Abstract
We assessed the hypothesis that black-blood steady-state free precession (SSFP) would provide coronary wall images comparable to images from TSE and have better performance than TSE under conditions of fast heart rate. With IRB approval, thirty participants without a history of coronary artery disease (19 men, 11 women, 26–83 y/o) were scanned with a 1.5 T MR scanner. Cross-sectional black-blood images of the proximal portions of coronary arteries were acquired with a two-dimensional (2D), double inversion recovery (DIR) prepared TSE sequence and a 2D DIR SSFP sequence on the same planes. Image quality (ranked with a 4-point system, scored from 0 to 3), vessel wall area and thickness, signal-to-noise ratio (SNR) of the wall and contrast-to-noise ratio (CNR, wall to lumen) were compared between SSFP and TSE with SPSS software (v 13.0). Totally 28 scans were completed. For SSFP and TSE, there was no difference in image quality. SSFP had a higher SNR (23.7 ± 10.1 vs. 14.4 ± 5.2, P < 0.001) and wall–lumen CNR (8.8 ± 4.5 vs. 6.7 ± 3.2, P = 0.001). Good agreements between measured wall area (r = 0.701, P < 0.001) and thickness (r = 0.560, P < 0.001) were found. For 10 participants with heart rate more than 80 beats/min, the image quality of SSFP was higher than TSE (P = 0.016). SSFP provided image quality and measurement accuracy that was comparable to TSE. With its higher performance under fast heart rate conditions, SSFP may break through the existing thresholds for heart rate and extend clinical applicability of coronary wall MR imaging to a larger population.
Keywords: Magnetic resonance imaging, Coronary wall, Fast heart rate
Introduction
Coronary artery disease (CAD) is the leading cause of death in the United States [1]. Currently, the diagnosis of CAD is mainly based on detection of atherosclerotic plaques in coronary artery, including morphological changes such as vessel narrowing and positive remodeling. Both features are significantly related to increased clinical events. Various invasive and noninvasive methods have been developed to screen atherosclerotic lesions in the coronary system. Magnetic resonance (MR) imaging has emerged as a noninvasive approach for directly investigating functional and morphological abnormalities of the coronary arteries [2]. Black-blood two-dimensional (2D) fast (turbo) spin-echo (FSE, TSE) sequence has been used as a conventional MR imaging sequence for coronary atherosclerotic plaque imaging [2]. Generally, data are acquired in a segmented fashion during multiple cardiac cycles. Cardiac motion, which is strongly influenced by heart rate, is a major source of vessel wall signal loss and imaging artifacts. Unfortunately, the TSE sequence is very sensitive to cardiac motion. Generally, if the heart rate cannot be controlled satisfactorily, the TSE coronary wall MR imaging sequence may lead to suboptimal image quality. Such a limitation therefore impedes its practice application in routine clinical practice [3].
Steady-state free precession (SSFP) is a fast MR imaging sequence [4]. Despite its identical spatial resolution and imaging time, SSFP can acquire data in a shorter time window than that required for TSE in each cardiac cycle. Such a characteristic is extremely valuable in cardiac imaging, which is adversely affected by cardiac motion. We hypothesized that 2D black-blood SSFP imaging would provide coronary wall images of adequate quality and quantitative measurements comparable to measurements made from TSE and, furthermore, would be more suitable for imaging patients with high heart rates. The aim of our study was to prospectively evaluate a 2D double inversion recovery (DIR)—prepared SSFP MR imaging sequence for black-blood coronary wall imaging and to estimate its merit in CAD detection.
Materials and methods
Participants
With the approval of our institutional review board (IRB), thirty volunteers (19 men, 11 women, age range 26–83 years, heart rate 52–90 beats/min, body weight 55–97 kg) underwent coronary artery scanning from December 2009 to May 2010. We excluded participants with contraindications to MR scanning. Written informed consent was obtained from all participants before scans. The study was compliant with the Health Insurance Portability and Accountability Act (HI-PAA). A fast heart rate was defined as ≥80 beats/min.
