Abstract
Background
Pulmonary hypertension (PH) is a life-threatening disorder characterized by increased pulmonary artery pressure, remodeling of the pulmonary vasculature, and right ventricular failure. Loss of endothelium-derived nitric oxide (NO) and prostacyclin (PGI2) contributes to PH pathogenesis and current therapies are targeted to restore these pathways. Phosphodiesterases (PDEs) are a family of enzymes that break down cGMP and cAMP which underpin the bioactivity of NO and PGI2. The PDE5 inhibitor (PDE5i) sildenafil is licensed for PH, but a role for PDE2 in lung physiology and disease has yet to be established. Herein, we investigated whether PDE2 inhibition modulates pulmonary cyclic nucleotide signaling and ameliorates experimental PH.
Methods and Results
The selective PDE2 inhibitor BAY 60-7550 augmented atrial natriuretic peptide (ANP) and treprostinil -evoked pulmonary vascular relaxation in isolated arteries from chronically hypoxic rats. BAY 60-7550 prevented the onset of both hypoxia- and bleomycin-induced PH, and produced a significantly greater reduction in disease severity when given in combination with a neutral endopeptidase inhibitor (enhances endogenous natriuretic peptides), the PGI2 analogue treprostinil, inorganic nitrate (NO donor), or a PDE5i. Proliferation of pulmonary artery smooth muscle cells from PAH patients was reduced by BAY 60-7550, an effect further enhanced in the presence of ANP, NO and treprostinil.
Conclusions
PDE2 inhibition elicits pulmonary dilation, prevents pulmonary vascular remodeling, and reduces the RVH characteristic of PH. This favorable pharmacodynamic profile is dependent on natriuretic peptide bioactivity, and is additive with PGI2 analogues, PDE5i, and NO. PDE2 inhibition represents a viable, orally-active therapy for PH.
Keywords: Pulmonary hypertension, phosphodiesterase, natriuretic peptide, nitric oxide, prostacyclin, cGMP, cAMP, vasodilatation, endothelium, hypoxia
INTRODUCTION
Pulmonary hypertension (PH) is a life-threatening, multi-factorial disorder characterized by increased pulmonary vascular resistance and remodeling of the small pulmonary arteries, which precipitate right ventricular hypertrophy (RVH) and failure1, 2. Despite therapeutic innovation, including the introduction of prostacyclin (PGI2) analogues3, endothelin receptor antagonists4, and phosphodiesterase 5 inhibitors (PDE5i)5 mortality remains unacceptably high6, 7 As such, there is a clear unmet medical need for improved drug efficacy in this disorder.
Loss of endothelial nitric oxide (NO)- and PGI2- driven cyclic guanosine-3′,5′-monophosphate (cGMP) and cyclic adenosine-3′,5′-monophosphate (cAMP) signaling is a hallmark of PH, particularly the pulmonary arterial hypertension (PAH) sub-group (WHO Group 1)1. Within the pulmonary circulation, cyclic nucleotides are responsible for mediating endothelium-dependent dilation, thereby maintaining pulmonary vascular homeostasis, but also have salutary actions on pulmonary vascular remodeling, fibrosis, and RV function8, 9. Thus, augmenting cyclic nucleotide signaling represents an appealing, proven strategy for improving therapy, and underpins the efficacy of PDEi, PGI2 analogues and soluble guanylate cyclase (sGC) stimulators9. Moreover, combinations of cGMP (e.g. PDE5i) and cAMP-elevating agents (e.g. iloprost or epoprostenol) exert an additive, if not synergistic, beneficial effect in PH patients10.
Cyclic nucleotide phosphodiesterases (PDEs) are homologous enzymes that facilitate the breakdown of cAMP and/or cGMP11. Within the lung, cGMP-hydrolyzing PDE5 is the most abundant isoform12 and enzyme expression and activity are up regulated in pre-clinical models of PH and patients with the disease13, 14. The expression and activity of additional PDE isoforms, including PDE1, PDE3, PDE4 and PDE10, are also altered in the pulmonary vasculature of PH patients15, 16, and isoform selective inhibitors are effective in pre-clinical models of PH17-20. One PDE isozyme that has received little or no attention in the setting of PH is PDE2. This ‘cGMP-stimulated’ PDE metabolizes cAMP and cGMP and, akin to PDE5, possesses a GAF domain21 within its N-terminus11 that acts as a positive feedback loop to expedite cyclic nucleotide hydrolysis in the presence of cGMP. PDE2 (and splice variants thereof) is expressed in a wide variety of cells and tissues in the cardiovascular system (e.g. myocardium, platelets, endothelium)11, and also found in the lung, including pulmonary artery smooth muscle cells from patients with PH15, 16. Indeed, the enzyme is functionally active in the pulmonary circulation since inhibition of this isoform has been shown to modulate microvascular permeability22, 23 and acute hypoxic vasoconstriction in vitro24.
We therefore hypothesized that in PH, pulmonary PDE2 activity curtails cytoprotective cGMP- and cAMP- signaling (since it metabolizes both cyclic nucleotides)11, and exacerbates pathology. In accord, we investigated the effects of the selective PDE2i, BAY 60-7550 (in vitro IC50 = 4.7nM; >50-fold selectivity over PDE1, and >100-fold selectivity over other PDE isozymes25), on pulmonary vascular dynamics and pulmonary vascular smooth muscle proliferation in vitro, and etiologically distinct pre-clinical models of PH, to identify beneficial activity of the molecule per se, interactions with endogenous pulmonary protective mediators, and additive effects with existing therapies.
METHODS
All studies conformed to the UK Animals (Scientific Procedures) Act 1986 and had approval from a local ethics committee within Barts & The London School of Medicine. Treatment groups, doses and route of administration for in vivo studies are outlined in Supplemental Table 1. Mice were randomly assigned to each drug treatment.
Hypoxia-induced PH
Male mice (C57BLK/6J; Charles River, UK), or Wild-type (WT) and natriuretic peptide receptor (NPR)-A knockout (KO) littermates (male, 20-25g; C57BLK/6J background) were placed inside a normobaric chamber26 with 10% oxygen for either 3 weeks with drug treatment from day 1 (Groups 1-6, Supplemental Table 1) or 5 weeks hypoxia with drug treatment from day 14 (i.e. after onset of overt PH to assess the potential of drugs to reverse established pathology; Groups 1-4 & 7-14, Supplemental Table 1). Age-matched normoxic control mice were housed in room air.
Bleomycin-induced PH
A second, etiologically distinct model of PH was used to validate the efficacy of BAY 60-7550 in reducing disease severity. Male mice (C57BLK/6J; Charles River, UK) were exposed to bleomycin (2mg/kg, 1ml/kg volume) once by oropharangeal instillation26 under light isofluorane-induced anesthesia (1.5% isofluorane, 0.2ml/min oxygen). Controls were similarly instilled with sterile saline (1ml/kg). Drug treatments were administered daily over a 3 week period, starting on the day of bleomycin administration.
