Abstract
Objective
The potential of stem cells to repair compromised cartilage tissues such as in osteoarthritis (OA) depends strongly on how transplanted cells respond to factors secreted from the residing OA chondrocytes. This study determines the effect of morphogenetic signals from OA chondrocytes on chondrogenic differentiation of human mesenchymal stem cells (hMSCs).
Methods
Effect of OA chondrocyte secreted morphogens on chondrogenic differentiation of hMSCs was evaluated using a co-culture system involving both primary and passaged OA chondrocytes. The findings were compared against hMSCs cultured in OA chondrocytes conditioned medium. Gene expression, biochemical assays, and immunofluorescent staining were used to characterize the chondrogenic differentiation of hMSCs. Mass spectrometry analysis was used to identify the soluble factors. Numerical analysis was carried out to model the concentration profile of soluble factors within the hMSC-laden hydrogels.
Results
The hMSCs co-cultured with primary OA chondrocytes underwent chondrogenic differentiation even in the absence of growth factors; however the same effect could not be mimicked using OA chondrocytes conditioned medium or expanded cells. Additionally, the co-cultured environment down-regulated hypertrophic differentiation of hMSCs. Mass spectrometry analysis demonstrates cell-cell communication and chondrocyte phenotype-dependent effects on cell-secreted morphogens.
Conclusion
The experimental findings along with the numerical analysis suggest a crucial role of soluble morphogens and their local concentrations on the differentiation pattern of hMSCs in a three-dimensional environment. This concept of utilizing a small number of chondrocytes to promote chondrogenic differentiation of hMSCs while preventing their hypertrophic differentiation could be of great of importance in formulating effective stem cell-based cartilage repair.
Keywords: cell-cell interactions, chondrogenesis, cartilage tissue engineering, hypertrophy, mesenchymal stem cells
Harnessing the ability of mesenchymal stem cells (MSCs) to undergo chondrogenic differentiation has a great potential in cartilage tissue engineering. However, utilizing MSCs for cartilage tissue repair is still a daunting task due to our current inability to efficiently and reproducibly regulate their tissue specific differentiation in a spatial and temporal manner. The main concerns involve the extent of cartilage specific matrix formation, heterogeneous differentiation, and expression of hypertrophic markers1-4. In native tissue, stem cell commitment and tissue morphogenesis are regulated by the local environment, comprising of both soluble and insoluble factors5. In particular, cell-cell interactions have been implicated in a plethora of biological phenomena spanning embryogenesis, tissue morphogenesis, and repair6-10. For tissue engineering, the ability of soluble morphogens to regulate stem cell differentiation can be exploited in various ways such as exogenous supplementation of cytokines and growth factors or adaptation of co-culture systems that promote cell-cell interactions either through direct contact and/or diffusion of cell-secreted soluble factors9, 11-15. Despite wide recognition of the functional importance of cell-cell interactions in stem cell chondrogenesis, the underlying molecular mechanism remains elusive.
Previous studies have revealed the ability of terminally differentiated chondrocytes isolated from healthy tissues to direct chondrogenic differentiation of stem cells12, 13, 16-20, but the effect of chondrocytes isolated from diseased tissues on stem cell chondrogenesis is unclear. The potential of using stem cells to repair compromised tissues (e.g. OA tissue) is highly dependent upon how these cells respond to the morphogens secreted by the cells residing within the targeted tissue. Recent findings suggest that articular cartilage contains progenitor cells with multi-potentiality, a cell source that could potentially contribute to the tissue repair21, 22. However, it is widely anticipated that the OA chondrocyte secreted factors such as the inflammatory factors have a detrimental effect on the residing progenitor cells thus impeding the in vivo repair process. In this study, we evaluated the effect of OA chondrocytes-secreted morphogens on chondrogenic differentiation of bone marrow derived MSCs using a co-culture system, wherein the hMSCs-laden hydrogels and human OA chondrocytes were not in physical contact but could exchange cell-secreted factors.
The OA chondrocytes promoted chondrogenic differentiation of MSCs while down-regulating collagen type I and X expression. This effect could not be achieved using conditioned medium from primary OA chondrocytes or co-culturing with passaged OA chondrocytes, implicating the importance of intercellular communication and chondrocyte phenotype for inducing chondrogenic differentiation of MSCs. This is supported by mass spectrometry analysis, which identified unique cell secreted factors in the co-culture system. The concept of utilizing a small number of terminally differentiated cells to promote tissue specific differentiation of stem cells could find application in engineering various hierarchically complex tissues and may be explored towards efficacious stem cell-based therapeutics for cartilage tissue repair.
Materials and Methods
Culture of hMSCs
p7043L hMSC cell line was obtained from Tulane University and expanded till 4th passage in growth medium [high glucose DMEM (Gibco: Invitrogen, Carlsbad, CA) supplemented with 16.7% FBS (Premium Select; Atlanta Biologicals, Atlanta, GA), 1% LGlutamine (Gibco) and 1% penicillin-streptomycin (Gibco)], and used in the experiments.
