Abstract
Purpose of review
This review will provide an overview of the translational regulation of globin mRNAs and integrated stress response during erythropoiesis by heme-regulated eIF2α kinase (HRI). HRI is an intracellular heme sensor that coordinates heme and globin synthesis in erythropoiesis by inhibiting protein synthesis of globins and heme biosynthetic enzymes during heme deficiency.
Recent findings
It has been demonstrated recently that HRI also activates the eIF2αP-ATF4 integrated stress response in primary erythroid precursors to combat oxidative stress. During chronic iron/heme deficiency in vivo, this HRI-eIF2αP-ATF4 signaling is necessary both to reduce oxidative stress and to promote erythroid differentiation. Augmenting eIF2αP signaling by the small molecule salubrinal, which inhibits dephosphorylation of eIF2αP, reduces excess α-globin synthesis and induces translation of ATF4 mRNA in mouse β-thalassemic erythroid precursors. Intriguingly, salubrinal treatment of differentiating human CD34+ cells in culture increases fetal hemoglobin production with a concomitant decrease of adult hemoglobin by a post-transcriptional mechanism.
Summary
HRI-eIF2αP-ATF4 stress signaling is important not only to inhibit excess globin synthesis during erythropoiesis, but is also critical for adaptation to oxidative stress and for enhancing effective erythropoiesis. Modulation of this signaling pathway with small chemicals may provide a novel therapy for hemoglobinopathy.
Keywords: HRI-eIF2αP signaling, erythroid differentiation, oxidative stress, thalassemia, fetal hemoglobin
Introduction
Regulation of transcription in erythropoiesis has been under extensive investigation with groundbreaking findings. In contrast, translational regulation during erythroid differentiation has received less attention. The greater importance of mRNA translation in regulating gene expression has been recognized recently owing to the advancement of mass spectrometry-based proteomics methodologies [1]. By quantification of mammalian gene expression globally, it has been demonstrated that protein abundance in the cell is controlled mainly at the translation level [2]. Furthermore, a global gene expression study of erythroid precursors at different stages of differentiation demonstrates that the majority of mRNA expressed during terminal stages of erythropoiesis is already activated at the earlier proerythroblast stage [3**], highlighting the potential role of translational regulation of many erythroid mRNAs during terminal differentiation.
Heme plays very important roles in hemoglobin synthesis and erythroid differentiation [4–6*]. In addition to serving as a prosthetic group for hemoglobin, heme also acts a signaling molecule by binding to the two heme-binding domains of HRI to modulate translation in erythroid precursors (Fig. 1A) [7,8]. HRI is a heme-regulated kinase that phosphorylates the α-subunit of eIF2 in heme deficiency, impairing another round of translational initiation and thereby inhibiting translation (Fig. 1B) [8–10**]. During erythroid differentiation, HRI is necessary to coordinate translation of globin mRNAs with the availability of heme for the production of large amounts of hemoglobin in red blood cells (RBCs). In HRI deficiency, excess globins synthesized during iron/heme deficiency precipitate and cause proteotoxicity [11,12]. The molecular mechanisms by which heme regulates erythroid differentiation are still not well understood.
Fig. 1. Family of eIF2α kinase and the integrated stress response of eIF2αP signaling.