MR imaging system
All MR scans were performed with a 1.5-T whole-body MR imaging system (Magnetom Espree; Siemens Healthcare, Erlangen, Germany) with a high-performance gradient system (maximum gradient amplitude, 40 mT/m; maximum slew rate, 200 mT/m/ms). A six-channel cardiac phase array coil was used for radiofrequency signal reception.
MR imaging protocol
Cardiac localization (general)
A three-plane fast localization sequence was run for anatomic orientation for the whole scan. A black-blood Half Fourier Acquisition Single Shot Turbo Spin Echo (HASTE) sequence was then run to identify the two-chamber, four-chamber, short-axis views. A four-chamber cine (with single breathhold) was run to identify quiescent phase for coronary scan.
Whole-heart coronary MR angiography (for accurate slice orientation)
An electrocardiogram (ECG)-triggered, fat-saturated, segmented three-dimensional (3D) SSFP sequence was employed for the whole-heart coronary MRA. A 40-ms T2-preparation was used to increase the blood-myocardium contrast. The 3D k-space data were collected using centric order in the phase-encoding direction and linear order in the partition-encoding direction. Seventy-two transverse slices were acquired and sinc-interpolated to 144 slices, each 0.75 mm thick. The in-plane spatial resolution was 0.55 × 0.55 mm2 (interpolated from 1.1 × 1.1 mm2). Other imaging parameters included: repetition time (TR) and echo time (TE) = 3.7 and 1.7 ms; flip angle = 90°; lines per heartbeat = 25–33; readout bandwidth = 870 Hz/pixel; parallel acquisition factor = 2 in the phase-encoding direction; field of view (FOV) = 320 mm2; matrix = 288 × 288. Imaging time was 8–10 min, assuming 35–45% respiratory gating efficiency.
Coronary artery wall imaging: TSE
The 3D multiplanar reformations were performed on the MRA images to localize the left main coronary artery (LM), the proximal left anterior descending coronary artery (LAD), and the right coronary artery (RCA). Once the coronary arteries were localized, cross-sectional black-blood images of their proximal portions were acquired using a 2D, navigator-gated, ECG-triggered, DIR prepared TSE sequence under free breathing. To improve contrast between the coronary wall and epicardial fat, a spectral-selective adiabatic inversion-recovery (SPAIR) pulse was used to suppress the fat signal. Imaging parameters included: TR = 800 ms, TE = 33 ms, echo train length = 10–13, bandwidth = 305 Hz/pixel, matrix = 448 × 448, FOV = 420 × 420 mm; slice thickness = 4.0 mm. Trigger pulse = 2. No parallel imaging was used. To overcome respiratory motion-induced image artifacts, a motion adaptive navigator (NAV) technique was used with end-expiratory imaging defined by the dome of the right hemidiaphragm. In all cases, data acquisition was performed during mid-diastole (rest period) of the cardiac cycle. Based on coronary landmarks, we acquired one cross-sectional slice each in the RCA, LM and LAD at locations perpendicular to the long axis of each vessel and 5 mm from the origin.
Coronary artery wall imaging: SSFP
A 2D, NAV gated, ECG-gated, DIR prepared SSFP sequence was run within 15 min of TSE (before or after). Cross-sectional images were acquired at the same position as the TSE images. Imaging parameters included: TR = 780 ms, TE = 2.45 ms, FOV = 420 × 420 mm2; slice thickness = 4.0 mm; readout matrix = 448 × 448, resulting in a 0.9 × 0.9 × 4.0 mm3 voxel size. Trigger pulse = 2. No parallel imaging was used. The same number of k-space lines was acquired in each cardiac cycle as in the TSE sequence, and DIR was again used to null the signal from the blood. For fat saturation suppression, SPAIR was used. Inversion time (TI): 200 ms. Bandwidth: 531 Hz/Pixel. NAV and the shimming box were set at the same position as TSE. The imaging was optimized for individual patients to avoid exceeding the coronary rest period duration and to begin after the onset of the coronary rest period, as defined on the four-chamber cine images.