Isolation of OA chondrocytes
The OA tissue fragments (Scripps Green Hospital, La Jolla, CA) were collected according to an Institutional Review Board approved protocol. The OA chondrocytes were extracted by digesting the cartilage chips with type II collagenase (Worthington Biochemical Corporation, Lakewood, NJ) for 14 h at 37°C and 5% CO2. The cell suspension was filtered through 70 μm nylon mesh and the isolated chondrocytes were washed with PBS containing 1% penicillin-streptomycin, followed by chondrocyte culture medium (high glucose DMEM supplemented with 10mM HEPES (Gibco), 0.1mM non-essential amino acid (G i b c o ) , 0 . 4 m M L-proline (Sigma), 50 μg/ml ascorbic acid (Sigma), 1% penicillin/streptomycin, 10% FBS, and 1mM sodium pyruvate (Gibco)). The cell viability was assessed using a trypan blue (Gibco) exclusion test. For passaged OA cells, the isolated chondrocytes were seeded at a cell density of 7500 cells/cm2 and cultured in chondrocyte medium for 7 days, after which the medium was changed twice weekly. The cells were trypsinized at 80% confluency.
Encapsulation of hMSCs
A precursor solution was prepared by mixing 10% (w/v) PEGDA23 (Mw of PEG: 3400 g/mol) in sterile PBS containing 1% penicillin/streptomycin. The photoinitiator (Irgacure D 2959, Ciba Specialty Chemicals) solution in 70% ethanol was added to the polymer solution resulting in a final concentration of 0.05% (w/v). P4 hMSCs were suspended in the above solution at a density of 20 million cells/mL. 65 μL of this mixture was pipetted into a cylindrical mold and photopolymerized under 365-nm ultraviolet (UV) light at 4 mW/cm2 for 5 minutes. The resulting cell-laden hydrogels (hMSC constructs) at equilibrium had a size of 4 mm diameter and 3.4 mm length.
Chondrogenic differentiation of hMSCs
OA chondrocytes were plated in a 24 well culture plate at a cell density of 7500 cells/cm2 and cultured in chondrocyte medium for 7 days. A transwell insert (0.4 μm pore size) containing the hMSC constructs was fitted into the well containing the monolayer of OA chondrocytes (Figure S1B). The co-culture was grown in chondrogenic medium (high glucose DMEM containing 100 nM dexamethasone (Sigma), 50 μg/ml ascorbate-2-phosphate (Sigma), 40 μg/ml proline (Sigma), 100 μg/ml sodium pyruvate, 1% penicillin streptomycin, and ITS-Premix (6.25 ng/ml insulin, 6.25 mg transferrin, 6.25 ng/ml selenious acid, 1.25 mg/ml bovine serum albumin, and 5.35 mg/ml linoleic acid; BD Biosciences, CA) with or without 10 ng/ml TGF-β1(Fitzgerald Industries International, MA). In the case of co-culture conditions, the inserts containing hMSC-laden hydrogels were transferred into wells containing freshly plated OA chondrocytes every seven days of culture. For conditioned medium (Figure S1C), the monolayer of primary chondrocytes were cultured for 7 days following which the medium was replaced with high glucose DMEM (without FBS) and the conditioned medium was collected after 48 hrs. The conditioned DMEM was then used to prepare chondrogenic medium by supplementing with chondrogenic inducing soluble factors as described above. The hMSC-laden hydrogels cultured in chondrogenic medium containing TGF-β1 in the absence of chondrocytes or chondrocyte-secreted factors (conditioned medium) were used as control (Fig. S1A). For studies involving PTHrP (human 1-34 PTHrP) (Sigma) and cyclopamine (LC Laboratories, MA), the chondrogenic medium was supplemented with 3×10-7 M and μrespectively. The culture medium was changed on alternate days.
Histology and Immunofluorescent staining
hMSC constructs were fixed overnight in 4% paraformaldehyde (pH 7.4) at room temperature and transferred to 70% ethanol at 4°C until processing. hMSC constructs were then embedded in OCT and cryosectioned into 30-50 μm sections that were stained with Safranin-O. For immunofluorescent staining, sections were blocked in blocking buffer (3% BSA and 0.1% Triton-X 100 in 1X PBS) for 1 hour and incubated with rabbit polyclonal antibodies against collagen types II or X (Fitzgerald Industries International, MA) at 1:300 dilutions. Sections were then incubated with either FITC- or Texas red conjugated goat anti-rabbit secondary antibody, Alexa Fluor 488 (all 1:250 dilutions) for 1 hour. Nuclei were counterstained with DAPI (Chemicon, MA) mounting medium and stored in the dark at −20 °C, and images were collected by using a Zeiss fluorescent microscope, Axio Observer A1. Live-dead assay (Invitrogen) was used for detecting cell viability within the PEGDA hydrogels24. The percentage of collagen type X positive cells was estimated by counting the cells with collagen type X in their periphery relative to the total cells (DAPI staining) in at least six different fields of images from three samples (n=3) and two independent experiments.
Biochemical Assays
Hydrogels were collected at different time points, lyophilized, and digested with papainase solution (1ml/ construct; Worthington Biomedical). The DNA content of the hMSC constructs was measured using the DNA Picogreen assay kit (Invitrogen) following the manufacturer's protocol. The GAG content was quantified by using 1,9-dimethyldimethylene blue spectrophotometric assays at a wavelength of 525nm25. Total collagen content was determined by measuring the hydroxyproline content of the constructs after acid hydrolysis and reaction with chloramine-T and p-dimethylaminobenzaldehyde as described26.