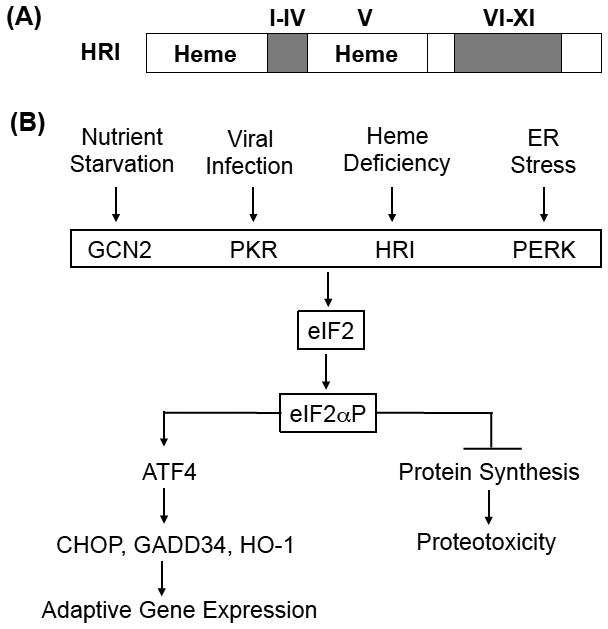
(A) Protein structure of HRI. There are two heme binding domains in HRI, the N-terminus and the kinase insertion region. The Roman numerals above the gray regions denote the conserved kinase domains of eIF2α kinases. (B) Integrated stress response. In mammalian cells, there are four eIF2α kinases that are activated in response to four major stress, nutrient starvation, viral infection, heme deficiency and ER stress. Activated eIF2α kinases phosphorylate eIF2α and inhibit protein synthesis to prevent proteotoxocity of unfolded protein occurs in ER stress and globin inclusions in heme deficiency. In addition, eIF2αP selectively enhances the translation of ATF4 mRNA. ATF4 then initiates an adaptive gene expression to mitigate stress.
Regulation of reactive oxygen species (ROS) levels and oxidative stress is extremely important in erythropoiesis. Starting at the basophilic erythroblast stage, erythroid precursors synthesize large amounts of hemoglobin. Consequently, iron uptake for heme biosynthesis also increases, potentially generating ROS through the iron-catalyzed Fenton reaction [13]. Oxidative stress occurring in β–thalassemia is one source of major complications in this disease and in other red cell disorders such as sickle cell anemia (SCA). Besides heme deficiency, HRI is also activated by oxidative stress and denatured cytoplasmic proteins [14], both of which occur in thalassemia [15]. Indeed, HRI is required to reduce the phenotypic severity of the Hbbth1/th1 mouse model of β-thalassemia intermedia (lacking both copies of β-globin major) [12]. The molecular basis of erythroid cell adaptation to oxidative stress is not fully understood.
Persistent fetal hemoglobin (HbF) expression is known to lessen the severity of β-thalassemia and SCA in patients [16–18**]. Pharmacological reactivation of endogenous γ-globin genes for enhancement of HbF production has become a translational holy grail in correcting both of these disorders [19]. Nevertheless, the molecular mechanisms of actions of HbF-inducing compounds are not well understood and may go beyond DNA demethylation and histone deacetylation. Lowrey and his colleagues have proposed that cell stress signaling including HRI-eIF2αP pathway may be a unified common mechanism for activating HbF expression by theses compounds [20].
In this review, recent advancement of HRI in promoting erythroid differentiation, mitigating oxidative stress and enhancing HbF production via translational regulation will be presented.
Integrated stress response of eIF2αP signaling in primary erythroblasts
Phosphorylation of eIF2α by eIF2α kinases elicits an integrated stress response (ISR) under various stress conditions, and is conserved from yeast to humans (Fig. 1B) [21]. In mammalian cells, four eIF2α kinases, HRI, PKR, GCN2 and PERK, are expressed in distinct tissues to combat different stresses. PKR responds to viral infection [22] while GCN2 senses nutrient starvations [23]. PERK is activated by endoplasmic reticulum (ER) stress [24], and HRI is inhibited by heme [7,8]. All four eIF2α kinases respond to oxidative and environmental stresses.
In addition to inhibiting protein synthesis of highly translated mRNAs, eIF2α phosphorylation also selectively increases translation of certain poorly translated mRNAs for adaptation to stress (Fig. 1B). This coordinated translational regulation is coined as ISR [10,25,26]. As shown in Fig. 2, translational up-regulation requires upstream open reading frames (uORFs) in the 5’UTR of these unique mRNAs, most notably ATF4 (activating transcription factor 4) [27–29**]. Under non-stressed conditions, these uORFs restrict the translation at the downstream initiating AUG codon encoding ATF4 protein. Upon stress, phosphorylation of eIF2α reduces pool of functional eIF2 and slows down the initiation to permit translation start site at the coding sequence of ATF4 mRNA.
Fig. 2. Up-regulation of the translation of ATF4 mRNA by eIF2αP upon stress.