Image evaluation and data processing
Images were transferred to an imaging workstation (Dell, Studio XPS 435T) with off-line use of a freely available image processing software (SEGMENT, version 1.8 for research use only; Lund University, Sweden) [5]. Images of the coronary wall were zoomed and up-sampled ten folds. Images were ranked with a 4-point system and required consensus from two authors. The scoring system can be described thus: in Grade 0, no vessel wall could be seen; in Grade 1, the vessel wall could barely be identified with reference images; in Grade 2, the vessel wall was seen clearly with some signal loss; lastly, in Grade 3, the vessel wall was distinct from the surrounding tissue and was free of signal loss. A grade of 1, 2 or 3 was considered eligible for quantitative analysis/comparison.
The outer and inner borders of eligible coronary walls (Grade 1, 2, 3) were manually drawn by one author with 4 years of experience in vessel analysis. The area of the vessel wall was defined as the difference between the area of the outer wall and the area of the lumen (outer wall area—luminal area). The mean thickness of the vessel wall was obtained from measurements at four points (the 12, 3, 6, and 9 o’clock positions). The signal to noise ratio (SNR, SIwall/SDnoise) of the vessel wall and the contrast-to-noise ratio (CNR, (SIwall-SIlumen)/SDnoise) of the coronary artery wall versus lumen were also calculated [6, 7].
Statistical methods
The SNR and CNR of the two imaging techniques (SSFP vs. TSE) at the same imaging position were compared with a paired t test. The image scores of two techniques were compared with Wilcoxon rank sum test. A Pearson’s Correlation Coefficient and Bland–Altman plot were applied to evaluate how well measurements of vessel wall area and wall thickness correlated for SSFP versus TSE images. Statistical analysis was performed with SPSS software (Version 13.0, Chicago, Ill). For all calculations and results, a P < 0.05 level was defined as statistically significant.
Results
General image quality
Two participants were excluded because they were unable to tolerate the long scan time (75 min). In total, 84 pairs of coronary images were acquired from 28 participants. For the SSFP sequence, the acquisition of 13 k-space lines cost 57 ms, while the TSE sequence required 93 ms for the same number of k-space lines. For the TSE sequence, 21 RCA, 25 LM and 24 LAD images were eligible for quantitative analysis (Grades 1, 2, 3). For the SSFP sequence, 22 RCA, 26 LM and 23 LAD images were eligible for analysis. There was no statistical difference in image quality score between the coronary artery branches (P > 0.05). Sixty pairs of images (both TSE and SSFP with score 1, 2, 3) were eligible for quantitative comparison. There was no significant difference between image quality in SSFP versus TSE (P = 0.431). A pair of representative images is shown in Fig. 1. Compared to TSE, SSFP had a higher mean coronary wall SNR (23.7 ± 10.1 vs. 14.4 ± 5.2, P < 0.001) and wall–lumen CNR (8.8 ± 4.5 vs. 6.7 ± 3.2, P < 0.001) Fig. 2a.
Fig. 1.

A 66 y/o male, heart rate 57 beats/min. The right coronary artery (RCA, grade 3) can easily be seen on both TSE (a) and SSFP (b)
Fig. 2.
a SSFP had higher SNR (23.7 ± 10.1 vs. 14.4 ± 5.2, P < 0.001) and CNR (8.8 ± 4.5 vs. 6.7 ± 3.2, P < 0.001) than TSE. b Correlations between measured vessel wall area on SSFP versus TSE. Areas of vessel wall (Area of vessel-Area of lumen) was median correlated (r = 0.701, P < 0.001). c Bland–Altman plot of wall area measurements. d Wall thicknesses measured on SSFP versus TSE were median correlated (r = 0.560, P < 0.001). e Bland–Altman plot of wall thickness measurements
There was no statistical difference in coronary wall area (20.04 ± 5.43 mm2 vs. 20.51 ± 5.55 mm2, P = 0.271) or average wall thickness (1.41 ± 0.25 mm vs. 1.42 ± 0.22 mm, P = 0.584) between SSFP and TSE. Excellent agreement between wall area measurements on SSFP and TSE was observed, with a Pearson correlation coefficient of 0.701 (P < 0.001). Thickness measurements (at four points on the wall) also agreed well for SSFP versus TSE, with a Pearson correlation coefficient of 0.560 (P < 0.001), respectively. These are shown in Fig. 2b–e.