RT-PCR and Real-Time PCR
Total RNA was extracted with TRIzol and reverse transcribed into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen). RT-PCR and real-time PCR were performed as previously described27. The PCR primers are listed in Supplementary Table 1.
Mass spectrometry analysis
The cells (chondrocytes alone (P0 OA or P1 OA), hMSCs alone, hMSC-P0 OA chondrocyte co-culture, and hMSC-P1 OA chondrocyte co-culture) were cultured for 7 days following which the medium was replaced with high glucose DMEM (without FBS) and the conditioned medium was collected after 48 hrs. The conditioned medium was used for mass spectrometry analysis as described in the Supplementary Text.
Statistical Analysis
Data are expressed as mean +/− standard deviation (SD). Statistical significant difference between test groups was determined by one-way ANOVA at a confidence limit of 95%. In addition, pair wise comparisons were made after ANOVA using Tukey's test. PRISM software was used for graphing and performing all the statistical analysis; in the figures, “*” represents p<0.05 and “**” represents p<0.01 in terms of statistical significance
Results
Characterization of OA chondrocytes
OA chondrocytes were isolated from five female donors with age varying from 54 to 72 years as described above. Primary OA chondrocytes expressed collagen type X and ALP markers in addition to cartilage specific markers such as aggrecan and collagen type II (see Supplementary Figure S2A), which is consistent with other reported studies28,29. The primary OA chondrocytes took 5-7 days to attach to the tissue culture treated plates and they retained a round morphology even 6 days after seeding (see Supplementary Figure S2B). In contrast, passaged OA chondrocytes (P1 OA) attached within 24 hours of culturing (see Supplementary Figure S2C) and underwent de-differentiation upon ex vivo expansion as indicated by a reduction in safranin-O and alcian blue staining (see Supplementary Figure S2D-G). Similarly, PCR analysis showed a down-regulation of collagen type II and aggrecan expression in ex vivo expanded OA chondrocytes (data not shown).
Differentiation of hMSCs
Our previous studies have indicated that the morphogens from healthy, primary chondrocytes can induce chondrogenic differentiation of stem cells12,13,27,30. Here we investigated whether morphogens from OA chondrocytes, isolated from human OA tissues, also have the potential to direct chondrogenic differentiation of hMSCs. To this end, hMSCs were encapsulated within poly(ethylene glycol diacrylate) (PEGDA, Mn 3400 g/mol) hydrogels and co-cultured with primary OA chondrocytes using a transwell co-culture system with a semi-permeable membrane of pore size 0.4 μm (see Supplementary Figure S1B for schematic).
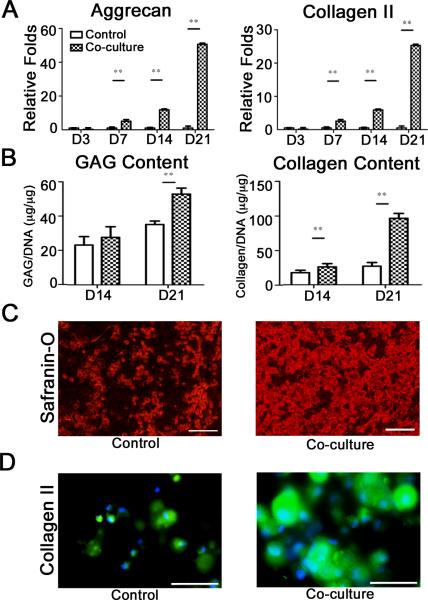
Comparison of co-cultured hMSC-laden hydrogels with those grown in the absence of OA chondrocyte-mediated soluble factors (control; see Supplementary Figure S1A) showed significant differences in their differentiation pattern, although both the conditions resulted in chondrogenic differentiation of hMSCs. A time dependent increase in cartilage specific markers of hMSCs was observed in both culture conditions (see Supplementary Figure S1D-F). The real-time PCR analysis showed higher expression levels of aggrecan and collagen type II for co-cultured hMSCs compared to their respective control cultures (Figure 1A). These findings were further supported by biochemical assays, which showed higher glycosaminoglycan (GAG) and collagen contents in co-cultured hMSC constructs compared to their control counterparts (Figure 1B). Histological analysis of co-cultured hMSCs displayed dense sulfated extracellular matrix, richly staining for Safranin-O compared to control cultures (Figure 1C and see Supplementary Figure S1G,H). In addition, the hyaline nature of the engineered cartilage in co-cultured hMSC constructs was also apparent from the intense staining for collagen type II (Figure 1D); figure 1D also shows the collagen type II staining for the control hMSC constructs.
Figure 1.
Chondrogenic differentiation of hMSCs co-cultured with P0 OA chondrocytes. Gene expressions for aggrecan and collagen type II (A) as a function of culture time. Quantification of glycosaminoglycan and collagen content (B) normalized to DNA. Safranin-O for control and co-culture (C) and collagen II for control and co-culture (D) staining after 21 days of culture, indicating cartilage tissue formation. Scale bar: 100 μm.
