | 0.03 ± 0.03 | 0.49 ± 0.17 | 5.93 ± 6.64 |
| 0.10 - 2.25 | 0.01 - 0.08 | 0.27 - 0.76 | 0.56 - 18.52 | |
| intestine | 0.55 ± 0.77 | 0.56 ± 0.76 | 0.39 ± 0.09 | 4.31 ± 2.98 |
| 0.11 - 2.11 | 0.09 - 2.09 | 0.22 - 0.49 | 0.45 - 8.74 | |
| heart | 0.49 ± 0.67 | 0.12 ± 0.14 | 0.47 ± 0.14 | 5.92 ± 3.63 |
| 0.10 - 1.84 | 0.04 - 0.41 | 0.41 - 0.59 | 0.29 - 10.69 | |
| stomach | 0.43 ± 0.57 | 0.06 ± 0.08 | 0.34 ± 0.08 | 4.17 ± 2.84 |
| 0.21 - 1.59 | 0.01 - 0.22 | 0.25 - 0.43 | 2.24 - 8.80 | |
| other | 0.39 ± 0.51 | 0.19 ± 0.16 | 0.36 ± 0.06 | 4.51 ± 2.70 |
| 0.12 - 1.43 | 0.05 - 0.50 | 0.27 - 0.42 | 0.71 - 8.90 | |
| lungs | 0.35 ± 0.49 | 0.17 ± 0.21 | 0.45 ± 0.18 | 7.07 ± 4.85 |
| 0.10 - 1.34 | 0.03 - 0.59 | 0.27 - 0.78 | 0.74 -12.76 | |
| brain | 0.28 ± 0.27 | 0.09 ± 0.08 | 0.23 ± 0.02 | 3.32 ± 1.71 |
| 0.10 - 0.81 | 0.03 - 0.25 | 0.20 - 0.26 | 0.61 - 5.49 | |
| reproductive tract | 0.26 ± 0.34 | 0.01 ± 0.01 | 0.34 ± 0.08 | 7.17 ± 5.00 |
| 0.06 - 0.94 | 0.00 - 0.03 | 0.21 - 0.43 | 1.00 -13.34 | |
| bone | 0.13 ± 0.19 | 0.20 ± 0.23 | 0.30 ± 0.08 | 15.87 ± 14.98 |
| 0.02 - 0.51 | 0.02 - 0.65 | 0.18 - 0.39 | 1.20 - 42.92 | |
| THg Body Burden | 11.58 ± 10.20 |
μg/g wet weight; mg
THg = total mercury; TSe = total selenium; ND = not determined
Figure 2.
Mean (± standard deviation) percentage of sum of total mercury (THg) concentration (μg/g; grey), THg body burden (mg; white), and tissue mass (g; black) are presented for 12 compartments for 6 Steller sea lion (Eumetopias jubatus) pups. Pelt was comprised of hair, dermis, and epidermis. “Other” was comprised of tongue, esophagus, and diaphragm. “Intestines” were comprised of pancreas, small and large intestine.
Figure 3.
High [THg] (A) and Low [THg] (B) vs. THg tissue burden. Total mercury (THg) tissue concentration (ww, μg/g) in relation to THg tissue burden (mg) for tissues of 6 Steller sea lion (Eumetopias jubatus) pups. Spearman correlations (rs) were assessed using R programming.
Figure 4.
THg burden (A) and [THg] (B) of tissues vs. muscle [THg]. Total mercury (THg) tissue concentration (ww, μg/g) and total mercury tissue burden (mg) of non-muscle tissue compartments of 6 Steller sea lion (Eumetopias jubatus) pups in relation to muscle. Spearman correlations (rs) were assessed using R programming.
Figure 5.
THg burden (A) and [THg] (B) of tissues vs. hair. Total mercury (THg) non-hair tissue concentration (ww, μg/g) and total mercury non-hair tissue burden (mg) in tissue compartments of 6 Steller sea lion (Eumetopias jubatus) pups in relation to total mercury concentrations in hair. Spearman correlations (rs) were assessed using R programming.