Performance of SSFP and TSE on participants with different heart rate
Thirty pairs of images from ten participants with fast heart rates (mean 83.5 ± 3.2 beats/min) were analyzed. The mean image quality score for SSFP was higher than for TSE (P = 0.016). On the other hand, for cases with low heart rates (18 participants, defined as heart rate <80 beats/min, mean 62.9 ± 7.3 beats/min), TSE seemed to have higher image scores (without statistic significance) than SSFP did (P = 0.095). Participants with fast heart rates had a shorter mean rest period than those with low heart rates (56 ± 23 ms vs. 217 ± 58 ms, P < 0.001). Representative images from cases with high heart rates ≥80 beats/min are shown in Figs. 3 and 4.
Fig. 3.
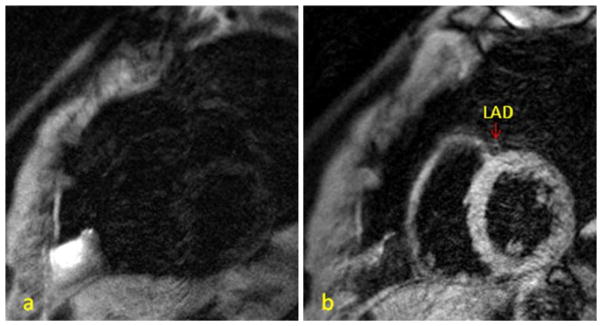
A 76 y/o male with heart rate 88 beats/min. The rest period was 40 ms/cardiac cycle. a TSE: No coronary wall can be found. Grade 0. b SSFP: The left anterior descending coronary artery (LAD) can be seen clearly. Grade 3
Fig. 4.

A 71 y/o male with heart rate 82 beats/min; rest period was 70 ms/cardiac cycle. a TSE: The left main coronary artery (LM) can be identified, but the border is not clear, and signal loss is seen. Grade 1. b SSFP: LM can be seen easily with a clear border. Grade 3
Discussion
In this study, we successfully evaluate two coronary MR techniques in a population with fast heart rate. SSFP seems to have a better performance under conditions of fast heart rate.
As early as 2000, Fayad et al. [8] reported the application of 2D black-blood MR imaging to evaluate the coronary wall in eight healthy participants and five patients with coronary stenosis, using traditional X-ray angiography as a reference. However, cardiac motion has always been considered to be a major technical impediment to clinical use of coronary wall MR imaging. Heart rate has become one of the main factors restricting individuals from such examinations. For coronary vessel wall imaging, k-space data is acquired during the rest period of a cardiac cycle. The length of the rest period, which is generally negatively related to the heart rate, varies greatly among individuals [9, 10].
Currently, DIR-prepared TSE is accepted as the conventional sequence for black-blood coronary wall imaging [8, 11, 12]. It requires short echo trains to limit signal decay (T2 decay) caused by transverse relaxation and to minimize cardiac motion during signal readout [13, 14], resulting in a relatively long imaging time. For TSE coronary wall MR imaging, the reduced number of k-space lines (lower acquisition time during signal cardiac cycle) acquired per cardiac cycle can accommodate the short rest period caused by a high heart rate, but the longer gross imaging time required is a substantial disadvantage for clinical examination since it results in more unexpected motion artifacts and irregular breathing modes, which reduce image quality.