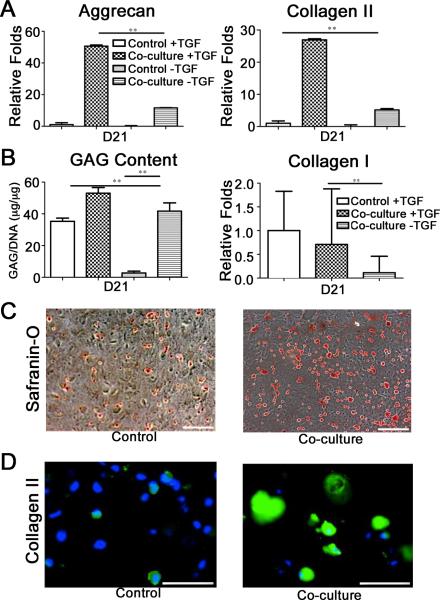
Interestingly, co-cultured hMSCs exhibited chondrogenic differentiation even in the absence of exogenous TGFβ1 as revealed by gene expression and cartilage specific extracellular matrix synthesis (Figure 2). hMSCs co-cultured with OA chondrocytes in the absence of exogenous TGFβ1 showed higher expression levels of aggrecan and collagen type II, while down-regulating collagen type I expression, compared to their corresponding control cultures (Figure 2A,B). Biochemical analysis for GAG accumulation (Figure 2B) as well as proteoglycan and collagen type II staining (Figure 2C,D) further support the chondrogenic differentiation of co-cultured hMSC constructs in the absence of exogenous TGFβ1. These findings underscore the potential role of OA chondrocytes-secreted morphogens in promoting chondrogenic differentiation of hMSCs.
Figure 2.
Chondrogenic differentiation of hMSCs co-cultured with P0 OA chondrocytes in the absence of TGF-β1. Comparative gene expressions with control cultures as a function of culture time: (A) aggrecan, (A) collagen type II, and (B) collagen type I. (B) Quantification of glycosaminoglycan normalized to DNA. Safranin-O for control and co-culture (C) and collagen type II staining for control and co-culture (D) of the hMSC constructs after 21 days of culture; scale bar is 100 μm.
Unlike co-culture, hMSC constructs grown in primary OA chondrocytes-conditioned medium (see Supplementary Figure S1C) showed down-regulation of aggrecan and collagen type II gene expressions (Figure 3A), corroborated by Safranin-O staining for proteoglycans and immunofluorescent staining for collagen type II (Figure 3B,C), while exhibiting an up-regulation of collagen type I expression (Figure 3A). We also evaluated the effect of ex vivo-expanded P1 OA chondrocytes on chondrogenic differentiation of hMSCs. Similar to conditioned medium, co-cultures using P1 OA chondrocytes promoted collagen type I expression of hMSCs, while down-regulating collagen type II and aggrecan expressions (Figure 3D).
Figure 3.
Chondrogenic differentiation of hMSCs in P0 OA chondrocyte conditioned medium and P1 OA chondrocyte co-culture system. Real-time PCR for aggrecan, collagen type II, and collagen type I (A) of hMSCs constructs cultured in P0 OA chondrocyte conditioned medium as a function of culture time. Safranin-O (B) and collagen type II (C) staining of hMSC constructs grown in P0 OA chondrocyte conditioned medium after 21 days of culture; scale bar is 100 μm. Real-time PCR for aggrecan, collagen type II, and collagen type I (D) of hMSCs co-cultured with P1 OA chondrocytes as a function of culture time.
In addition to promoting chondrogenic differentiation of hMSCs, the co-culture mediated environment involving primary OA chondrocytes had a significant effect on hypertrophic differentiation of hMSCs. Detectable levels of collagen type X were observed in the control cultures after seven days and increased with time (Figure 4A). However, the hMSCs in the co-culture environment had a minimal collagen type X expression throughout the culture time (Figure 4A). The gene expression profile was further confirmed by immunofluorescent staining, which revealed that fewer than 18% of cells were collagen type X positive (Figure 4B). Interestingly, the number of positive cells increased from the periphery towards the center of the hydrogel to the extent that the majority of the positive cells were in fact confined to the center (Figure 4B). Conversely most of the hMSCs in the control cultures were stained positive for collagen type X (>82%) (Figure 4B).
Figure 4.
Chondrogenic differentiation of hMSCs co-cultured with P0 chondrocytes in various supplements. Gene expression profile for collagen X (A) is quantified as a function of culture time. (B) Positive collagen type X staining for control and co-cultured hMSC constructs at 21 day of culture. The C and P in the co-culture staining indicate the center and the periphery of the cylindrical construct, respectively and the arrow indicates the radial direction. Scale bar: 100 μm. Relative gene expressions for aggrecan and collagen type II (C), collagen type X and collagen type I (D) after 21 days of culture.