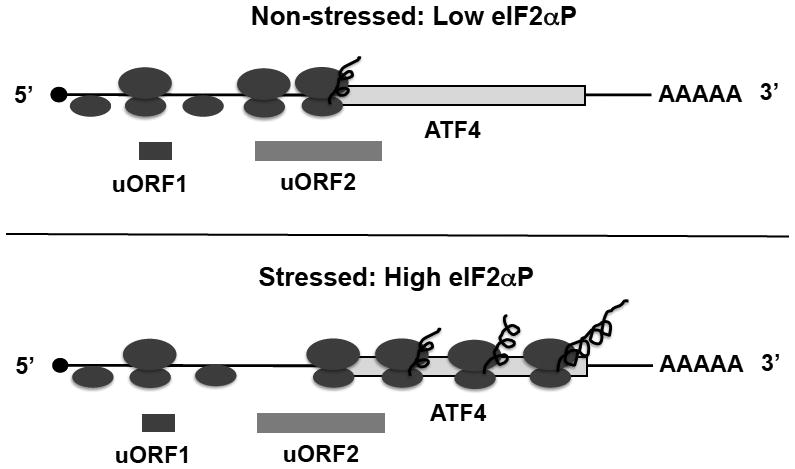
In the 5’UTR of ATF4 mRNA, there are two uORFs that are preferentially translated under non-stressed conditions and prevent the downstream translational initiation at the coding sequence of ATF4 mRNA. As initiating 40S ribosomal subunits scan from the cap structure, translation starts at the uORF1. After termination of translation, the 40S subunit remains associated with mRNA and reinitiates efficiently at uORF2 under non-stressed conditions. Upon stress, elevated eIF2αP impairs the reinitiation of 40S at uORF2 due to limiting functional eIF2. Thus, 40S continues to scan downstream and initiates at the AUG codon of coding sequence of ATF4 mRNA permitting the synthesis of ATF4 protein.
A major target gene activated by ATF4 is the transcription factor, C/EBP homologous protein-10 (CHOP). CHOP is up-regulated transcriptionally in a wide variety of cells upon many stresses [30,31]. Induction of CHOP leads to expression of GADD34 (Fig. 1B), which recruits eIF2αP for dephosphorylation by PPase1 [32–35]. This action of GADD34 in regenerating active eIF2 is necessary for the recovery of protein synthesis of stress-induced gene expression that occurs late in the stress response [36]. Upon ER stress, ISR has been shown to up-regulate expression of genes directly involved in redox homeostasis to mitigate oxidative stress [25]. Increased ROS levels were observed in cells with impaired ISR signaling resulting from mutations in eIF2α phosphorylation [37], or from deletion of PERK [25].
In the erythroid lineage, HRI expression increases during differentiation with higher expression in the hemoglobinized erythroblasts (Fig. 3) [38]. Starting at the basophilic erythroblast stage, HRI is the predominant eIF2α kinase and is expressed at two orders of magnitude higher than the other three eIF2α kinases ([3**] and J. Velazquez and J-J Chen unpublished results). Recently, it has been demonstrated that HRI activates the eIF2αP-ATF4 signaling pathway upon oxidative stress in primary erythroblasts (Fig. 3) [29**]. Hri−/− erythroblasts suffer from increased ROS and apoptosis upon acute oxidative stress induced by exposure to sodium arsenite. During chronic iron deficiency in vivo, HRI is also necessary to reduce oxidative stress. ROS levels in RBCs and erythroid precursors were dramatically elevated during iron deficiency in Hri−/− mice, but not in Hri+/+ mice. Furthermore, the induction of heme oxygenase1 (HO-1) and other antioxidant genes upon acute oxidative stress in erythroblasts is dependent on HRI and ATF4. RBCs from Hri−/− and Atf4−/− mice were more sensitive to H2O2-induced oxidative stress and exhibited increased ROS levels when compared to RBCs from Hri+/+ and Atf4+/− mice. These findings indicate that HRI-ATF4 signaling may also be required during erythroid development. Impairment of this pathway may generate RBCs that are more sensitive to oxidative insult [29**]. Thus, HRI-eIF2αP-ATF4 signaling provides the third signaling axis to combat oxidative stress in addition to the two known pathways mediated by Foxo3 and Nrf2 [39–41].