Mouse haemodynamics
Mice were anaesthetized using isofluorane (1.5%, 0.2ml/min oxygen) & maintained at 37°C. The right ventricular systolic pressure (RVSP) and mean arterial blood pressure (MABP) were measured using a Mikrotip® pressure catheter (size 1F, SPR-1000, Millar Instruments, Houston, TX, USA) and RVH was calculated by weight of RV to left ventricle + septum ratio (RV/(LV+S))26. Plasma was obtained from centrifugation of whole blood (10,000xg, 2min) and assayed for cGMP (cGMP Direct Biotrak EIA, GE Healthcare, Buckinghamshire, UK) and cAMP (cAMP ELISA, Enzo Life Sciences, Exeter, UK).
Immunohistochemistry
Serial sections (4μm) were used for trichrome blue staining and α smooth muscle actin (αSMA) immunohistochemistry. For the latter, sections were stained with mouse monoclonal anti αSMA antibody (DAKO, UK, 1:1000 dilution), followed by biotinylated anti-mouse secondary antibody. Immunoreactivity was detected using the ABC-peroxide based system (DAKO, UK). Stained slides were imaged by Nanozoomer Virtual Microscopy (Hamamatsu, Welwyn Garden City,UK).
Morphological analysis
Transverse formalin fixed lung sections were stained with van Gieson’s elastic (EVG) method. Pulmonary arterial muscularisation was then assessed as we have described previously27, 28 (see Supplemental Methods).
Vascular function
Aortic and pulmonary artery (3rd order) rings, isolated from hypoxic (2 weeks, 10% O2) or normoxic (Control) rats (male, Sprague-Dawley, 225-275g) were set up for isometric tension measurement, as we have described previously26. For this set of experiments, rat vessels were used to permit concomitant analysis of aorta and pulmonary arteries from the same animals. Vessels were pre-contracted with an EC80 concentration of U46619 and endothelial function determined by relaxation responses to acetylcholine (10μmol/L). Relaxation curves were then constructed for either atrial natriuretic peptide (ANP; 0.01nmol/L-0.3μmol/L), the NO donor spermine NONOate (S-NO; 1nmol/L-30 μmol/L) or the PGI2 analogue treprostinil (1nmol/L-3 μmol/L) in the presence or absence of the PDE1i vinpocetine (30μmol/L), PDE2i BAY 60-7550 (0.1μmol/L), PDE3i milrinone (10μmol/L) or PDE5i sildenafil (3μmol/L). Relaxation to BAY 60-7550 (1nmol/L-3 μmol/L) per se was also assessed.
Cell proliferation
Growth of human distal pulmonary artery smooth muscle cells isolated from patients with idiopathic pulmonary arterial hypertension (IPAH) or control cells from adults undergoing transplant or lung resection for suspected malignancy, were monitored as we have described previously29 following treatment with BAY 60-7550 (1μmol/L), ANP (1μmol/L), DETA-NONOate (10μmol/L), or treprostinil (1μmol/L), alone or in combination.
RT-PCR & Immunoblotting
cDNA was prepared from pulmonary arteries from normoxic and hypoxic rats, and pulmonary artery smooth muscle cells isolated from patients with IPAH and control cells (as above) and analyzed for PDE2A expression using quantitative real-time PCR over 40 cycles (see Supplemental Methods for primer sequence and PCR conditions). PDE2A protein expression was determined by immunoblot using primary anti-PDE2A antibody (Santa Cruz Biotechnology, USA; 1:500) and secondary horse-radish peroxidase conjugated anti-goat IgG antibody (Santa Cruz Biotechnology; 1:10,000). Bands were quantitated by densitometry using ImageJ and normalized to the loading control (anti-actin, 1:20,000, Millipore, Watford, UK. secondary antibody horse-radish peroxidase conjugated anti-mouse IgG, Dako, Cambridge, UK).
PDE2 activity & NO production
PDE2 activity in cytosolic extracts from rat pulmonary arteries and human pulmonary artery smooth muscle cells was determined by the production of 5′-GMP using a commercially available kit (Enzo Life Sciences, Exeter, UK). Total PDE activity was determined using the non-selective PDEi 3-isobutyl-1-methylxanthine (IBMX, 300μmol/L) and specific PDE2 activity calculated as the reduction in 5′-GMP formation in the presence of BAY 60-7550 (1μmol/L).
Plasma nitrite (NO2−) levels, as an index of vascular eNOS activity30, were determined by ozone chemiluminescence as we have described previously28
Data analysis
Results are expressed as mean±s.e.mean, and P<0.05 denotes significance. The n value denotes the number of animals used in each group. Statistical analyses were performed using GraphPad Prism version 5 as described in each Figure legend.
RESULTS
PDE2 plays a key role in regulating the vasoreactivity of pulmonary arteries
Rats exposed to 2 weeks hypoxia exhibited substantial RVH (Supplemental Figure 1A) and pulmonary artery (Supplemental Figure 1B), but not aortic (Supplemental Figure 1C), endothelial dysfunction compared with normoxic animals, confirming the induction of a PH phenotype. Incubation with BAY 60-7550 sensitized pulmonary arteries from both normoxic (Figure 1A) and hypoxic (Figure 1B) rats to ANP. Spermine-NONOate-evoked responses were also increased in the presence of BAY 60-7550 (Figure 1C), yet this effect was abolished in hypoxic animals (Figure 1D). PDE2 inhibition increased the potency of treprostinil in pulmonary vessels from hypoxic (Figure 1F), but not normoxic (Figure 1E) rats.
Figure 1.
Concentration-response curves to atrial natriuretic peptide (ANP), spermine-NONOate (S-NO), and treprostinil in pulmonary arteries in the absence or presence of BAY 60-7550 (BAY, 0.1 μmol/L) isolated from normoxic (A, C, E; n=4-6) and hypoxic (2 weeks 10% O2; B, D, F; n=3-8) rats. Data are presented as mean ± SEM. Curves are compared using two-way analysis of variance with repeated measures. *P<0.05, ***P<0.001 BAY vs. Control.
In the presence of BAY 60-7550, there were no differences in ANP-evoked relaxation (Figure 2A & B) in aortae from normoxic or hypoxic rats. However, in aortae from normoxic rats, BAY 60-7550 enhanced relaxation by Spermine-NONOate (Figure 2C); an effect absent in arteries of hypoxic rats. Interestingly, treprostinil did not induce relaxation in the aorta of normoxic rats, in the absence or presence of BAY 60-7550 (Figure 2E). In hypoxic rat aorta, treprostinil had little or no relaxant activity per se but its vasorelaxant potency was markedly enhanced by BAY 60-7550 (Figure 2F).
Figure 2.