Next, we evaluated the role of PTHrP (parathyroid harmone-related protein), a known suppressor of hypertrophic differentiation of MSCs and chondrocytes, on chondrogenic differentiation of hMSCs31,32. Real-time PCR analysis revealed that co-cultured hMSCs exogenously supplemented with TGFβ1 and PTHrP showed higher expressions of aggrecan and collagen type II compared to co-cultures containing only TGFβ1 (Figure 4C). A similar trend was observed in the case of non-co-cultured hMSCs constructs (Figure 4C). Exogenous supplementation of PTHrP down-regulated collagen type X expression in control cultures (Figure 4D). The effect of PTHrP was easily reversed by supplementing the culture medium with alkaloid cyclopamine (Figure 4C,D), which has been shown to inhibit Hedgehog signal transduction33,34. In addition to down-regulating cartilage specific markers, cyclopamine up-regulated collagen type I expression (Figure 4D). As seen from Figure 4D, the cyclopamine induced up-regulation of collagen type X was significant even in co-cultures devoid of any PTHrP supplementation. Co-cultured hMSC constructs grown in presence of exogenous TGFβ1 with or without PTHrP showed almost equal amounts of GAG and collagen (see Supplementary Figure S3A,B). But in the presence of cyclopamine, the hMSC constructs had significantly lower GAG amounts but similar collagen content (see Supplementary Figure S3A,B). The similar amounts of collagen in both the cultures could be attributed to the fact that the collagen measurements do not distinguish between collagen types I and II.
In contrast to collagen type X staining where the strongest intensity was found at the center of co-cultured hMSC constructs, the intensity of Safranin-O staining was highest at the peripheral regions of the constructs and progressively decreased towards the center (Figure 3B). This trend in Safranin-O staining was especially prominent in the case of hMSC constructs cultured in conditioned medium. We hypothesize that the observed differentiation pattern of hMSCs encapsulated within the hydrogels is caused by the local concentration of soluble morphogens. To examine this hypothesis, we have modeled the concentration profile of soluble morphogens across the cylindrical hMSC construct (of radius R and height H) by solving the mass transfer equation:
| (1) |
where CA is the concentration of the morphogen, DAB is the diffusion coefficient of the morphogen in the aqueous solution, and v is the flow velocity of the culture medium. We assume that the rate of consumption of the morphogen by the cells per unit volume of the construct RA is governed by first order reaction kinetics: RA = –kCA, where k is the consumption rate constant of the soluble factors by the cells. We also assume that no bulk flows are generated within the construct (v = 0) and that steady state has been reached, given that the culture time exceeds the nominal diffusion time.
The above equation yields the following simplified equation in cylindrical coordinates by further assuming that the construct is isotropic in the θ direction:
| (2) |
and use the following four boundary conditions:
| (3) |
where Cbulk is the concentrations of the morphogen at the periphery, i.e., r = R and z = H/2 direction. Note that symmetry conditions at the center of the constructs impose the remaining two boundary conditions. We used the finite difference method to solve the above equations and obtain the concentration profile. The analyses indicate a decrease in concentration of soluble morphogens from the periphery to the center in both the radial and axial direction of the cylindrical hMSC constructs (Figure 5A). The computed concentration profiles correlates strongly with the observed staining pattern, thus confirming our hypothesis that the local morphogen concentration dictates the differentiation of hMSCs within the hydrogel.
Figure 5.
Diffusion Modeling and Mass Spectrometry Analysis. Normalized concentration profile of a generic morphogen is shown as a contour map for cross sections at increasing heights from the center of the gel (A). Venn diagram (B) showing comparison of soluble factors between the groups: (i) P4 hMSC : P0 OA : P0 OA-hMSC and (ii) P4 hMSC : P1 OA : P1 OA-hMSC. The italized numbers of smaller font size exclusively compare soluble factors between P0 and P1 OA chondrocytes.
We next employed mass spectrometry analysis to identify protein based unique soluble factors present in different cultures and also to determine the effect of chondrocyte phenotype on cell-secreted morphogens. The candidate identification should shed light onto the molecular mechanisms underlying the chondrogenic induction of hMSCs in a co-culture system. We employed a combinatorial approach: (i) chondrocytes alone (P0 OA or P1 OA), (ii) hMSCs alone, (iii) hMSC-P0 OA chondrocyte co-culture, and (iv) hMSC-P1 OA chondrocyte co-culture. The total number of identified factors was higher in the co-cultures compared to cells cultured alone; 9 and 10 factors for P0 and P1 OA chondrocytes alone, respectively, 9 factors for P4 hMSCs alone, 26 factors for hMSCs- P1 OA chondrocytes co-culture, and 60 factors for hMSCs-P0 OA chondrocytes co-culture (see Supplementary Table 2). Some of the soluble factors in hMSCs- P1 OA chondrocytes co-culture were found to be cartilage specific extracellular matrix proteins such as hyaluronan and proteoglycan fractions. A Venn diagram analysis was used to identify the factors that are distinct from those that are shared amongst the cultures. As seen from Figure 5B, there were 53 proteins unique to the hMSC-P0 OA chondrocytes co-culture, 14 in hMSC-P1 OA chondrocyte co-culture, 4 in hMSC alone with respect to the P0 OA chondrocytes, 3 in hMSC alone with respect to the P1 OA chondrocytes, 3 in P0 OA chondrocytes alone, and 4 in P1 OA chondrocytes alone. Among the 53 unique factors identified in the hMSC-P0 OA chondrocyte co-culture system, 44 candidates were extracellular histones (different isoforms of H2A and H2B), and the remaining candidates were extracellular matrix proteins and growth factors.