Fig. 3. The HRI-eIF2αP-ATF4 signaling during erythropoiesis.
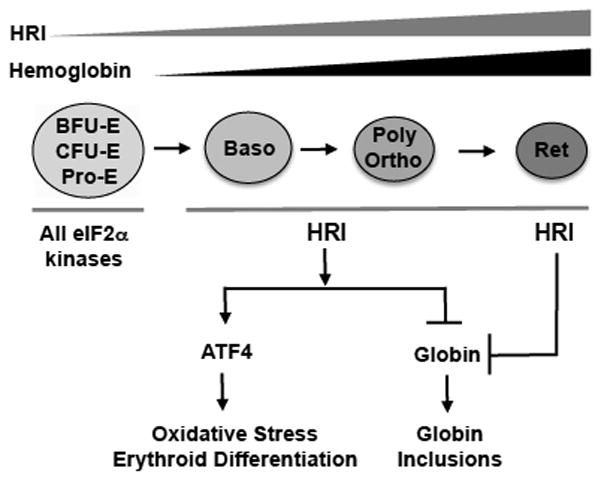
The expression of HRI increases during erythroid differentiation from BFU-E to reticulocytes. HRI is the major eIF2α kinase in the erythroid lineage, and is indispensable to coordinate heme and globin synthesis. Additionally, HRI activate ATF4 signaling pathway to mitigate oxidative stress in nucleated erythroblasts. This HRI-ATF4 pathway is activated during erythropoiesis and is necessary to promote erythroid differentiation. At the earlier stages of erythropoiesis before basophilic erythroblasts, other eIF2α kinases and HRI are both required for the proliferation of the erythroid precursors.
HRI-eIF2αP-ATF4 signaling necessary for erythroid differentiation
Beyond regulation of globin translation, HRI is also necessary to reduce ineffective erythropoiesis during iron/heme deficiency and in β-thalassemia [11,12]. Recently, it has been shown that the ineffective erythropoiesis occurring in Hri−/− mice during iron deficiency is due primarily to the profound inhibition of erythroid differentiation at the basophilic erythroblast stage [29**], which is also observed in several other mouse models of stress erythropoiesis including β-thalassemia [42] and in mice deficient of Rb [43,44] or Stat5a/5b [45].
While Hri−/− fetal liver (FL) displayed a mild defect in erythroid differentiation in vivo under normal iron sufficient conditions [38], Hri−/− FL erythroid progenitors showed a significant inhibition of erythroid differentiation at the basophilic erythroblast stage when cultured and differentiated ex vivo, recapitulating the inhibition of erythroid differentiation in vivo during iron deficiency [29**, 38]. The HRI-eIF2αP-ATF4 pathway is also activated during ex vivo differentiation of erythroid precursors and during erythroid differentiation of mouse erythroleukemic (MEL) cells [29**]. Furthermore, knockdown of ATF4 in MEL cells resulted in inhibition of erythroid differentiation. Thus, the HRI-ATF4 signaling pathway may be necessary for inducing transcription of genes required for erythropoiesis starting at the basophilic erythroblast stage. As summarized in Fig. 3, HRI not only inhibits globin translation in nucleated erythroblasts, but also increases ATF4 translation to mitigate oxidative stress and to promote erythroid differentiation. At the enucleated reticulocyte stage, the role of HRI is to regulate globin translation to prevent excessive globin synthesis, which is cytotoxic and increases oxidative stress. Both of these functions of HRI are necessary for optimal erythroid maturation to prevent anemia.
Dephosphorylation of eIF2αP in erythropoiesis
The steady state of eIF2αP in vivo is regulated by the equilibrium of eIF2α kinases and eIF2αP phosphatase (PPase1). The constitutively expressed CReP [46] and stress-induced GADD34 [32–35] are the two regulatory proteins that recruit eIF2αP to PPase1 for dephosphorylation (Fig. 4). Studies of targeted disruptions of GADD34 and CReP genes also revealed the role of eIF2αP in erythropoiesis and development [47,48]. Under normal conditions, GADD34−/− mice develop mild microcytic anemia with slight splenomegaly similarly to Hri−/− mice [11]. However, GADD34−/− mice do not recover completely from iron deficiency anemia upon repletion of iron due to the sustained high eIF2αP level and inhibition of globin synthesis [47].