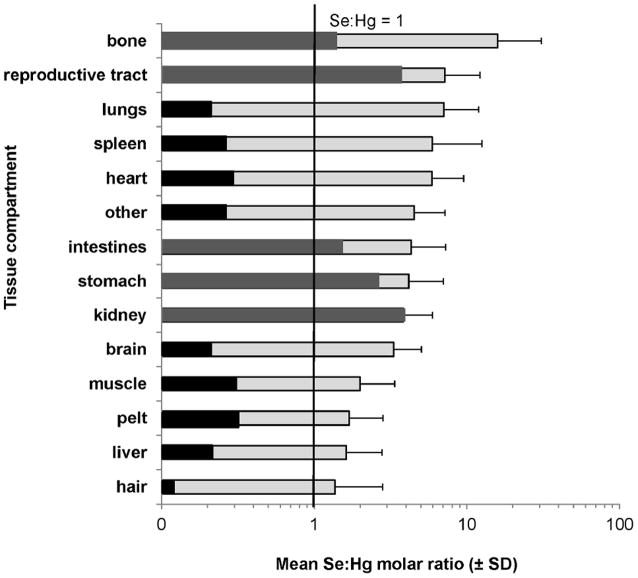
Mean Se:Hg molar ratios demonstrated a high variability among tissues. Bone had the highest mean Se:Hg molar ratio and hair had the lowest mean Se:Hg molar ratio (Figure 6). In 5 of 6 pups the Se:Hg molar ratios in all tissues ranged from 0.90 to 42.92. In the pup with the highest hair [THg] (pup no. 6; emaciated), the Se:Hg molar ratios in 9 of 14 tissues were 0.7 or less.
Figure 6.
Mean (± standard deviation) Se:Hg molar ratio in various tissues of 6 Steller sea lion (Eumetopias jubatus) pups (light grey). Se:Hg molar ratios for the case study (dark grey represents tissues above 1 for the pup with the highest [THg]; tissues in black are below 1 for the pup with the highest [THg]) and are included in the mean.
Discussion
Total Hg concentrations
Although [THg] in the tissues of these carcasses cannot be considered representative of the western DPS Steller sea lion population because we are limited to six individuals, they do span the range of hair [THg] found in live captured pups in this population and provide a unique opportunity to study how THg is distributed among different tissue compartments of sea lion pups based on both mass (mg of THg) and concentration (μg/g, ww of THg). Hair [THg] has been used as an indicator of Hg exposure in Steller sea lion pups due to ease of collection in live capture studies [2,12] and has been shown to correlate closely to circulating blood [THg] in young pups [1].
In the current study, hair [THg] in 3 of the 6 pups was equal to or exceeded the threshold for [THg] in hair set by the EPA (11μg/g, [40,41]) for humans illustrating recent concerns of Hg exposure in Steller sea lions [1,2]. Similar to the findings of Brookens et al. [35] in harbor seals (Phoca vitulina), hair had the highest [THg] and bone the lowest [THg] for all 6 pups. Hair is an excretory pathway for Hg [21,35], while bone may serve as a reservoir for some trace metals (such as lead) but previous studies have shown that Hg is not readily retained in bone to the same extent as other metals [35].
Sea lions in the current study are < 2 months of age and likely have not developed significant demethylation mechanisms. Therefore it is likely that a high proportion of the THg in the liver and kidney of the 6 pups is MeHg+ [20,42]. Liver and kidney [THg] have not been extensively studied in sea lion pups. Sydeman and Jarman [43] and Holmes et al. [9] are among the few published studies to date that evaluated [THg] in various tissues of Steller sea lion pups. In the current study 5 of 6 pups were males and had higher [THg] in brain, heart and lungs when compared to the Aleutian Island males from Holmes et al. [9]. These same 5 pups had lower [THg] in kidney and liver but similar concentrations in testes to those found in the Aleutian Island males from Holmes et al. These differences may be age dependent as the Aleutian Island males were approximately 1 year old from Holmes et al. [44] and the males in the current study were < 2 months old. Sydeman and Jarman [43] reported higher liver [THg] in Steller sea lion pups when compared to the pups in the current study. Mercury is known to bioaccumulate in liver and to some extent in kidney resulting in higher [THg] with increased age. In adult South American sea lions, liver [THg] have been reported [45] more than 100-fold greater than [THg] in pup liver from this and previous studies [9,43].