For coronary wall scans in general, the “rest period” for coronary artery imaging is determined on a standard four-chamber cine acquired before vessel wall imaging. However, motion of the different parts of the coronary tree is not always synchronized [9]. Heart rate and the length of the “rest period” may vary during the scan. Therefore, only part of the “rest period”, which would otherwise have been used to set up parameters, is really eligible for imaging. DIR-prepared, SSFP sequences (e.g., TrueFISP, FFE, FIESTA) are more time efficient than TSE for vessel wall imaging. When SSFP is applied to coronary wall MR imaging, a shorter data acquisition window is required (a shorter time is needed to acquire the same number of k-space lines per cardiac cycle) than for a 2D DIR–prepared TSE sequence; this falls much more easily into the “rest period” within a single cardiac cycle, especially when the “rest period” is shorter, as in high heart rate conditions. Hence, time efficiency emerges as a valuable characteristic of SSFP for cardiac imaging and is the theoretical basis of our working hypothesis.
Furthermore, SSFP sequence has a gradient structure that is balanced in all directions (readout, phase-encoding and partition-encoding), which allows for a high flip angle without substantial signal decay. Compared to TSE (FSE), SSFP acquires images at a faster speed, using a higher readout bandwidth and shorter repetition time (TR). Both a balanced gradient structure and a shorter acquisition time may result in less intravoxel dephasing and signal loss caused by cardiac motion [15]. In our study, the 2D DIR-prepared SSFP sequence showed the coronary wall at an image quality similar to the TSE (FSE) sequence. It also offered similar vessel wall measurements compared to TSE. When heart rate was higher than 80 beats/min, SSFP exploited its advantages of time efficiency and insensitivity to motion, yielding even better image quality than TSE did.
Although we can say without exaggeration that “time is image quality” on coronary wall imaging, image quality is not merely determined by “time”. From the viewpoint of clinical use, various indices should be considered comprehensively [16]. For example, in addition to some traditional indices, such as motion artifact, SNR and CNR, fat suppression should not be ignored since it seriously affects visibility of the coronary wall. In our study, we chose SPAIR as the fat suppression strategy for both SSFP and TSE, based on our experience from our daily work. SPAIR only excites fat protons and uses frequency-selective inversion pulses based on the resonance frequency of fat. Compared to several other traditional fat suppression techniques, SPAIR is relatively insensitive to motion due to its use of non-slice-selective inversion recovery pulses and adjustable TI [17, 18].
On the other hand, we want to point out that SSFP seems not to be a definitely better choice than TSE in coronary wall imaging. Although our results showed a remarkable increase of SNR and CNR with the SSFP sequence, we did not find a corresponding improvement in image quality with SSFP. In fact, the image quality of TSE seemed to be superior to SSFP in participants with a slow heart rate (<80 beats/min). Although the difference was not considered to be statistically significant (P = 0.095) in our study, it still suggests that the “advantage” of SSFP for better image quality is probably a conditional merit partly because the intrinsic image contrast of TrueFISP is determined by the T2/T1-ratio of the tissue [19]. Therefore, with SSFP, less contrast will be shown between vessel wall and myocardium than with TSE. Thus, it would be unwise to arbitrarily conclude that SSFP is “good” or “bad” without considering alternate circumstances.