Fig. 4. Dephosphorylation of eIF2αP in the regulation of the HRI-eIF2αP-ATF4 signaling.
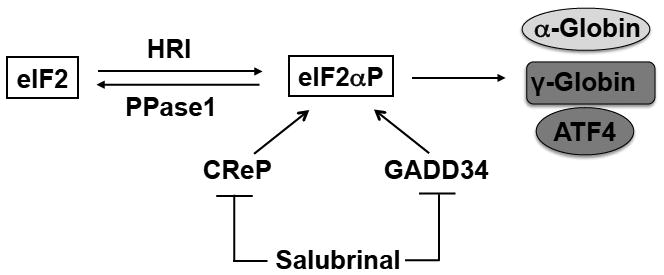
The homeostasis of cellular eIF2αP level is controlled not only by the activation of HRI, but also by dephosphorylation of eIF2αP by PPase1 in order to regenerate active eIF2. Salubrinal, a small chemical molecule, is a selective inhibitor of dephosporylation of eIF2αP by interfering with the recruitment of eIF2αP to PPase1. Thus, treatment of cells with salubrinal results in increased eIF2αP level and the strength of its ISR resulting in reduction of α-globin protein synthesis and induction of ATF4 in mouse β-thalassemic erythroid precursors. In human erythroid cells, γ-globin synthesis is increased upon salubrinal treatment. These triple actions of salubrinal in erythroid precursors make it an extremely attractive candidate for treatment of β-thalassemia and other hemoglobinopathy.
CReP−/− mice had much severer phenotypes; significant growth retardation, impaired erythropoiesis and postnatal death on the first day of birth. Furthermore, the anemia of CReP−/− embryos cannot be rescued by deletion of HRI or PERK alone [48], suggesting that more than one eIF2α kinase is necessary for proliferation of erythroid progenitors in the FL. In addition to HRI, other eIF2α kinases may also contribute to erythropoiesis by acting prior to the basophilic erythroblast stage to regulate cell proliferation (Fig. 3). Importantly, both inadequate eIF2αP signaling as in HRI and ATF4 deficiencies [11,49], and excessive eIF2αP signaling as in GADD34 and CReP deficiencies are associated with anemia. These results underscore the importance of the delicate balance of eIF2αP during erythropoiesis by transient and dynamic activation of eIF2α kinases and dephosphorylation of eIF2αP.
Augmentation of HRI-eIF2αP-ATF4 signaling by salubrinal
As described above, the HRI-eIF2αP-ATF4 stress response pathway is necessary to mitigate oxidative stress and to promote erythroid differentiation [29**]. Both of these essential processes are compromised in β-thalassemia. Indeed, HRI is activated and induces ISR in mouse β-thalassemic erythroid precursors [12,29*]. Salubrinal is a selective inhibitor of eIF2αP dephosphorylation by interfering with the recruitment of eIF2αP to PPase1 through GADD34 and CReP (Fig. 4) [50]. Recently, salubrinal has been tested for its capability to enhance the HRI signaling pathway in β-thalassemic erythroid precursors [29**]. Salubrinal is effective in increasing eIF2αP and reducing globin inclusions in β-thalassemic reticulocytes. Furthermore, salubrinal also enhances the eIF2αP signaling pathway in β-thalassemic erythroid precursors by increasing ATF4 and CHOP proteins (Fig. 4). These observations provide the foundation for exploiting the HRI-eIF2αP signaling pathway for treatment of thalassemia.