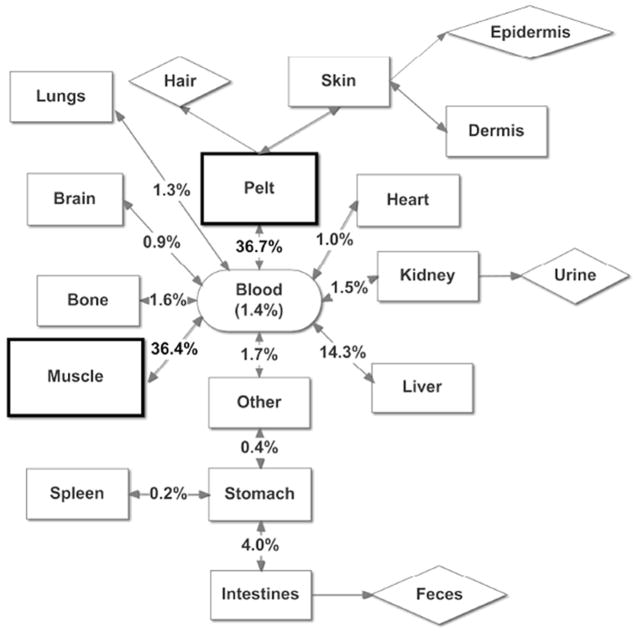
Mercury concentrations in blood, hair and muscle can provide information regarding Hg exposure but are not considered key target organs. Mercury concentrations in various tissues can be most useful when mass of the tissue (compartment) is taken into account so as to determine the actual burden. Total Hg body burdens were determined by the sum of all tissue compartment burdens. The ranking of total Hg burden of tissues in all 6 pups was similar to that found in Pacific harbor seal pups [35]. The top three tissue compartments with the highest % THg body burden were pelt, muscle and liver and the lowest % total Hg body burden was in bone. The combined % total Hg body burden in pelt and muscle for sea lion pups was slightly lower than what was found in Pacific harbor seal pups (greater than 75%) [35]. The conceptual diagram designed after [35] illustrates how THg is distributed in the body of Steller sea lion pups and where the highest THg burden occurs (Figure 7).
Figure 7.
Conceptual model for mean % of THg body burden. Mean percent of THg body burden in various tissue compartments of six Steller sea lion (Eumetopias jubatus) pups. All tissue compartments that exchange THg with blood are represented with rectangles; tissue compartments considered not to exchange THg with blood are represented with diamonds (e.g., eliminated). For comparison, mean proportion of THg in blood was estimated using mean THg concentrations of blood [1] and mean blood volume in wild Steller sea lion pups [50], and was not included in the total body burden of mercury measured in this study. Feces is qualifed as being in the colon. “Other” was comprised of tongue, esophagus, and diaphragm.
Hair [THg] was correlated with [THg] and with THg tissue burdens in target tissues such as the brain, heart, and kidney, indicating that hair [THg] is a good indicator of THg exposure and Hg tissue burden in pups of this age. The close association between muscle [THg] and both tissue [THg] and tissue THg burden of other tissue compartments also indicated that muscle was an adequate tissue for determining THg exposure and THg tissue burden; as was determined by Brookens et al. [35].
Se:Hg molar ratios
Previous studies on marine mammals indicate a strong correlation between Se and Hg mass and molar-based concentrations [25,26]. It has been hypothesized that an abundance of Se as compared to Hg on a molar basis, where Se:Hg molar ratio is well above 1, is important for potential amelioration of the adverse effects due to MeHg+ exposure and maintenance of Se-dependent processes [25,26,28,46]. One study showed that Se antagonism of Hg only occurred after specific Hg threshold concentrations had been reached [28]. In the current study, 3 of 6 pups reached the Hg threshold designated for humans (11μg/g) under acute Hg exposure scenarios. In 5 of 6 pups, Se:Hg molar ratios were equal to or higher than 0.90 in all tissues. The pup with the highest hair [THg] had ratios at 0.7 or less in liver, brain, heart, and spleen, among other tissues indicating a potentially inadequate Se supply for normal function and protection against Hg toxicosis.
