Abstract
Fragile X syndrome (FXS), a severe neurodevelopmental anomaly, and one of the earliest disorders linked to an unstable (‘dynamic’) mutation, is caused by the large (>200) CGG repeat expansions in the noncoding portion of the FMR1 (Fragile X Mental Retardation-1) gene. These expansions, termed full mutations, normally silence this gene's promoter through methylation, leading to a gross deficit of the Fragile X Mental Retardation Protein (FMRP) that is essential for normal brain development. Rare individuals with the expansion but with an unmethylated promoter (and thus, FMRP production), present a much less severe form of FXS.
However, a unique feature of the relationship between the different sizes of CGG expanded tract and phenotypic changes is that smaller expansions (<200) generate a series of different clinical manifestations and/or neuropsychological changes. The major part of this chapter is devoted to those FMR1 alleles with small (55-200) CGG expansions, termed ‘premutations’, which have the potential for generating the full mutation alleles on mother-offspring transmission, on the one hand, and are associated with some phenotypic changes, on the other. Thus, the role of several factors known to determine the rate of CGG expansion in the premutation alleles is discussed first. Then, an account of various neurodevelopmental, congnitive, behavioural and physical changes reported in carriers of these small expansions is given, and possible association of these conditions with a toxicity of the elevated FMR1 gene's transcript (mRNA) is discussed.
The next two sections are devoted to major and well defined clinical conditions associated with the premutation alleles. The first one is the late onset neurodegenerative disorder termed fragile X-associated tremor ataxia syndrome (FXTAS). The wide range of clinical and neuropsychological manifestations of this syndrome, and their relevance to elevated levels of the FMR1 mRNA, are described. Another distinct disorder linked to the CGG repeat expansions within the premutation range is fragile X-associated primary ovarian insufficiency (FXPOI) in females, and an account of the spectrum of manifestations of this disorder, together with the latest findings suggesting an early onset of the ovarian changes, is given.
In the following section, the most recent findings concerning the possible contribution of FMR1 ‘grey zone’ alleles (those with the smallest repeat expansions overlapping with the normal range i.e., 41-54 CGGs), to the psychological and clinical manifestations, already associated with premutation alleles, are discussed. Special emphasis has been placed on the possibility that the modest elevation of ‘toxic’ FMR1 mRNA in the carriers of grey zone alleles may present an additional risk for some neurodegenerative diseases, such as those associated with parkinsonism, by synergizing with either other susceptibility genes or environmental poisons.
The present status of the treatment of fragile X-related disorders, especially FXS, is presented in the last section of this chapter. Pharmacological interventions in this syndrome have recently extended beyond stimulants and antipsychotic medications, and the latest trials involving a group of GluR5 antagonists aim to ascertain if these substances have the potential to reverse some of the neurobiological abnormalities of FXS.
INTRODUCTION
The trinucleotide expansion of CGG repeats in the 5′ untranslated region (5′-UTR) of the fragile X mental retardation 1 gene (FMR1) located at Xq27.3 was sequenced in 1991.1 Although the phenotype of fragile X syndrome (FXS) was first described by Lubs,2 it was not until 1991 that those with FXS were found to have >200 CGG repeats described as a full mutation in FMR1. Individuals who were carriers of smaller expansions between 55 to 200 CGG repeats (premutation) were originally thought to be unaffected clinically. However, in 1991 an elevated rate of premature ovarian failure (POF) was documented in carriers compared to controls3 and later confirmed by many other groups (reviewed in refs. 4,5).POF has been renamed the fragile X-associated primary ovarian insufficiency (FXPOI) to emphasize the association with the premutation and also the occasional ability of women to reproduce such that the ovary has not completely failed.6 Subsequently in 2001, the fragile X-associated tremor ataxia syndrome (FXTAS) was discovered in aging carriers7,8 and it includes not only tremor and ataxia but also neuropathy, autonomic dysfunction, neuropsychiatric problems and cognitive decline sometimes leading to dementia.9,10 This chapter delineates the history and development of the spectrum of involvement in these fragile X-associated disorders, with special emphasis on premutation (PM) carriers.
As the role of elevated FMR1 mRNA in carriers of the PM alleles11 has been researched and the concept of RNA toxicity leading to FXPOI or FXTAS has been developed12,13 a variety of additional phenotypes has been described in those carriers. They include developmental problems in a subgroup of young male carriers including autism, autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), shyness, anxiety and seizures.10,14-18 In many adults with the premutation including both males and females, psychopathology is common including anxiety and depression compared to controls.19-24 Cognitive changes, particularly executive function deficits, cna begin before the onset of FXTAS in carriers25 and there is evidence of early white matter disease reflected in diffusion tensor imaging changes before the onset of FXTAS.26 Most recently autoimmune dysfunction including fibromyalgia and hypothyroidism has been found to be more common in carriers compared to controls.22,27 Therefore a large spectrum of involvement associated with the premutation constitutes a newly emerging group of disorders leading to new avenues in research and clinical management.