Our study had several limitations. First, the thickness of the coronary wall may have been overestimated due to the limited spatial resolution of coronary wall MR imaging [20]. It would have been useful to compare measurements from both the 2D DIR-prepared SSFP and TSE sequences against a “gold standard” such as intravascular ultrasound (IVUS). Unfortunately, such invasive examinations are not available for most healthy participants. Since evaluation of TSE images have proven to be repeatable and acceptable [21], it is reasonable to use it as a “reference” here. Second, the sample of participants with heart rates ≥80 beats/min included only 10 subjects. As a matter of fact, it was difficult for us to enroll participants with heart rates ≥80 beats/min before the study began because of the recruiting system we had been using. Therefore, we only compared image quality but not accuracy of measurements on participants with fast heart rate because insufficient eligible images pairs (both SSPP and TSE image were scored 1, 2 or 3) in this subgroup could not result in statistic significance. However, good agreements between SSFP and TSE based on general participants had proved that SSFP is a qualify selection for coronary wall imaging. Third, many parameters, such as the mode of k-space sampling, were not considered in the comparison. We acknowledge that many physical and physiological parameters may affect image quality in coronary wall MR imaging. Since our aim was to compare two different sequences, a simple, fixed protocol was used. Further experiments will be needed to study the relationships between subtle parameters in coronary wall MR imaging. Fourth, we did not evaluate any pathological changes of the coronary wall. Although we intended to recruit aged participants in order to increase the likelihood of positive findings, we were still unable to identify pathological changes of the vessel wall, such as atherosclerotic plaques. This was due to the limited spatial resolution of current MRI techniques. However, our results confirmed the capability of SSFP coronary wall MR imaging for acquiring indices that are important for evaluating CAD, such as coronary wall area and wall thickness.
As an eligible option of coronary wall MR imaging, 2D DIR-prepared SSFP provides image quality and measurement accuracy that is comparable to those acquired with 2D DIR TSE. With its higher performance under fast heart rate conditions, SSFP may break through the existing thresholds for heart rate and may extend the clinical applicability of coronary wall MR imaging to a larger population.
Acknowledgments
This study is supported by a Clinical Research Program award of American Heart Association (10CRP3050051) and a grant from the National Institute of Health (R01HL089695).
Footnotes
Conflict of interest Two co-authors, XB and SZ, are employees of SIEMENS Healthcare. However, this study is under control of faculty/staff of Northwestern University.
Contributor Information
Kai Lin, Department of Radiology, Northwestern University, 737 N Michigan Avenue, Suite 1600, Chicago, IL 60611, USA.
Xiaoming Bi, Cardiovascular MR R&D, Siemens Healthcare, 737 N Michigan Avenue, Suite 1600, Chicago, IL 60611, USA.
Kirsi Taimen, Department of Radiology, Northwestern University, 737 N Michigan Avenue, Suite 1600, Chicago, IL 60611, USA.
Sven Zuehlsdorff, Cardiovascular MR R&D, Siemens Healthcare, 737 N Michigan Avenue, Suite 1600, Chicago, IL 60611, USA.
Biao Lu, Department of Radiology, Northwestern University, 737 N Michigan Avenue, Suite 1600, Chicago, IL 60611, USA. Department of Radiology, Beijing Anzhen Hospital, Capital Medical University, 100029 Beijing, China.
James Carr, Department of Radiology, Northwestern University, 737 N Michigan Avenue, Suite 1600, Chicago, IL 60611, USA.