Total Se concentrations
Sea lions are piscivorous diving mammals and tend to have higher Se concentrations than non-diving mammals through their marine fish diet. This is an advantage for a marine mammal because Se is a major component of Se-dependent glutathione peroxidase and other enzymes which help to alleviate the production of reactive oxygen species (ROS) caused by episodes of ischemia (restriction of blood flow) and reperfusion (return of blood flow) that occur in diving mammals [33,47]. Most Steller sea lion pups are thought to be weaned at approximately 1 year of age and while they are capable of entering the water and swimming soon after birth, they do not engage in diving for foraging purposes as young pups. Se in the liver and kidney of young of the year animals (< 1 year old) is thought to be an early store of Se from placental transfer to the fetus, this along with Se in milk are the only sources of Se available until the young animals can derive their own Se sources through foraging. Some studies in humans and dairy cattle indicate that milk is a good source of Se for young mammals and it is representative of Se obtained through maternal diet [48,49].
Selenium tissue concentrations have not been previously published for adult Steller sea lions. On average the total Se concentrations ([TSe]) in the liver of the 6 pups (mean: 1.79 +/- 2.06 μg/g) were slightly higher than what was found for Pacific harbor seal pups (mean: 0.75 +/- 0.05 μg/g) [42] and Steller sea lion pups (0.98 μg/g) [43]. In adult South American sea lions, [TSe] in liver [45] were more than 10-fold greater than [TSe] in liver of pups from this and previous studies [9,42,43]. Despite the high variability in tissue [THg] among the 6 pups, [TSe] was within a narrow range particularly in brain, heart, and skeletal muscle. As an essential element Se is under homeostatic control and is critical to the function antioxidant defense mechanisms and function of enzymes.
Conclusion
Hair had the highest [THg] in all 6 Steller sea lion pups as compared to other tissue compartments. Since these pups were only 1-2 months of age, the hair (lanugo) sampled was a good indicator of Hg exposure via maternal placental transfer and a good indicator of individual Hg tissue burdens. The percent of total Hg body burden for many organ compartments in Steller sea lion pups was similar to that found in Pacific harbor seals [35]. The Se:Hg molar ratios were between 0.9 and 43 in all tissues of 5 of the 6 pups. The pup with the highest [THg] in all tissues had Se:Hg molar ratios of 0.7 or less in 9 of 14 tissues (including brain, heart, liver and spleen) indicating that this animal may have limited Se-dependent oxidative stress and/or Hg toxicosis protection.
Highlights.
Hair [THg] ranged from 5.99 – 59.17μg/g.
The highest [THg] determined was in hair and the lowest [THg] was in bone.
The top 3 highest % of THg body burden for 6 pups was pelt, muscle and liver.
Se:Hg molar ratios in all tissues ranged from 0.14 to 42.92.