Discussion
Although MSCs are entering clinical trials for orthopedic applications, the use of MSCs for cartilage repair is hindered by various factors such as the quality and quantity of MSC-derived matrix and expression of hypertrophic markers by MSCs undergoing chondrogenesis1,3,4. A number of studies have demonstrated the effect of chondrocytes-secreted morphogens from healthy chondrocytes on chondrogenic differentiation of stem cells13,18,20,27. In contrast, this study reports a comprehensive and systematic evaluation of the effect of OA chondrocytes-secreted morphogens on chondrogenic differentiation of hMSCs. We evaluated the effect of OA chondrocyte phenotypes (P0 versus P1) and intercellular communication (co-culture vs. conditioned medium) on the differentiation of hMSCs. We also identified the culture condition dependent cells-secreted morphogens using mass spectrometry. Taken together the results from the various culture conditions and a simple diffusion model within a 3D construct, we propose that the differentiation of hMSCs within a three-dimensional environment is dependent upon the soluble morphogens and their spatial concentration.
The transwell co-culture system described here allows for cell-cell communication in the form of soluble factors without direct cell-cell contact thus mimicking to a certain extent the in vivo cartilage environment in which the transplanted cells will be surrounded by native chondrocytes residing within the tissue. hMSCs co-cultured with P0 OA chondrocytes exhibited higher levels of cartilage-specific markers and produced more extracellular matrices compared to control cultures. The co-culture condition was sufficient to induce chondrogenic differentiation of hMSCs even in the absence of any exogenous growth factors, which are generally required for chondrogenic differentiation of hMSCs. This is in contrary to the hypothesis that the OA chondrocyte secreted factors inhibit chondrogenic differentiation of progenitor cells. This could be due to various reasons such as the context dependency where the OA cells are isolated from their degenerated tissue environment.
A number of studies have demonstrated that MSCs undergoing chondrogenic differentiation typically become hypertrophic chondrocytes1, 2, 4. In contrast to control cultures supplemented with exogenous TGFβ1, hypertrophic markers such as collagen type X were rarely detected in hMSCs co-cultured with P0 OA chondrocytes under similar conditions. This observation is in agreement with other studies that showed suppression of collagen type X expression in differentiating MSCs in the presence of healthy cartilage-derived factors14, 20. An exogenous supplementation of PTHrP down-regulated the collagen type X expression in control cultures. This effect could be easily reversed by cyclopamine treatment, showing agreement with previously reported studies that demonstrated the role of PTHrP in chondrocyte hypertrophy through activation of Hedgehog signaling32, 35.
The effect of co-culture could not be recapitulated by exposing hMSCs to P0 OA chondrocytes-conditioned medium. Similarly, co-culture with P1 OA chondrocytes was also insufficient to mimic the results of P0 OA co-culture. This could be attributed to various reasons such as changes in the concentration of secreted factors and/or altogether different cell-secreted morphogens. The culture condition dependent differentiation of hMSCs along with their spatial differentiation pattern suggests that the differentiation of hMSCs within the 3D hydrogel is dependent upon both cell secreted factors and their local concentration within the hydrogel. The possible effect of local concentration of soluble factors on differentiation of MSCs may also explain the reported results demonstrating osteogenic36,37 and chondrogenic differentiation12,14,38 of MSCs in response to the chondrocyte secreted morphogens. Indeed a recent study by Mo et al. reported that the differentiation of MSCs to cartilaginous vs. osseous phenotype was dependent upon the ratio of MSCs to chondrocytes39. An increased ratio (1:2) was shown to lead to chondrogenesis over osteogenesis, suggesting a possible role of concentration of chondrocytes-secreted factors on directing chondrogenic differentiation of co-cultured MSCs.
Our mass spectrometry analysis indicated a culture condition dependent difference in the cells-secreted morphogens. In addition to a large number of matrix proteins and growth factors, the P0 OA chondrocyte co-culture contained a significant number of extracellular histones. Studies have shown that besides their structural role in chromatin, histones exhibit hormone-like activities when they are present in extracellular and extranuclear environments40,41. Brown et al reported that histones H2A and H2B possess growth hormone (GH)-releasing activity in vitro; growth hormones have been shown to play an important role in cartilage tissue formation42. Extracellular and extranuclear functions of histones have also been demonstrated in various disease models such as leukemia, arthritis, etc43-45. A number of studies have implicated extracellular H2A and H2B in thyroid hormone signaling41,46. Thyroid hormones play an important role in hypertrophic differentiation of chondrocytes and matrix calcification47-49. The role of PTHrP on thyroid hormone mediated hypertrophy of chondrocytes has been demonstrated by a number of studies31,50. Given the unique but large number of histones in the P0 OA co-culture and their role in enhancing the secretion of growth hormone (GH) and thyroid hormone signaling, we surmise that the extracellular histones observed in co-culture conditions might be one of the factors contributing to the observed chondrogenic differentiation of hMSCs when co-cultured with P0 OA chondrocytes. However, a definitive explanation for the mechanism and the role of extracellular histones requires further investigation. It is also important to note that a number of other morphogenic factors — growth factors, hormones, and extracellular matrix components — could have contributed to the observed chondrogenesis of hMSCs either individually and/or synergistically with other morphogens. Furthermore, it could also be possible that the mass spectrometry analysis could not detect some of the morphogens due to their half-life or due to the sensitivity of the measurement used.