Concentration-response curves to atrial natriuretic peptide (ANP), spermine-NONOate (S-NO), and treprostinil in aorta in the absence or presence of BAY 60-7550 (BAY, 0.1 μmol/L) isolated from normoxic (A, C, E; n=5-13) and hypoxic (2 weeks 10% O2; B, D, F; n=5-8) rats. Data are presented as mean ± SEM. Curves are compared using two-way analysis of variance with repeated measures. ***P<0.001 BAY vs. Control.
In contrast to PDE2i (Figure 1A-D), inhibition of PDE1 or PDE3 did not alter ANP or Spermine-NONOate-induced relaxation (Supplemental Table 2).
PDE2 inhibition decreases right ventricular pressure and hypertrophy in two murine models of PH
Hypoxic vehicle-treated mice developed augmented RVSP (Figure 3A) and increased RV/(LV+S) (Figure 3C) compared with normoxic animals. Hypoxic mice treated with BAY 60-7550 had significantly reduced RVSP and RV/(LV+S) (Figure 3A, C), both of which remained commensurate with control values. Hypoxic mice had more than twice the number of muscularised small pulmonary arteries compared with normoxic controls; PDE2 inhibition prevented this morphological pathology (Figure 3G, H).
Figure 3.
Right ventricular systolic pressure (RVSP; A & B), right ventricle/left ventricle+septum ratio (RV/(LV+S); C & D), and mean arterial blood pressure (MABP; E & F) in normoxic (Nx, n=6-18), vehicle-treated hypoxic (3 weeks 10% O2; n=10-11) or BAY-60-7550-treated (n=11-14) hypoxic mice (A, C, E) and saline- (n=15), vehicle- (Veh; n=21) or BAY-60-7550-treated (n=15) mice exposed to bleomycin (1mg/kg; B, D, F). Muscularisation of pulmonary small arteries (G) and representative images showing pulmonary vessel muscularisation using anti-alpha smooth muscle actin staining (H; scale bar = 20 μm, red stain). All mice were treated with either veh (0.5% carboxymethylcellulose + 10% polyethylene glycol) or BAY 60-7550 (BAY, 10 mg/kg/day) by oral gavage for 3 weeks. Data are presented as mean ± SEM. Statistical analysis by one-way analysis of variance. *P<0.05, **P<0.01 vs. Veh, #P<0.05, # #P<0.01, # # #P<0.001 vs. Nx or Saline as determined by Bonferroni post-hoc comparisons (2 in total: Nx/Saline v Veh and Veh v BAY).
Bleomycin-treated mice had increased RVSP (Figure 3B) and RV/(LV+S) ratio (Figure 3D) compared with saline-treated animals. Akin to the hypoxia model, both RVSP (Figure 3B) and RV/(LV+S) ratio (Figure 3D) were lower in BAY 60-7550-treated mice. Importantly, in both models, MABP was similar in hypoxic mice in the absence and presence of BAY 60-7550 (Figure 3E, F), suggesting that this drug exhibits a degree of pulmonary selectivity.
Obligatory role of natriuretic peptide bioactivity in the beneficial effects of PDE2 inhibition in PH
In WT mice, the RVSP was lower in BAY 60-7550-treated hypoxic mice compared with vehicle treated WT animals (Figure 4A) but this effect was abolished in NPR-A KO mice (Figure 4A). Likewise the ability of BAY 60-7550 to prevent the RVH in hypoxic WT mice was lost in NPR-A KO animals (Figure 4C), suggesting that natriuretic peptide-generated cGMP is regulated by PDE2. Vehicle-treated NPR-A KO mice had elevated MABP compared with vehicle-treated WT animals (Figure 4E), but PDE2 inhibition per se did not alter MABP.
Figure 4.
Right ventricular systolic pressure (RVSP; A & B), right ventricle/left ventricle+septum ratio (RV/(LV+S); C & D), and mean arterial blood pressure (MABP; E & F) in wildtype (WT) and natriuretic peptide receptor A (NPR-A) knockout (KO) mice, and WT animals receiving Nω-nitro-l-arginine methyl ester (l-NAME; 100 mg/kg/day in the drinking water) in hypoxia (3 weeks 10% O2) -induced PH. Mice were administrated vehicle (Veh; n=10 WT, n=8 KO, n=15 WT + L-NAME) or BAY 60-7550 (BAY; 10 mg/kg/day, n=10 WT, n=7 KO, n=10 WT + L-NAME). Data are presented as mean ± SEM. Statistical analysis by one-way analysis of variance. #P<0.05 vehicle-treated KO/l-NAME vs. vehicle-treated WT, *P<0.05, **P<0.01, ***P<0.001 vs. Veh, as determined by Bonferroni post-hoc comparisons (3 in total: WT Veh v KO Veh, and Veh v BAY in both WT & KO groups).
To assess the effect of the NO pathway on the beneficial activity of BAY 60-7550, WT mice were treated with the NOS inhibitor, l-NAME, for the duration of hypoxia. The ability of BAY 60-7550 to prevent the increased RVSP was maintained in L-NAME-treated animals (Figure 4B), suggesting that an intact NO pathway is not necessary for PDE2 inhibition to be effective. BAY 60-7550 also caused a similar reduction in RVH in L-NAME-treated mice, although the NOS inhibitor per se caused an apparent reduction in the RV/(LV+S) ratio, at least in part due to a modest left ventricular hypertrophy resulting from blockade of systemic NO production31 (Figure 4D). L-NAME treatment caused elevated MABP and a significant reduction in the plasma nitrite (NO2−) concentrations (index of vascular NO production30; Control=1.04±0.06μmol/L, L-NAME=0.66±0.04μmol/L, P<0.001, n=8), confirming effective inhibition of eNOS activity, but BAY 60-7550-treatment did not alter MABP in these mice (Figure 4F).
Interaction between PDE2 inhibition and natriuretic peptides in PH
The preceding experiments ascertained a pivotal role for natriuretic peptides in the beneficial activity of PDE2i in experimental models of PH. We have recently reported that augmentation of endogenous natriuretic peptide levels using the neutral endopeptidase (NEP; an enzyme that hydrolyses and terminates the biological activity of endogenous natriuretic peptides32) inhibitor ecadotril synergistically interacts with the PDE5i sildenafil to ameliorate PH26. Therefore, we investigated whether increasing endogenous natriuretic peptides with ecadotril would also increase the efficacy of BAY 60-7550.