Debiao Li, Email: d-li2@northwestern.edu, Department of Radiology, Northwestern University, 737 N Michigan Avenue, Suite 1600, Chicago, IL 60611, USA.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics–2010 update: a report from the American heart association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Miao C, Chen S, Macedo R, et al. Positive remodeling of the coronary arteries detected by magnetic resonance imaging in an asymptomatic population: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2009;53(18):1708–1715. doi: 10.1016/j.jacc.2008.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkowitz SJ, Macedo R, Malayeri AA, et al. Axial black blood turbo spin echo imaging of the right ventricle. Magn Reson Med. 2009;61(2):307–314. doi: 10.1002/mrm.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koktzoglou I, Chung YC, Carroll TJ, et al. Three-dimensional black-blood MR imaging of carotid arteries with segmented steady-state free precession: initial experience. Radiology. 2007;243(1):220–228. doi: 10.1148/radiol.2431060310. [DOI] [PubMed] [Google Scholar]
- 5.Heiberg E, Sjögren J, Ugander M, et al. Design and validation of segment—a freely available software for cardiovascular image analysis. BMC Medical Imaging. 2010;10:1. doi: 10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi X, Deshpande V, Simonetti O, Laub G, Li D. Three-dimensional breathhold SSFP coronary MRA: a comparison between 1.5T and 3.0T. J Magn Reson Imaging. 2005;22(2):206–212. doi: 10.1002/jmri.20374. [DOI] [PubMed] [Google Scholar]
- 7.Stuber M, Botnar RM, Fischer SE, et al. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med. 2002;48:425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 8.Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102(5):506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Vidan E, Bergman GW, et al. Cardiac motion of coronary arteries: variability in the rest period and implications for coronary MR angiography. Radiology. 1999;213(3):751–758. doi: 10.1148/radiology.213.3.r99dc41751. [DOI] [PubMed] [Google Scholar]
- 10.Jahnke C, Paetsch I, Achenbach S, et al. Coronary MR imaging: breath-hold capability and patterns, coronary artery rest periods, and beta-blocker use. Radiology. 2006;239(1):71–78. doi: 10.1148/radiol.2383042019. [DOI] [PubMed] [Google Scholar]
- 11.Spuentrup E, Ruebben A, Mahnken A, et al. Artifact-free coronary magnetic resonance angiography and coronary vessel wall imaging in the presence of a new, metallic, coronary magnetic resonance imaging stent. Circulation. 2005;111(8):1019–1026. doi: 10.1161/01.CIR.0000156462.97532.8F. [DOI] [PubMed] [Google Scholar]
- 12.Botnar RM, Stuber M, Kissinger KV, et al. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102:2582–2587. doi: 10.1161/01.cir.102.21.2582. [DOI] [PubMed] [Google Scholar]
- 13.Constable RT, Gore JC. The loss of small objects in variable TE imaging: implications for FSE, RARE, and EPI. Magn Reson Med. 1992;28:9–24. doi: 10.1002/mrm.1910280103. [DOI] [PubMed] [Google Scholar]
- 14.Listerud J, Einstein S, Outwater E, Kressel HY. First principles of fast spin echo. Magn Reson Q. 1992;8:199–244. [PubMed] [Google Scholar]
- 15.Deshpande VS, Shea SM, Laub G, et al. 3D magnetization-prepared true-FISP: a new technique for imaging coronary arteries. Magn Reson Med. 2001;46(3):494–502. doi: 10.1002/mrm.1219. [DOI] [PubMed] [Google Scholar]
- 16.Malayeri AA, Macedo R, Li D, et al. Coronary vessel wall evaluation by magnetic resonance imaging in the multi-ethnic study of atherosclerosis: determinants of image quality. J Comput Assist Tomogr. 2009;33(1):1–7. doi: 10.1097/RCT.0b013e3181648606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauenstein TC, Sharma P, Hughes T, et al. Evaluation of optimized inversion-recovery fat-suppression techniques for T2-weighted abdominal MR imaging. J Magn Reson Imaging. 2008;27(6):1448–1454. doi: 10.1002/jmri.21350. [DOI] [PubMed] [Google Scholar]
- 18.Udayasankar UK, Martin D, Lauenstein T, et al. Role of spectral presaturation attenuated inversion-recovery fat-suppressed T2-weighted MR imaging in active inflammatory bowel disease. J Magn Reson Imaging. 2008;28(5):1133–1140. doi: 10.1002/jmri.21574. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs F, Laub G, Othomo K. TrueFISP: technical considerations and cardiovascular applications. Eur J Radiol. 2003;46:28–32. doi: 10.1016/s0720-048x(02)00330-3. [DOI] [PubMed] [Google Scholar]
- 20.Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MR IMAGING, with implications for coronary and vulnerable plaque imaging. Magn Reson Med. 2008;60:1020–1028. doi: 10.1002/mrm.21758. [DOI] [PubMed] [Google Scholar]
- 21.Hazirolan T, Gupta SN, Mohamed MA, et al. Reproducibility of black-blood coronary vessel wall MR imaging. J Cardiovasc Magn Reson. 2005;7(2):409–413. doi: 10.1081/jcmr-200053464. [DOI] [PubMed] [Google Scholar]