Acknowledgments
We thank the Alaska Ecosystems program at the National Marine Mammal Laboratory, NMFS, NOAA for their field support during the collection of pups, Drs. C. Lieske and C. Hansen for their assistance during necropsies and J.M. Castellini for her laboratory support and assistance. This research was approved under MMPA Permit number 14325 held by ADF&G. Funding was provided by Alaska Department of Fish and Game, Steller Sea Lion Research Program through a NOAA cooperative research agreement and funding from the State of Alaska and through the National Institute of General Medical Sciences of the National Institutes of Health under Award Number P20GM103395.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rea LD, Correa L, Castellini J, Fadely BS, O’Hara TM. Maternal Steller sea lion diets elevate fetal mercury concentrations in an area of population decline. Sci Total Environ. 2013;454-455:277–282. doi: 10.1016/j.scitotenv.2013.02.095. [DOI] [PubMed] [Google Scholar]
- 2.Castellini J, Rea LD, Lieske CL, Beckmen KB, Fadely BS, Maniscalco JM, O’Hara TM. Mercury Concentrations in Hair from Neonatal and Juvenile Steller Sea Lions (Eumetopias jubatus): Implications Based on Age and Region in this Northern Pacific Marine Sentinel Piscivore. Ecohealth. 2012;9:267–277. doi: 10.1007/s10393-012-0784-4. [DOI] [PubMed] [Google Scholar]
- 3.Basu N, Head J. Mammalian wildlife as complementary models in environmental neurotoxicology. Neurotoxicol Teratol. 2010;32:114–119. doi: 10.1016/j.ntt.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Basu N. Piscivorous mammalian wildlife as sentinels of methylmercury exposure and neurotoxicity in humans. In: Ceccatelli S, Aschner M, editors. Methylmercury Neurotox. Springer US; Boston, MA: 2012. pp. 357–370. [Google Scholar]
- 5.Atkinson S, DeMaster D, Calkins DG. Anthropogenic causes of the western Steller sea lion (Eumetopias jubatus) population decline and their threat to recovery. Mamm Rev. 2008;38:1–18. [Google Scholar]
- 6.DeMaster D. Results of Steller Sea Lion Surveys in Alaska June-July 2011. Vol. 1. Seattle, WA: 2011. p. 18. [Google Scholar]
- 7.Merrick RL, Brown R, Loughlin T. A comparison of Steller sea lion, Eumetopias jubatus , pup masses between rookeries with increasing and decreasing populations. Fish Bull. 1995;93:753–758. [Google Scholar]
- 8.Lander ME, Fritz LW, Johnson DS, Logsdon MG. Population trends of Steller sea lions (Eumetopias jubatus) with respect to remote sensing measures of chlorophyll-a in critical habitat. Mar Biol. 2012;160:195–209. [Google Scholar]
- 9.Holmes AL, Wise SS, Goertz CEC, Dunn JL, Gulland FMD, Gelatt T, Beckmen KB, Burek K, Atkinson S, Bozza M, Taylor R, Zheng T, Zhang Y, Aboueissa A, Wise JP. Metal tissue levels in Steller sea lion (Eumetopias jubatus) pups. Mar Pollut Bull. 2008;56:1416–1421. doi: 10.1016/j.marpolbul.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Loughlin T, York A. An accounting of the sources of Steller sea lion (Eumetopias jubatus) mortality. Mar Fish Rev. 2000;64:40–45. [Google Scholar]
- 11.Trites AW, Donnelly CP. The decline of Steller sea lions (Eumetopias jubatus) in Alaska: a review of the nutritional stress hypothesis. Mamm Rev. 2003;33:3–28. [Google Scholar]
- 12.Beckmen KB, Duffy LK, Zhang X, Pitcher KW. Mercury concentrations in the fur of Steller sea lions and northern fur seals from Alaska. Mar Pollut Bull. 2002;44:1130–1135. doi: 10.1016/s0025-326x(02)00167-4. [DOI] [PubMed] [Google Scholar]
- 13.Eto K. Minamata disease. Neuropathology. 2000;20:S14–S19. doi: 10.1046/j.1440-1789.2000.00295.x. [DOI] [PubMed] [Google Scholar]