Mice treated with ecadotril alone had RVSP (Figure 5A) and RV/(LV+S) (Figure 5C) similar to hypoxic vehicle-treated mice. In combination with BAY 60-7550, the hypoxia-induced increases in RVSP and RVH were attenuated, essentially back to control levels (Figure 5A, C). MABP was not altered by ecadotril or the ecadotril/BAY 60-7550 combination (Figure 5E). When administered alone, the effect of BAY 60-7550 on established PH was smaller than that achieved when given prophylactically (Figure 5B, H) with a significantly reduced RVSP only achieved at a dose of 100mg/kg/day (Figure 5H); 10-fold higher than that necessary to prevent the onset of PH (Figure 3A). However, in the presence of ecadotril, BAY 60-7550 reversed the RVSP (Figure 5B) and RVH (Figure 5D) to a greater extent than either drug alone. This dual therapy did not alter MABP (Figure 5F). The beneficial effect of PDE2i/NEPi combination therapy appeared to be underpinned by cGMP production, as only dual treatment was able to significantly enhance plasma cGMP levels (Figure 5G).
Figure 5.
Right ventricular systolic pressure (RVSP; A,B, H), right ventricle/left ventricle+septum ratio (RV/(LV+S); C & D), and mean arterial blood pressure (MABP; E & F) in hypoxia-induced PH. In A, C, E mice were exposed to normoxia (Nx; n=6) or 3 weeks hypoxia (10% O2) with drug treatment from day 1. In B, D, F, G & H animals were exposed to normoxia (Nx; n=15) or 5 weeks hypoxia (10% O2) with drug treatment at day 14. Hypoxic mice received one of vehicle (Veh; n=6-15), BAY 60-7550 (BAY, 10mg/kg/day, n=8-12; 30 mg/kg/day, n=10; or 100 mg/kg/day, n=9), ecadotril (E; 60mg/kg/day; n=6-10) or a combination of both (at the same doses; n=8-9). Plasma cGMP concentrations from animals exposed to 5 weeks hypoxia (10% O2; G). Data are presented as mean ± SEM. Statistical analysis by one-way analysis of variance. *P<0.05, **P<0.01 vs. Veh, # #P<0.01, # # #P<0.001 vs. Nx as determined by Bonferroni post-hoc comparisons (4 in total: Nx v Veh, and Veh v each treatment group).
PDE2 inhibition promotes the salutary effects of NO and PGI2 in PH
Experiments in l-NAME-treated mice suggested that the attenuation of RVSP with PDE2 inhibition is not dependent on endogenous NO. However, we investigated the possibility that a pharmacological interaction between PDE2 inhibition and NO bioactivity may be evident in reversing hypoxia-induced PH. In support of this concept, treatment with inorganic nitrate (which we have shown previously to ameliorate hypoxia-induced PH28), at a dose that was ineffective per se, significantly attenuated the RVSP in combination with PDE2 inhibition (Figure 6A). However, this treatment effect was less pronounced against the corresponding RVH (Figure 6C).
Figure 6.
Right ventricular systolic pressure (RVSP; A & B), right ventricle/left ventricle+septum ratio (RV/(LV+S); C & D), mean arterial blood pressure (MABP; E & F), and plasma cAMP concentration (G) in mice exposed to normoxia (Nx) or 5 weeks hypoxia (10% O2) with drug treatment from day 14. Animals received vehicle (Veh; n=15), sham-surgery plus vehicle (sham, n=4), BAY-60-7550 (BAY; 10 mg/kg/day; n=8), inorganic nitrate (N; 150mg/kg/day; n=7), treprostinil (T; 20mg/kg/day; n=8) or a combination of BAY plus nitrate or BAY plus treprostinil (at the same doses). Data are presented as mean ± SEM. Statistical analysis by one-way analysis of variance. *P<0.05, vs. Veh, # # #P<0.001 vs. Nx as determined by Bonferroni post-hoc comparisons (4 in total: Nx v Veh/Sham, and Veh/Sham v each treatment group).
In parallel experiments, since PDE2 hydrolyses both cGMP and cAMP and PDE2i augmented the vasorelaxant activity of treprostinil in vitro, we explored if BAY 60-7550 could potentiate the pharmacodynamic effect of treprostinil and reverse established PH. A very similar pattern of activity was observed such that in combination, BAY 60-7550 and treprostinil were able to reverse the hypoxia-induced increase in RVSP, whilst neither intervention as monotherapy produced a significant effect (Figure 6B). Again, the benefit of dual therapy was less evident against RVH (Figure 6D). Neither combination resulted in a significant decrease in MABP (Figure 6E, F). The efficacy of the PDE2i/treprostinil combination appeared to be linked to cAMP accumulation since the dual therapy resulted in a doubling in plasma cAMP levels that was greater than either drug alone (Figure 6G).
PDE2 expression and activity in rodent and human PH
PDE2 mRNA and protein expression were significantly reduced in the pulmonary arteries of hypoxic animals compared to normoxic controls (Figure 7A, B). Enzyme activity trended towards a lower level but was not significantly different between normoxic and hypoxic arteries (Figure 7C). Expression of PDE2 mRNA was also down-regulated in cells from patients with IPAH compared to cells from normal controls (Figure 7D). PDE2 activity in cells from IPAH patients was commensurate with that seen in the pulmonary arteries of animals with hypoxia-induced PH (Figure 7E); however, the PDE2 activity observed in pulmonary vascular smooth muscle cells from normal individuals was markedly lower than that in the pulmonary arteries from normoxic control animals, entailing that overall there is little or no reduction in PDE2 activity in PH patients. At the concentration used for in vitro evaluation, BAY 60-7550 caused a significant reduction in PDE activity in both rat pulmonary arteries and pulmonary vascular smooth muscle cells confirming effective inhibition of PDE2 (Figure 7C, E).
Figure 7.
PDE2A mRNA (A, D) and protein (B) expression, and activity (defined as 5′-GMP formation inhibitable by BAY 60-7550 [1μmol/L]; C, E), in isolated pulmonary arteries from normoxic (Nx) and hypoxic (Hx) rats (2 weeks 10% O2; n=3-8; A, B, C) and pulmonary vascular smooth muscle cells from normal individuals and PAH patients (n=5; D, E). Proliferation of human pulmonary artery smooth muscle cells from PAH patients in the absence (Control; n=9) or presence of BAY 60-7550 (BAY; 1μmol/L; n=9), atrial natriuretic peptide (ANP; 1μmol/L; n=4), DETA-NONOate (DETA-NO; 10μmol/L; n=5), treprostinil (T; 3μmol/L; n=4) or combinations thereof (at the same concentrations; F, G, H). Data are shown as mean ± SEM. Statistical analysis by unpaired Students T-test (A-E) or two-way analysis of variance (F, G, H). *P<0.05, **P<0.01, ***P<0.001 versus control/normoxia/normal, #P<0.05, # #P<0.01 vs. BAY, ≠P<0.05 versus treprostinil.