Most recently, Hahn and Lowrey have shown that salubrinal increases HbF production with a concomitant decrease of HbA in differentiating human CD34+ cells by a post-transcriptional mechanism [51**,52*]. Salubrinal increases eIF2αP and shifts the polysome profile to monosomes, indicative of reduced translation. The role of eIF2αP in induction of HbF is further supported by additional two eIF2αP modulating pharmacological agents, BTdCPU for activating HRI [53] and guanabenz for inhibiting eIF2αP dephosphorylation [54], as well as by knocking down GADD34 and CReP expression. Importantly, this eIF2αP-mediated pathway works synergistically with two clinical therapeutics, azacytidine and hydroxyurea (HU), to induce higher levels of HbF that is achieved by single agent alone.
Although there is no known human disease associated with an HRI mutation to date, there is a positive association of two SNPs in the Ppp1r15a gene (encoding GADD34) with the induction of HbF by HU in SCA patients reported recently in the 2012 ASH Annual Meeting [55*]. These two recent studies underscore the additional novel role of HRI-eIF2αP signaling in the induction of HbF that may be exploited for treatment of hemoglobinopathy.
Conclusion
Translational up-regulation of ATF4 mRNA by HRI-eIF2αP signaling is important for mitigating oxidative stress and for promoting erythroid differentiation. However, ATF4 mRNA may not be the only target of HRI-eIF2αP signaling during erythropoiesis. Recently, an extremely powerful method for analyzing in vivo translation genome-wide, ribosome profiling, has been developed [56]. Ribosome profiling studies of mouse embryonic stem cells have uncovered translation initiations at many uORFs, both at AUG codon and non-AUG codons. Additionally, translation initiations at these mRNAs are altered upon differentiation [57]. Defining these translationally regulated and long-sought target mRNAs of eIF2αP signaling by ribosome profiling in erythroid precursors will reveal additional protein components in regulating erythropoiesis. Reduction of globin inclusions and induction of ATF4 and HbF by the HRI-eIF2αP signaling provide strong bases for targeting this pathway for novel pharmaceutical therapy of hemoglobinopathy.
Key points.
The HRI-eIF2αP-ATF4 signaling is required for mitigating oxidative stress and for effective erythroid differentiation.
Salubrinal augments HRI-eIF2αP-ATF4 signaling in β-thalassemic erythroid precursors
Salubrinal induces HbF production.
The HRI-eIF2αP-ATF4 signaling pathway is a potential pharmaceutical target for treatment of hemoglobinopathy.
Acknowledgments
The work was supported in part by grants from National Institute of Health, DK78442 and DK87984.
Funding: The research at author’s laboratory is supported by NIH, DK78442 and DK87984.
Footnotes
Conflict of Interests:
There is no conflict of interests.
References
- 1.Vogel C. Translation’s coming of age. Mol Syst Biol. 2011;7:498. doi: 10.1038/msb.2011.33. Epub 2011/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature. 2011;473(7347):337–42. doi: 10.1038/nature10098. Epub 2011/05/20. [DOI] [PubMed] [Google Scholar]
- 3**.Kingsley PD, Greenfest-Allen E, Frame JM, Bushnell TP, Malik J, McGrath KE, et al. Ontogeny of erythroid gene expression. Blood. 2013;121(6):e5–e13. doi: 10.1182/blood-2012-04-422394. Epub 2012/12/18 This is the first paper examining the global gene expression of distinct cell population during both primitive and definirtive erythropoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89(1):1–25. [PubMed] [Google Scholar]
- 5.Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J Exp Med. 2005;205(4):297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- 6*.Chung J, Chen C, Paw BH. Heme metabolism and erythropoiesis. Current opinion in hematology. 2012;19(3):156–62. doi: 10.1097/MOH.0b013e328351c48b. Epub 2012/03/13. This is an excellent review on heme and iron transport during erythropoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J-J. Heme-regulated eIF-2α kinase. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Springs Harbor: Cold Spring Harbor Laboratory Press; 2000. pp. 529–46. [Google Scholar]