- 14.Bennett PM, Jepson PD, Law RJ, Jones BR, Kuiken T, Baker JR, Rogan E, Kirkwood JK. Exposure to heavy metals and infectious disease mortality in harbour porpoises from England and Wales. Environ Pollut. 2001;112:33–40. doi: 10.1016/s0269-7491(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 16.Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2011;52:113–143. [PubMed] [Google Scholar]
- 17.Scheuhammer AM, Meyer MW, Sandheinrich MB, Murray MW. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO A J Hum Environ. 2007;36:12–19. doi: 10.1579/0044-7447(2007)36[12:eoemot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Wintle NJP, Duffield DA, Barros NB, Deceased, Jones RD, Rice JM. Total mercury in stranded marine mammals from the Oregon and southern Washington coasts. Mar Mammal Sci. 2011;27:E268–E278. [Google Scholar]
- 19.Dietz R, Riget F, Born EW. An assessment of selenium to mercury in Greenland marine animals. Sci Total Environ. 2000;245:15–24. doi: 10.1016/s0048-9697(99)00430-1. [DOI] [PubMed] [Google Scholar]
- 20.Dehn LA, Sheffield G, Thomas DL, Bratton GR, Taylor R, O’Hara TM. Trace elements in tissues of phocid seals harvested in the Alaskan and Canadian Arctic: influence of age and feeding ecology. Can J Zool. 2005;83:726–746. [Google Scholar]
- 21.Lieske CL, Moses SK, Castellini J, Klejka J, Hueffer K, O’Hara TM. Toxicokinetics of mercury in blood compartments and hair of fish-fed sled dogs. Acta Vet Scand. 2011;53:66. doi: 10.1186/1751-0147-53-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cernichiari E, Toribara TY, Lian L, Marsh DO, Berlin MW, Myers GJ, Cox C, Shamlaye CF, Choisy O, Davidson P, Clarkson TW. The biological monitoring of mercury in the Seychelles study: Methylmercury and human health. Neurotoxicology. 1995;16:613–627. [PubMed] [Google Scholar]
- 23.Dietz R, Sonne C, Basu N, Braune B. What are the toxicological effects of mercury in Arctic biota? Sci Total Environ. 2013;443:775–790. doi: 10.1016/j.scitotenv.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Poulin J, Gibb H. Mercury: Assessing the environmental burden of disease at national and local levels. World Health Organization, (WHO Environment; Geneva: 2008. [Google Scholar]
- 25.Koeman J, Peeters W, Koudstaal-Hol C, Tjioe P, De Goeij J. Mercury-Selenium Correlations in Marine Mammals. Nature. 1973;245:385–386. doi: 10.1038/245385a0. [DOI] [PubMed] [Google Scholar]
- 26.Koeman J, Van de ven W, De Goeij J, Tjioe P, Van Haaften J. Mercury and selenium in marine mammals and birds. Sci Total Environ. 1975;3:279–287. doi: 10.1016/0048-9697(75)90052-2. [DOI] [PubMed] [Google Scholar]
- 27.Wang A, Barber D, Pfeiffer C. Protective effects of selenium against mercury toxicity in cultured Atlantic spotted dolphin (Stenella plagiodon) renal cells. Arch Environ Contam Toxicol. 2001;41:403–409. doi: 10.1007/s002440010266. [DOI] [PubMed] [Google Scholar]
- 28.Khan M, Wang F. Mercury- selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem. 2009;28:1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- 29.Battin EE, Brumaghim JL. Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys. 2009;55:1–23. doi: 10.1007/s12013-009-9054-7. [DOI] [PubMed] [Google Scholar]
- 30.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 31.Gailer J. Arsenic–selenium and mercury–selenium bonds in biology. Coord Chem Rev. 2007;251:234–254. [Google Scholar]
- 32.Zenteno-Savín T, Clayton-Hernández E, Elsner R. Diving seals: are they a model for coping with oxidative stress? Comp Biochem Physiol Part C. 2002;133:527–536. doi: 10.1016/s1532-0456(02)00075-3. [DOI] [PubMed] [Google Scholar]
- 33.Zenteno-Savín T, Vazquez-Medina JP, Cantu-Medellin N, Ponganis PJ, Elsner R. Ischemia/reperfusion in diving birds and mammals: how they avoid oxidative damage. In: Abele D, Vazquez-Medina JP, Zenteno-Savin T, editors. Oxidative Stress Aquat Ecosyst. 1. Blackwell Publishing Ltd.; 2012. pp. 178–190. [Google Scholar]