PDE2 inhibition regulates the proliferation of pulmonary artery smooth muscle cells from patients with IPAH
As PH is characterized by dysregulated proliferation of pulmonary artery smooth muscle cells (PASMCs)29, and to provide proof-of-concept in the human disease, we assessed the effect of PDE2 inhibition on growth of PASMCs from patients with IPAH. Compared with untreated control cells, proliferation of PASMCs from IPAH patients was significantly reduced by BAY 60-7550 (Figure 7F, G, H). This anti-proliferative effect was enhanced by combination with ANP (Figure 7F), DETA-NONOate (Figure 7G) and treprostinil (Figure 7H). The effect of dual therapy was additive, if not synergistic, as treatment of cells with PDE2i/ANP or PDE2i/treprostinil caused a greater reduction in cell growth than PDE2 inhibition alone (Figure 7F, H).
Additive beneficial effect of PDE2i and PDE5i in PH
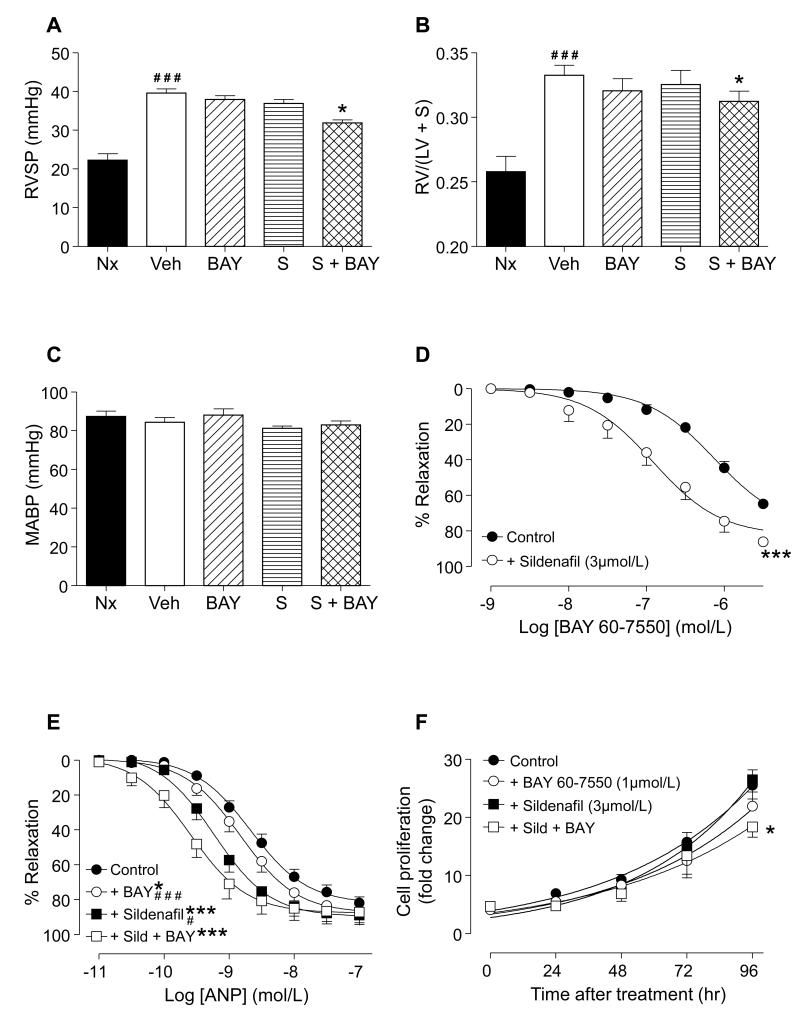
Since PDE5i are first-line therapy for PH, we investigated whether PDE2 inhibition sustains a therapeutic effect in the presence of PDE5i. In established PH, sildenafil alone did not cause a significant reduction in RVSP (Figure 8A), RVH (Figure 8B) or MABP (Figure 8C). However, treatment with sildenafil plus BAY 60-7550 attenuated the rise in RVSP and RVH compared with both vehicle and PDE2i -treated mice (Figure 8A, B). MABP was not reduced by any of these treatments, alone or in combination (Figure 8C). The beneficial effect of the PDE2i/PDE5i combination on RVSP was mirrored in isolated pulmonary arteries from hypoxic rats. In this setting, BAY 60-7550 alone caused concentration-dependent relaxation which was substantially enhanced after incubation with sildenafil (Figure 8D). Incubation with both BAY 60-7550 and sildenafil also substantially increased the sensitivity of pulmonary arteries to ANP; an effect that was greater than that elicited by either drug alone (Figure 8E). Finally, the combination of sildenafil plus BAY 60-7550 decreased IPAH patient PASMC proliferation to a greater extent than either drug administrated independently (Figure 8F).
Figure 8.
Right ventricular systolic pressure (RVSP; A), right ventricle/left ventricle+septum ratio (RV/(LV+S); B), and mean arterial blood pressure (MABP; C) in normoxic mice (Nx; n=6) or hypoxic (5 weeks 10% O2) animals treated with vehicle (Veh; n=13), BAY 60-7550 (BAY, 10 mg/kg/day; n=9), sildenafil (S; 30 mg/kg/day; n=9) or a combination of sildenafil plus BAY (at the same doses; S + BAY, n=10). Concentration-responses curves to BAY 60-7550 (D) and atrial natriuretic peptide (ANP; E) in isolated pulmonary arteries from hypoxic (2 weeks 10% O2) rats. Proliferation of human pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension in the absence (Control; n=9) and presence of BAY 60-7550 (1μmol/L; n=9), sildenafil (3umol/L; n=3) or a combination thereof (at the same concentrations; n=3) (F). Data are shown as mean ± SEM. Statistical analysis by one-way analysis of variance with Bonferroni post-hoc comparisons (4 in total: Nx v Veh, and Veh v each treatment group; A, B, C) or two-way analysis of variance (D, E, F). *P<0.05, ***P<0.001 versus vehicle control, ≠P<0.05 vs. BAY, #P<0.05, ###P<0.001 versus sildenafil + BAY.
DISCUSSION
The strategy of promoting pulmonary cyclic nucleotide signaling, by both cGMP (e.g. PDE5 inhibition5, sGC stimulation33) and cAMP (PGI2 analogues3), is clinically effective in PH. Moreover, drug combinations which target both cyclic nucleotide systems have additive or synergistic effects to diminish disease severity10. In addition to the clinical efficacy of PDE5i, blockade of additional PDEs has shown promise in experimental models of PH, including PDE117, PDE318, PDE419, and PDE1020. In distinct contrast, there is a paucity of information regarding a role for PDE2 in pulmonary physiology and PH. As a therapeutic target in PH, PDE2 is particularly attractive since it metabolizes both cGMP and cAMP11 implying that blockade of this enzyme will concomitantly promote signaling by both cyclic nucleotides. In accord with this hypothesis, herein we demonstrate that the selective PDE2 inhibitor, BAY 60-7550 produces a prophylactic salutary activity in two pre-clinical models of PH, and reverses multi aspects of pathology in hypoxia-induced PH, impacting on pulmonary vasoconstriction, remodeling, and RVH. These positive outcomes were augmented in the presence of interventions, including approved therapies for PH, that bolster cGMP (i.e. sildenafil, inorganic nitrate) and cAMP signaling (i.e. treprostinil). Finally, inhibition of PDE2 prevents the hyper-proliferative phenotype of pulmonary artery smooth muscle cells from patients with PAH.