In summary, we have demonstrated that the primary OA chondrocytes enhance chondrogenic differentiation of hMSCs in co-culture experiments. Results from this study demonstrate the effects of both cell secreted factors and their local concentration on differentiation pattern of hMSCs in a three-dimensional environment. The culture condition dependent differentiation of MSCs provides new insights into how tissue specific soluble factors may be directed to engineer complex tissues from stem cells that exhibit a differentiation potential towards multiple tissues. Additionally, these findings suggest the potential of harnessing OA chondrocytes-MSCs interaction for improved cartilage tissue regeneration. The culture models and methodologies adopted in this study can be easily extended to other tissue systems.
Supplementary Material
Acknowledgements
The authors thank Drs. Ayala and Sangaj for their critical review of the paper. We acknowledge Drs. Sah and Bugbee for OA chondrocytes and valuable discussions. We also acknowledge discussions on finite difference analysis with Dr. Arya. Aung acknowledges the summer research fellowship from Calit2. The hMSCs used in this study were provided by the Tulane Center for Gene Therapy through a grant from NCRR of the NIH (#P40RR017447).
Footnotes
Author contributions:
A.A., G.G., and S.V: designed and performed the experiments
A.A., G.G., and S.V: analyzed the experimental
results G.M: performed mass spectrometry experiments and data analysis
A.A., G.G., and S.V: wrote the paper
References
- 1.Tuan RS. Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis Rheum. 2006;54:3075–3078. doi: 10.1002/art.22148. [DOI] [PubMed] [Google Scholar]
- 2.Steinert AF, Ghivizzani SC, Rethwilm A, et al. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179–189. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Pelttari K, Winter A, Steck E, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 5.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 6.Dale B, Gualtieri R, Talevi R, et al. Intercellular communication in the early human embryo. Mol Reprod Dev. 1991;29:22–28. doi: 10.1002/mrd.1080290105. [DOI] [PubMed] [Google Scholar]
- 7.Bryant SV, Gardiner DM. Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Dev Biol. 1992;152:1–25. doi: 10.1016/0012-1606(92)90152-7. [DOI] [PubMed] [Google Scholar]
- 8.Hirschi KK, D'Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. EXS. 1997;79:419–428. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- 9.Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci U S A. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CP, Lu J, Seon H, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009;9:1740–1748. doi: 10.1039/b818401a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sui Y, Clarke T, Khillan JS. Limb bud progenitor cells induce differentiation of pluripotent embryonic stem cells into chondrogenic lineage. Differentiation. 2003;71:578–585. doi: 10.1111/j.1432-0436.2003.07109001.x. [DOI] [PubMed] [Google Scholar]
- 12.Hwang NS, Varghese S, Elisseeff J. Derivation of chondrogenically-committed cells from human embryonic cells for cartilage tissue regeneration. PLoS One. 2008;3:e2498. doi: 10.1371/journal.pone.0002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang NS, Varghese S, Lee HJ, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008;105:20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grassel S, Ahmed N. Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front Biosci. 2007;12:4946–4956. doi: 10.2741/2440. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Sakai D, Yamamoto Y, et al. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 28:623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- 16.Yang HN, Park JS, Na K, et al. The use of green fluorescence gene (GFP)-modified rabbit mesenchymal stem cells (rMSCs) co-cultured with chondrocytes in hydrogel constructs to reveal the chondrogenesis of MSCs. Biomaterials. 2009;30:6374–6385. doi: 10.1016/j.biomaterials.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 17.Hoben GM, Willard VP, Athanasiou KA. Fibrochondrogenesis of hESCs: growth factor combinations and cocultures. Stem Cells Dev. 2009;18:283–292. doi: 10.1089/scd.2008.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WH, Lai MT, Wu AT, et al. In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 2009;60:450–459. doi: 10.1002/art.24265. [DOI] [PubMed] [Google Scholar]
- 19.Varghese S, Theprungsirikul P, Ferran A, et al. Chondrogenic differentiation of human embryonic germ cell derived cells in hydrogels. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2643–2646. doi: 10.1109/IEMBS.2006.259710. [DOI] [PubMed] [Google Scholar]
- 20.Fischer J, Dickhut A, Richter W, et al. Articular chondrocytes secrete PTHrP and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 21.Grogan SP, Miyaki S, Asahara H, et al. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11:R85. doi: 10.1186/ar2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koelling S, Kruegel J, Irmer M, et al. Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell. 2009;4:324–335. doi: 10.1016/j.stem.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Aung A, Liao LQ, et al. A novel single precursor-based biodegradable hydrogel with enhanced mechanical properties. Soft Matter. 2009;5:3831–3834. [Google Scholar]