- 34.Pitcher KW, Burkanov VN, Calkins DG, Le Boeuf BJ, Mamaev EG, Merrick RL, Pendleton GW. Spatial and temporal variation in the timing of births of Steller sea lions. J Mammal. 2001;82:1047–1053. [Google Scholar]
- 35.Brookens TJ, O’Hara TM, Taylor RJ, Bratton GR, Harvey JT. Total mercury body burden in Pacific harbor seal, Phoca vitulina richardii, pups from central California. Mar Pollut Bull. 2008;56:27–41. doi: 10.1016/j.marpolbul.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Knott K, Boyd D, Ylitalo GM, O’Hara TM. Concentrations of mercury and polychlorinated biphenyls in blood of Southern Beaufort Sea polar bears (Ursus maritimus) during spring: variations with lipids and stable isotopes (15N,13C) values. Can J Zool. 2011:999–1012. doi: 10.1139/Z11-071. [DOI] [Google Scholar]
- 37.Moses S, Whiting A, Bratton GR, Taylor R, O’Hara TM. Inorganic nutrients and contaminants in subsistence species of Alaska: linking wildlife and human health. Int J Circumpolar Health. 2009;68:53–74. doi: 10.3402/ijch.v68i1.18294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knott K, Schenk P, Beyerlein S, Boyd D, Ylitalo GM, O’Hara TM. Blood-based biomarkers of selenium and thyroid status indicate possible adverse biological effects of mercury and polychlorinated biphenyls in Southern Beaufort Sea polar bears. Environ Res. 2011;111:1124–1136. doi: 10.1016/j.envres.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Correa L, Castellini JM, Wells RS, O’Hara T. Distribution of mercury and selenium in blood compartments of bottlenose dolphins (Tursiops truncatus) from Sarasota Bay, Florida. Environ Toxicol Chem. 2013;32:2441–8. doi: 10.1002/etc.2327. [DOI] [PubMed] [Google Scholar]
- 40.EPA. Mercury study report to congress volume v: Health effect of mercury and mercury compounds V 1997 [Google Scholar]
- 41.Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty Ra. Methylmercury poisoning in Iraq. Science (80) 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 42.Brookens TJ, Harvey JT, O’Hara TM. Trace element concentrations in the Pacific harbor seal (Phoca vitulina richardii) in central and northern California. Sci Total Environ. 2007;372:676–92. doi: 10.1016/j.scitotenv.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Sydeman W, Jarman W. Trace metals in seabirds, Steller sea lion, and forage fish and zooplankton from central California. Mar Pollut Bull. 1998;36:828–832. [Google Scholar]
- 44.Holmes P, James KaF, Levy LS. Is low-level environmental mercury exposure of concern to human health? Sci Total Environ. 2009;408:171–82. doi: 10.1016/j.scitotenv.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 45.Nigro M, Leonzio C. Intracellular storage of mercury and selenium in different marine vertebrates. Mar Ecol Prog Ser. 1996;135:137–143. [Google Scholar]
- 46.AMAP. Mercury in the Arctic. Oslo, Norway: 2011. AMAP Assessment 2011. [Google Scholar]
- 47.Vázquez-Medina JP, Zenteno-Savín T, Elsner R. Glutathione protection against dive-associated ischemia/reperfusion in ringed seal tissues. J Exp Mar Bio Ecol. 2007;345:110–118. [Google Scholar]
- 48.Panter K, James L. Natural plant toxicants in milk: a review. J Anim Sci. 1990;68:892–904. doi: 10.2527/1990.683892x. [DOI] [PubMed] [Google Scholar]
- 49.Tinggi U. Essentiality and toxicity of selenium and its status in Australia: a review. Toxicol Lett. 2003;137:103–10. doi: 10.1016/s0378-4274(02)00384-3. [DOI] [PubMed] [Google Scholar]
- 50.Richmond JP, Burns JM, Rea LD. Ontogeny of total body oxygen stores and aerobic dive potential in Steller sea lions (Eumetopias jubatus) J Comp Physiol B. 2006;176:535–45. doi: 10.1007/s00360-006-0076-9. [DOI] [PubMed] [Google Scholar]