Previous work exploring the contribution of PDE isozymes to pulmonary vascular physiology and PH-centric pathology has not advocated a major role for PDE2 based on expression levels and cyclic nucleotide turnover13, 15, 19. This study took a functional approach to evaluate the capacity of ‘classical’ cGMP-hydrolyzing PDEs (i.e. PDEs 1-3 and PDE5) to modulate the reactivity of aorta and pulmonary arteries from healthy (normoxic) animals, and littermates with hypoxia-induced PH. As established in pre-clinical models, PDE5 inhibition augmented responses to NO and ANP in the pulmonary artery, a capacity that plays a role in the therapeutic efficacy of this drug class in PH. Notably, neither PDE1 nor PDE3 inhibition was able to recapitulate the vasodilator capacity of sildenafil, indicating that these PDEs do not play a functional role in regulating vascular cGMP turnover (at least in this model system). However, evidence to support our hypothesis that PDE2 plays an active role in pulmonary physiology and PH was provided by the observation that BAY 60-7550 enhanced the vasorelaxant responses to ANP in pulmonary vessels in tissues from both normoxic and hypoxic animals, and increased responses to NO in tissues from normoxic rats. These data raise the possibility that in the hypoxic environment of PH, PDE2i increases pulmonary sensitivity to natriuretic peptides without promoting the vasorelaxant activity of cGMP in the systemic circulation, thereby bringing about a pulmonary specific vasodilator activity. This observation dovetails well with previous reports intimating a role for PDE2 in acute hypoxic vasoconstriction24 but not in regulating peripheral vascular tone34. Vascular reactivity studies also demonstrated that pulmonary PDE2 is involved in modulating cAMP signaling mechanisms, as we hypothesized based on the dual substrate utilization of this enzyme. Augmentation of the vasorelaxant activity of treprostinil by BAY 60-7550 was only apparent under hypoxic conditions, implying that akin to cGMP signaling, PDE2i will selectively promote cAMP signaling (in the lung) in PH.
Having determined that PDE2i produced a pulmonary-selective effect of vascular function, we examined if BAY 60-7550 is effective in two well-validated experimental models of PH; hypoxia- and bleomycin- induced. Prophylactic administration of BAY 60-7550 resulted in a significantly less severe phenotype, with lower RVSP, reduced RVH, and fewer muscularized pulmonary arteries. Indeed, treatment with BAY 60-7550 returned these indices of disease severity to near normal values. Importantly, the MABP in animals receiving BAY 60-7550 was not altered, mirroring the pulmonary-selective profile of PDE2 inhibition in isolated arteries. Notably, BAY 60-7550 alone produced a less pronounced effect on the altered pulmonary hemodynamics and RVH in established PH, with a dose-response relationship shifted at least 10-fold. This reduced efficacy matches that of sildenafil in pre-clinical models of PH (shown herein and26), and is commensurate with the small reduction in pulmonary artery pressure that sildenafil produces in PH patients5. Only using combined therapies, most effectively BAY 60-7550 plus ecadotril, but also BAY 60-7550 plus sildenafil, inorganic nitrate or treprostinil, was a significant salutary effect on RVSP and RVH evident. This advocates the use of such dual approaches in PH to optimize cyclic nucleotide signaling as a treatment strategy and improve on existing therapeutics based on these mechanisms.
We determined that the efficacy of BAY 60-7550 was dependent on intact natriuretic peptide bioactivity, but not on NO-dependent signaling, since the salutary effects of PDE2 inhibition were absent in mice lacking NPR-A (the cognate receptor for ANP and BNP), but maintained in animals treated chronically with L-NAME. As a logical extension, we also established that pharmacological augmentation of natriuretic peptide levels, using the NEP inhibitor ecadotril, in combination with BAY 60-7550 caused an additive, if not synergistic effect, on both the prevention and reversal of hypoxia-induced PH. The efficacy of the combination was dependent on cGMP production and pulmonary selective, providing further evidence that PDE2 inhibition has its most pronounced effect on natriuretic peptide-driven cGMP signaling in PH. These observations parallel recent data derived from studies in models of left ventricular hypertrophy (LVH) and heart failure in which compartmentalized cGMP pools, generated by sGC and particulate (pGC) guanylate cyclases (triggered by NO and natriuretic peptides, respectively) produce distinct effects on myocardial structure and function. These spatially restricted signaling mechanisms are also regulated by distinct PDE isozymes; sGC-generated cGMP is metabolized predominantly by PDE5 whereas pGC-synthesized cGMP is hydrolyzed primarily by PDE235, 36. It appears that a similar mechanism is active in the RV and pulmonary circulation; PDE2 inhibition promotes the cytoprotective functions of endogenous natriuretic peptides whereas it is necessary to drive the NO-dependent pathway pharmacologically before any effect of PDE2 blockade is observed. This latter phenomenon may result from spill-over of cGMP that would ordinarily be hydrolyzed by PDE5 into a PDE2 regulated pool. This also brings into the spotlight the additive activity between BAY 60-7550 and sildenafil revealed in the present study. This cooperative action was apparent at many different levels; pulmonary artery vasodilatation, RVH, and the proliferation of pulmonary vascular smooth muscle cells from PAH patients. Yet, a compounded effect was not observed in the periphery since MABP remained unchanged in the presence of both inhibitors. This cross-talk between PDE2 and PDE5 therefore appears to be specific to the heart and pulmonary circulation. We conclude that increasing cellular cGMP levels by pharmacological blockade of either PDE2 or PDE5 results in activation of the alternate isozyme as a result of cGMP binding to the analogous N-terminal GAF domains both enzymes possess11. Only with combined blockade of both PDE2 and PDE5 is it possible to optimize the beneficial effects of cGMP signaling. This finding is important from a therapeutic standpoint since sildenafil is a first-line treatment for PAH and drug efficacy is likely to be limited by activation of PDE2. Thus, evidence that PDE2 inhibition can produce additional activity above and beyond PDE5 inhibition alone is encouraging, and might explain why a significant cohort of PAH patients do not respond well to sildenafil, or experience a diminution of efficacy over time37; co-administration of a PDE2 inhibitor to this population may be superior.