- 24.Varghese S, Hwang NS, Canver AC, et al. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 26.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 27.Varghese S, Hwang NS, Ferran A, et al. Engineering musculoskeletal tissues with human embryonic germ cell derivatives. Stem Cells. 28:765–774. doi: 10.1002/stem.325. [DOI] [PubMed] [Google Scholar]
- 28.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
- 30.Hwang NS, Varghese S, Puleo C, et al. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212:281–284. doi: 10.1002/jcp.21052. [DOI] [PubMed] [Google Scholar]
- 31.Jiang J, Leong NL, Mung JC, et al. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis Cartilage. 2008;16:70–82. doi: 10.1016/j.joca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373:104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 33.Incardona JP, Gaffield W, Kapur RP, et al. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 34.Cooper MK, Porter JA, Young KE, et al. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 35.Harrington EK, Roddy GW, West R, et al. Parathyroid hormone/parathyroid hormone-related peptide modulates growth of avian sternal cartilage via chondrocytic proliferation. Anat Rec (Hoboken) 2007;290:155–167. doi: 10.1002/ar.20416. [DOI] [PubMed] [Google Scholar]
- 36.Thompson AD, Betz MW, Yoon DM, et al. Osteogenic differentiation of bone marrow stromal cells induced by coculture with chondrocytes encapsulated in three-dimensional matrices. Tissue Eng Part A. 2009;15:1181–1190. doi: 10.1089/ten.tea.2007.0275. [DOI] [PubMed] [Google Scholar]
- 37.Gerstenfeld LC, Cruceta J, Shea CM, et al. Chondrocytes provide morphogenic signals that selectively induce osteogenic differentiation of mesenchymal stem cells. J Bone Miner Res. 2002;17:221–230. doi: 10.1359/jbmr.2002.17.2.221. [DOI] [PubMed] [Google Scholar]
- 38.Bigdeli N, Karlsson C, Strehl R, et al. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells. 2009;27:1812–1821. doi: 10.1002/stem.114. [DOI] [PubMed] [Google Scholar]
- 39.Mo XT, Guo SC, Xie HQ, et al. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45:42–51. doi: 10.1016/j.bone.2008.07.240. [DOI] [PubMed] [Google Scholar]
- 40.Brown OA, Sosa YE, Goya RG. Histones as extracellular messengers: effects on growth hormone secretion. Cell Biol Int. 1997;21:787–792. doi: 10.1006/cbir.1998.0203. [DOI] [PubMed] [Google Scholar]
- 41.Reichhart R, Zeppezauer M, Jornvall H. Preparations of homeostatic thymus hormone consist predominantly of histones 2A and 2B and suggest additional histone functions. Proc Natl Acad Sci U S A. 1985;82:4871–4875. doi: 10.1073/pnas.82.15.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaksson OG, Ohlsson C, Nilsson A, et al. Regulation of cartilage growth by growth hormone and insulin-like growth factor I. Pediatr Nephrol. 1991;5:451–453. doi: 10.1007/BF01453680. [DOI] [PubMed] [Google Scholar]
- 43.Class R, Lindman S, Fassbender C, et al. Histone H1 suppresses tumor growth of leukemia cells in vitro, ex vivo and in an animal model suggesting extracellular functions of histones. Am J Clin Oncol. 1996;19:522–531. doi: 10.1097/00000421-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Jung N, Kim DS, Kwon HY, et al. Suppression of collagen-induced arthritis with histone H1. Scand J Rheumatol. 2000;29:222–225. doi: 10.1080/030097400750041352. [DOI] [PubMed] [Google Scholar]
- 45.Vani G, Devipriya S, Shyamaladevi CS. Histone H1 modulates immune status in experimental breast cancer. Chemotherapy. 2003;49:252–256. doi: 10.1159/000072450. [DOI] [PubMed] [Google Scholar]
- 46.Eberhardt NL, Ring JC, Johnson LK, et al. Regulation of activity of chromatin receptors for thyroid hormone: possible involvement of histone-like proteins. Proc Natl Acad Sci U S A. 1979;76:5005–5009. doi: 10.1073/pnas.76.10.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robson H, Siebler T, Stevens DA, et al. Thyroid hormone acts directly on growth plate chondrocytes to promote hypertrophic differentiation and inhibit clonal expansion and cell proliferation. Endocrinology. 2000;141:3887–3897. doi: 10.1210/endo.141.10.7733. [DOI] [PubMed] [Google Scholar]
- 48.Alini M, Kofsky Y, Wu W, et al. In serum-free culture thyroid hormones can induce full expression of chondrocyte hypertrophy leading to matrix calcification. J Bone Miner Res. 1996;11:105–113. doi: 10.1002/jbmr.5650110115. [DOI] [PubMed] [Google Scholar]
- 49.Wakita R, Izumi T, Itoman M. Thyroid hormone-induced chondrocyte terminal differentiation in rat femur organ culture. Cell Tissue Res. 1998;293:357–364. doi: 10.1007/s004410051127. [DOI] [PubMed] [Google Scholar]
- 50.Stevens DA, Hasserjian RP, Robson H, et al. Thyroid hormones regulate hypertrophic chondrocyte differentiation and expression of parathyroid hormone-related peptide and its receptor during endochondral bone formation. J Bone Miner Res. 2000;15:2431–2442. doi: 10.1359/jbmr.2000.15.12.2431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.