- 8.Chen JJ. Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias. Blood. 2007;109(7):2693–9. doi: 10.1182/blood-2006-08-041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–45. doi: 10.1016/j.cell.2009.01.042. Epub 2009/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Pavitt GD, Ron D. New insights into translational regulation in the endoplasmic reticulum unfolded protein response. In: Hershey, editor. Cold Spring Harb Perspect Biol. 2012. 2012. Apr 27, This is an important review on the up-regulation of the translation of ATF4 mRNA by uORFs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han AP, Yu C, Lu L, Fujiwara Y, Browne C, Chin G, et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 2001;20(23):6909–18. doi: 10.1093/emboj/20.23.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han AP, Fleming MD, Chen JJ. Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Invest. 2005;115(6):1562–70. doi: 10.1172/JCI24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008;10(11):1923–40. doi: 10.1089/ars.2008.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L, Han AP, Chen JJ. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol Cell Biol. 2001;21(23):7971–80. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivella S. Ineffective erythropoiesis and thalassemias. Current opinion in hematology. 2009;16(3):187–94. doi: 10.1097/MOH.0b013e32832990a4. Epub 2009/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus AP, Koch B, Burckett L. Two families showing interaction of haemoglobin C or thalassaemia with high foetal haemoglobin in adults. Br Med J. 1961;1(5237):1434–6. doi: 10.1136/bmj.1.5237.1434. Epub 1961/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conley CL, Weatherall DJ, Richardson SN, Shepard MK, Charache S. Hereditary persistence of fetal hemoglobin: a study of 79 affected persons in 15 Negro families in Baltimore. Blood. 1963;21:261–81. Epub 1963/03/01. [PubMed] [Google Scholar]
- 18**.Sankaran VG, Orkin SH. The switch from fetal to adult hemoglobin. Cold Spring Harbor perspectives in medicine. 2013;3(1):a011643. doi: 10.1101/cshperspect.a011643. Epub 2012/12/05. This an excellent review on induction of fetal hemoglobin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrine SP, Castaneda SA, Chui DH, Faller DV, Berenson RJ, Siritanaratku N, et al. Fetal globin gene inducers: novel agents and new potential. Annals of the New York Academy of Sciences. 2010;1202:158–64. doi: 10.1111/j.1749-6632.2010.05593.x. Epub 2010/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mabaera R, West RJ, Conine SJ, Macari ER, Boyd CD, Engman CA, et al. A cell stress signaling model of fetal hemoglobin induction: what doesn’t kill red blood cells may make them stronger. Exp Hematol. 2008;36(9):1057–72. doi: 10.1016/j.exphem.2008.06.014. Epub 2008/08/23. [DOI] [PubMed] [Google Scholar]
- 21.Ron D, Harding HP. eIF2α phosphorylation in cellular stress responses and disease. In: Mathews MNS, Hershey JWB, editors. Translational Control in Biology and Medicine. Cols Spring Harbor, NY: Cols Spring Harbor Laboratory Press; 2007. [Google Scholar]
- 22.Kaufman RJ. Double-stranded RNA-activated protein kinase PKR. In: Sonenberg NJH, Matthews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2000. pp. 503–28. [Google Scholar]
- 23.Hinnebusch AG. Translational control of GCN4: gene-specific regulation by phosphorylation of eIF2. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational Control. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1996. pp. 199–244. [Google Scholar]
- 24.Ron D, Harding HP. PERK and translational control by stress in endoplasmic reticulum. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Springs Harbor: Cold Spring Harbor Laboratory Press; 2000. pp. 547–60. [Google Scholar]
- 25.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–33. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 26.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. doi: 10.1126/science.1209038. Epub 2011/11/26. [DOI] [PubMed] [Google Scholar]
- 27.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 28.Harding HP, Novoa II, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 29**.Suragani RN, Zachariah RS, Velazquez JG, Liu S, Sun CW, Townes TM, et al. Heme-regulated eIF2alpha kinase activated Atf4 signaling pathway in oxidative stress and erythropoiesis. Blood. 2012;119(22):5276–84. doi: 10.1182/blood-2011-10-388132. Epub 2012/04/14. This work demonstrated that HRI activates eIF2αP-ATF4 signaling in primary erythroblasts and that ATF4 is necessary for mitigating oxidative stress and for effective erythropoiesis. This paper also showed the feasibility of salubrinal in enhancing ISR and as a potential candidate for treatment of thalassemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM, Ron D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16(8):4273–80. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–95. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–22. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kojima E, Takeuchi A, Haneda M, Yagi A, Hasegawa T, Yamaki K, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. Faseb J. 2003;17(11):1573–5. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- 34.Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21(20):6841–50. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23(4):1292–303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. Embo J. 2003;22(5):1180–7. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Bhattacharya S, Han A, Suragani RN, Zhao W, Fry RC, et al. Haem-regulated eIF2alpha kinase is necessary for adaptive gene expression in erythroid precursors under the stress of iron deficiency. Br J Haematol. 2008;143(1):129–37. doi: 10.1111/j.1365-2141.2008.07293.x. Epub 2008/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawatani Y, Suzuki T, Shimizu R, Kelly VP, Yamamoto M. Nrf2 and selenoproteins are essential for maintaining oxidative homeostasis in erythrocytes and protecting against hemolytic anemia. Blood. 2011;117(3):986–96. doi: 10.1182/blood-2010-05-285817. Epub 2010/10/28. [DOI] [PubMed] [Google Scholar]
- 40.Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117(8):2133–44. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu D, dos Santos CO, Zhao G, Jiang J, Amigo JD, Khandros E, et al. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev. 2010;24(15):1620–33. doi: 10.1101/gad.1942110. Epub 2010/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libani IV, Guy EC, Melchiori L, Schiro R, Ramos P, Breda L, et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia. Blood. 2008;112(3):875–85. doi: 10.1182/blood-2007-12-126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sankaran VG, Orkin SH, Walkley CR. Rb intrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev. 2008;22(4):463–75. doi: 10.1101/gad.1627208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spike BT, Dirlam A, Dibling BC, Marvin J, Williams BO, Jacks T, et al. The Rb tumor suppressor is required for stress erythropoiesis. Embo J. 2004;23(21):4319–29. doi: 10.1038/sj.emboj.7600432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98(12):3261–73. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- 46.Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163(4):767–75. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patterson AD, Hollander MC, Miller GF, Fornace AJ., Jr Gadd34 requirement for normal hemoglobin synthesis. Mol Cell Biol. 2006;26(5):1644–53. doi: 10.1128/MCB.26.5.1644-1653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A. 2009;106(6):1832–7. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99(3):736–45. doi: 10.1182/blood.v99.3.736. [DOI] [PubMed] [Google Scholar]
- 50.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–9. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 51**.Hahn CK, Lowrey CH. Eukaryotic initiation factor 2alpha phosphorylation mediates fetal hemoglobin induction through a post-transcriptional mechanism. Blood. 2013;122(4):477–85. doi: 10.1182/blood-2013-03-491043. Epub 2013/05/22. This paper demonstrated for the first time that increasing eIF2αP by salubrinal can induce r-globin expression most likely at the translational level. Importantly, salubrinal worked synergestically with other known r-globin inducing chemicals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Chen JJ, Perrine S. Stressing HbF synthesis: role of translation? Blood. 2013;122(4):467–8. doi: 10.1182/blood-2013-06-506139. Epub 2013/07/28. This is a commentary for the above referecnce of Han and Lowrey and highlights the new role of HRI in inducing fetal hemoglobin production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen T, Ozel D, Qiao Y, Harbinski F, Chen L, Denoyelle S, et al. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat Chem Biol. 2011 doi: 10.1038/nchembio.613. Epub 2011/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332(6025):91–4. doi: 10.1126/science.1201396. Epub 2011/03/10. [DOI] [PubMed] [Google Scholar]
- 55*.Sheedon VA, Howard TA, Sobo A, Nagasaswamy U, Crosby JR, Davis B. Genetic predictors of Hemoglobin F Response to Hydroxyurea in Sickle Cell Anemia. Blood. 2012 This abstract indicated that two SNPs in the GADD34 gene were positively correlated with iduction of HbF by hydroxyurea in sickle cell anemia patients. [Google Scholar]
- 56.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324(5924):218–23. doi: 10.1126/science.1168978. Epub 2009/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147(4):789–802. doi: 10.1016/j.cell.2011.10.002. Epub 2011/11/08. [DOI] [PMC free article] [PubMed] [Google Scholar]