Despite similarities between the right and left ventricles with respect to spatially-constrained cGMP signaling, an interesting dichotomy exists in terms of the pharmacodynamic effect of PDE2 inhibition. Recent work has revealed PDE2 to change its substrate profile in the left ventricle based on the dynamic levels of cGMP and cAMP. Thus, under physiological circumstances PDE2 hydrolyses natriuretic peptide generated cGMP almost exclusively, whereas in the presence of β-adrenergic activation PDE2 metabolizes predominantly cAMP, thereby augmenting adrenergic signaling35, 36, 38. In the failing heart, PDE2 expression and activity are up-regulated and enzyme inhibition appears to be detrimental because of the inotropic and chronotropic activity driven by cAMP is exacerbated39. This is in distinct contrast to the current study in which PDE2 inhibition is clearly beneficial in RVH associated with PH. The mechanisms underlying this right-left ventricle difference remain to be determined but might reside with the bioactivity of cAMP. Long term potentiation of sympathetic, cAMP-dependent pathways (e.g. β-agonists, PDE3 inhibitors) increases mortality in patients with left heart failure40, 41, whereas pharmacologically targeting cAMP signaling via the use of prostacyclin analogues offers a survival advantage in PAH patients3. Therefore, it is possible that PDE2 inhibition is beneficial in PH because it slows the breakdown of natriuretic peptide-driven cytoprotective cGMP first and foremost, but additionally reverses the cAMP signaling deficit (resulting from endothelial dysfunction and loss of prostacyclin functionality) to reduce pulmonary vascular resistance & remodeling thereby exerting an indirect, beneficial effect on the RV42. In addition, the RV possesses an inherently greater capacity, compared to the left ventricle, to recover structure and function in the face of substantially reduced afterload (e.g. pulmonary thromboendarterectomy in CETPH patients versus valve replacement in individuals with aortic stenosis). Thus, despite the fact that β -blockers slow RV deterioration in PH43, 44, the clinical outcome of augmenting cAMP likely depends on the relative impact of the pharmacological intervention on the pulmonary circulation versus the RV. Interestingly, the effects of BAY 60-7550 in combination with treprostinil were more pronounced on RVSP compared to RVH in the pre-clinical model employed herein, supporting a greater impact of cAMP elevation on the pulmonary circulation. This thesis also dovetails well with beneficial activity of inhibitors of PDE4, a cAMP-specific PDE, in pre-clinical models of PH and on the proliferation of pulmonary vascular smooth muscle cells from PH patients19, 29. Finally, the reported involvement of PDE2 in maintaining endothelial barrier function22, 23, intimates that PDE2i may possess an additional advantage by preserving pulmonary endothelial integrity and thereby decreasing pulmonary capillary permeability and edema.
This study also gleaned proof-of-concept data in the human disease by establishing a potent anti-proliferative effect of BAY 60-7550 in pulmonary vascular smooth muscle cells from patients with IPAH. In line with the in vitro vessel studies and experimental models of PH, the favorable activity of BAY 60-7550 was additive, if not synergistic, with NO, natriuretic peptides and treprostinil. Furthermore, we demonstrate that PDE2A mRNA and protein expression are reduced in pulmonary artery smooth muscle cells from PAH patients and pulmonary arteries from rats with hypoxia-induced PH. Importantly however, a commensurate drop in PDE2 activity was not apparent. We conclude that this is indicative of an innate defense mechanism that helps preserve cytoprotective cyclic nucleotide signaling in PH by reducing PDE2-mediated hydrolysis of cGMP (and cAMP) and maximizing the beneficial activity of NO, natriuretic peptide and PGI2. The sharp reduction in mRNA and protein expression only results in a subtle drop in enzyme activity, probably the result of the higher tissue cGMP background (resulting from the increased natriuretic peptide expression and bioactivity associated with PH) which activates PDE2 via interaction with its GAF-B domain. Regardless, this is in marked contrast to other PDE isozymes (e.g. PDE1, PDE3, PDE5, PDE10)15-17, 20 which have been reported to be up-regulated in PH.
In sum, this study provides convincing evidence in vitro and in vivo of the therapeutic potential of PDE2 inhibition in PH. The beneficial effect of PDE2i is dependent on endogenous natriuretic peptide bioactivity, but also produces additive effects with existing therapies, including PDE5i, PGI2 analogues and NO. The double-pronged mechanism of action inherent to PDE2 inhibition (i.e. promoting cGMP and cAMP signaling) is unique in terms of existing therapy for PH, which targets one or other cyclic nucleotide transduction system (i.e. PGI2 analogues, PDE5i), and therefore holds a theoretical advantage in treating the disease.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by British Heart Foundation Grant PG/10/077/28554. This study forms part of the translational programme of the National Institute for Health Research Biomedical Research Unit at Barts & The London.
Footnotes
Conflict of Interest Disclosures: LC has received research grants from United Therapeutics and acted as a consultant. AH has acted as a consultant/advisory board member for Bayer AG, Novartis, Merck and Palatin Technologies.
Journal Subject Codes: [18] Pulmonary circulation and disease, [95] Endothelium/vascular type/nitric oxide, [118] Cardiovascular pharmacology, [130] Animal models of human disease, [145] Genetically altered mice
References
- 1.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 2.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jobsis MM, Blackburn SD, Shortino D, Crow JW. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 4.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 5.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 7.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the reveal registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 8.Janssen W, Schermuly RT, Kojonazarov B. The role of cgmp in the physiological and molecular responses of the right ventricle to pressure overload. Exp Physiol. 2013;98:1274–1278. doi: 10.1113/expphysiol.2012.069138. [DOI] [PubMed] [Google Scholar]
- 9.Baliga RS, MacAllister RJ, Hobbs AJ. New perspectives for the treatment of pulmonary hypertension. Br J Pharmacol. 2011;163:125–140. doi: 10.1111/j.1476-5381.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: A randomized trial. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 12.Corbin JD, Beasley A, Blount MA, Francis SH. High lung pde5: A strong basis for treating pulmonary hypertension with pde5 inhibitors. Biochem Biophys Res Commun. 2005;334:930–938. doi: 10.1016/j.bbrc.2005.06.183. [DOI] [PubMed] [Google Scholar]
- 13.Murray F, MacLean MR, Pyne NJ. Increased expression of the cgmp-inhibited camp-specific (pde3) and cgmp binding cgmp-specific (pde5) phosphodiesterases in models of pulmonary hypertension. Br J Pharmacol. 2002;137:1187–1194. doi: 10.1038/sj.bjp.0704984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 15.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, Insel PA. Expression and activity of camp phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: Role for pde1. Am J Physiol Lung Cell Mol Physiol. 2007;292:L294–303. doi: 10.1152/ajplung.00190.2006. [DOI] [PubMed] [Google Scholar]
- 16.Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: Changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283:619–624. [PubMed] [Google Scholar]
- 17.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, Voswinckel R, Zhou J, Samidurai A, Klepetko W, Paddenberg R, Kummer W, Seeger W, Grimminger F. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: Target for reverse-remodeling therapy. Circulation. 2007;115:2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- 18.Dony E, Lai YJ, Dumitrascu R, Pullamsetti SS, Savai R, Ghofrani HA, Weissmann N, Schudt C, Flockerzi D, Seeger W, Grimminger F, Schermuly RT. Partial reversal of experimental pulmonary hypertension by phosphodiesterase-3/4 inhibition. Eur Respir J. 2008;31:599–610. doi: 10.1183/09031936.00002007. [DOI] [PubMed] [Google Scholar]
- 19.Phillips PG, Long L, Wilkins MR, Morrell NW. Camp phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2005;288:L103–115. doi: 10.1152/ajplung.00095.2004. [DOI] [PubMed] [Google Scholar]
- 20.Tian X, Vroom C, Ghofrani HA, Weissmann N, Bieniek E, Grimminger F, Seeger W, Schermuly RT, Pullamsetti SS. Phosphodiesterase 10a upregulation contributes to pulmonary vascular remodeling. PLoS One. 2011;6:e18136. doi: 10.1371/journal.pone.0018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aravind L, Ponting CP. The gaf domain: An evolutionary link between diverse phototransducing proteins. Trends Biochem Sci. 1997;22:458–459. doi: 10.1016/s0968-0004(97)01148-1. [DOI] [PubMed] [Google Scholar]
- 22.Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Burger A, Hatzelmann A, Tenor H, Schudt C, Krull M, Schutte H, Hippenstiel S, Suttorp N. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: Role in endothelial hyperpermeability. Blood. 2005;105:3569–3576. doi: 10.1182/blood-2004-07-2729. [DOI] [PubMed] [Google Scholar]
- 23.Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cgmp via phosphodiesterases 2 and 3. Circ Res. 2007;101:811–818. doi: 10.1161/CIRCRESAHA.107.154229. [DOI] [PubMed] [Google Scholar]
- 24.Haynes J, Jr., Killilea DW, Peterson PD, Thompson WJ. Erythro-9-(2-hydroxy-3-nonyl)adenine inhibits cyclic-3′,5′-guanosine monophosphate-stimulated phosphodiesterase to reverse hypoxic pulmonary vasoconstriction in the perfused rat lung. J Pharmacol Exp Ther. 1996;276:752–757. [PubMed] [Google Scholar]
- 25.Boess FG, Hendrix M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koenig G. Inhibition of phosphodiesterase 2 increases neuronal cgmp, synaptic plasticity and memory performance. Neuropharmacology. 2004;47:1081–1092. doi: 10.1016/j.neuropharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Baliga RS, Zhao L, Madhani M, Lopez-Torondel B, Visintin C, Selwood D, Wilkins MR, MacAllister RJ, Hobbs AJ. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:861–869. doi: 10.1164/rccm.200801-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L, Mason NA, Strange JW, Walker H, Wilkins MR. Beneficial effects of phosphodiesterase 5 inhibition in pulmonary hypertension are influenced by natriuretic peptide activity. Circulation. 2003;107:234–237. doi: 10.1161/01.cir.0000050653.10758.6b. [DOI] [PubMed] [Google Scholar]
- 28.Baliga RS, Milsom AB, Ghosh SM, Trinder SL, Macallister RJ, Ahluwalia A, Hobbs AJ. Dietary nitrate ameliorates pulmonary hypertension: Cytoprotective role for endothelial nitric oxide synthase and xanthine oxidoreductase. Circulation. 2012;125:2922–2932. doi: 10.1161/CIRCULATIONAHA.112.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falcetti E, Hall SM, Phillips PG, Patel J, Morrell NW, Haworth SG, Clapp LH. Smooth muscle proliferation and role of the prostacyclin (ip) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182 doi: 10.1164/rccm.201001-0011OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milsom AB, Fernandez BO, Garcia-Saura MF, Rodriguez J, Feelisch M. Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of no signaling products. Antioxid Redox Signal. 2012;17:422–432. doi: 10.1089/ars.2011.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubikat A, De Windt LJ, Zetsche B, Fabritz L, Sickler H, Eckardt D, Godecke A, Baba HA, Kuhn M. Local atrial natriuretic peptide signaling prevents hypertensive cardiac hypertrophy in endothelial nitric-oxide synthase-deficient mice. J Biol Chem. 2005;280:21594–21599. doi: 10.1074/jbc.M501103200. [DOI] [PubMed] [Google Scholar]
- 32.Kenny AJ, Stephenson SL. Role of endopeptidase-24.11 in the inactivation of atrial natriuretic peptide. FEBS Lett. 1988;232:1–8. doi: 10.1016/0014-5793(88)80375-2. [DOI] [PubMed] [Google Scholar]
- 33.Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, Keogh AM, Langleben D, Kilama MO, Fritsch A, Neuser D, Rubin LJ. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 34.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic gmp phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 35.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, Dostmann WR, Pozzan T, Kass DA, Paolocci N, Houslay MD, Zaccolo M. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an no/cgmp-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 37.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (esc) and the european respiratory society (ers), endorsed by the international society of heart and lung transplantation (ishlt) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 38.Moltzau LR, Meier S, Aronsen JM, Afzal F, Sjaastad I, Skomedal T, Osnes JB, Levy FO, Qvigstad E. Differential regulation of c-type natriuretic peptide-induced cgmp and functional responses by pde2 and pde3 in failing myocardium. Naunyn Schmiedebergs Arch Pharmacol. 2014 doi: 10.1007/s00210-013-0953-1. (ePub) [DOI] [PubMed] [Google Scholar]
- 39.Mehel H, Emons J, Vettel C, Wittkopper K, Seppelt D, Dewenter M, Lutz S, Sossalla S, Maier LS, Lechene P, Leroy J, Lefebvre F, Varin A, Eschenhagen T, Nattel S, Dobrev D, Zimmermann WH, Nikolaev VO, Vandecasteele G, Fischmeister R, El-Armouche A. Phosphodiesterase-2 is upregulated in human failing hearts and blunts beta-adrenergic responses in cardiomyocytes. J Am Coll Cardiol. 2013;62:1596–606. doi: 10.1016/j.jacc.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor CM, Gattis WA, Uretsky BF, Adams KF, Jr., McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M, Califf RM. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: Insights from the flolan international randomized survival trial (first) Am Heart J. 1999;138:78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 41.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, Hendrix GH, Bommer WJ, Elkayam U, Kukin ML, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The promise study research group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 42.Kerbaul F, Brimioulle S, Rondelet B, Dewachter C, Hubloue I, Naeije R. How prostacyclin improves cardiac output in right heart failure in conjunction with pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:846–850. doi: 10.1164/rccm.200611-1615OC. [DOI] [PubMed] [Google Scholar]
- 43.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–660. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 44.de Man FS, Handoko ML, van Ballegoij JJ, Schalij I, Bogaards SJ, Postmus PE, van der Velden J, Westerhof N, Paulus WJ, Vonk-Noordegraaf A. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ Heart Fail. 2012;5:97–105. doi: 10.1161/CIRCHEARTFAILURE.111.964494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.