LARGE EXPANSIONS OF CGG REPEAT—THE CAUSE OF FRAGILE X SYNDROME
Fragile X syndrome (FXS) was one of the earliest conditions found to be linked to the unstable mutation. It is the most common inherited form of intellectual disability caused by the large expansion in the FMR1 gene, where cognitive and behavioural impairments are associated with minor physical features.28 These expansions defined as ‘full mutation’ are usually associated with the methylation-coupled inactivation of FMR1 gene leading to gross deficit of the protein gene product, FMRP. 29,30 Abnormalities of FXS are primarily caused by the depletion of FMRP,31 and experimental evidence indicates that FMRP is essential for normal brain development.32-35 More specifically, studies based on rat brains have shown that FMRP is involved in synaptogenesis, especially in the cerebral cortex, cerebellum and hippocampus,36 and in modifying synaptic structure in response to environmental stimulation.37-40 FMRP is an RNA binding protein and it carries mRNAs to the synapse where it typically inhibits translation until stimulation occurs and then it allows translation.41 In the absence of FMRP there is dramatic up-regulation of protein production in the hippocampus.42 FMRP regulates the translation of hundreds of proteins and they include many of the proteins also associated with autism when they are mutated including Neuroligins 3 and 4, Neurorexins, SHANK3, amyloid precursor protein (APP), Arc, MAPKinase, CYFIP 1 and 2 and many more.43-48 There is also upregulation of mTOR in animals and humans with FXS49,50 similar to other forms of autism such as Tuberous Sclerosis.51 The molecular overlap between FXS and autism is likely the cause of the high rate of autism (30%) and also autism spectrum disorder (30%; ASD) in FXS.16,52-54 The behavioral phenotype also includes a high rate of anxiety disorders55 and ADHD.56-58 Significant mood instability leading to aggression or severe tantrums occurs in about 30 to 40% of adolescents or adults.28 This problem frequently leads to out of home placement and the use of psychotropic medication.
The physical phenotype of FXS includes a long face and prominent ears but approximately 30% of children will not have these features, although hyperextensible finger joints are seen in the majority along with double jointed thumbs. 28 Large testicles (macroorchidism) begin to occur at about 8 years of age and they reach their maximal size, typically at 2 to 3 times normal (60 ml) at about 15 to 16 years.59
Approximately 20% to 40% of individuals with FXS have mosaicism, meaning some cells with the premutation and some cells with the full mutation (size mosaicism) or a mixture of some cells with a lack of methylation and some cells with the full methylation (methylation mosaicism). Occasional individuals have a complete lack of methylation but a CGG repeat number >200. Those with a lack of methylation or those with a majority of their cells with the premutation typically have the highest levels of FMRP and they are often high functioning with an IQ > 70.31,60 Also, individuals with FXS and FMRP levels >30% to 50% of normal are likely to maintain an IQ above 70 as adults and demonstrate fewer physical features of FXS.31,61 Although these individuals seem to be better off in childhood and early adulthood, they may also have an elevated level of FMR1 mRNA because of an increased rate of translation compared to those with a full mutation that is fully methylated.62 The higher level of FMR1 mRNA may put them at risk for neurological disease with aging similar to those with the premutation.63 Hall et al63 reported a case of a male with a high level of mosaicism who experience signifiacnt neurodegeration in his 60s, although his diagnosis was consistent with Parkinson's disease (PD). Although there is concern for aging problems in those with mosaicism, a recent report of more than 60 individuals with FXS who were aging demonstrated no difference in the molecular studies in those who developed PD and those who did not.64 So far there has never been an individual with FXS who developed FXTAS, although PD is more common in aging in FXS than what is seen in the general population.64
Females with FXS are typically less affected than males because they have a second X chromosome. Their level of FMRP correlates with their activation ratio (the proportion of cells that have the normal X as the active X).30,31 Approximately 25 to 30 percent of females have an IQ below 70 but the majority of females have an IQ in the borderline or low normal range.65 The majority of girls or women with the full mutation experience some anxiety and common diagnoses are selective mutism, social phobia and specific phobia.55 ADHD is seen in at least 30% of women and executive function deficits are common even when ADHD is not apparent.66 Even women with a normal IQ and the full mutation can experience attentional problems and anxiety difficulties.67 Usually girls respond well to stimulant medication to treat ADHD and selective serotonin reuptake inhibitors (SSRIs) to treat anxiety.68 Counseling with a psychologist is also helpful for these problems especially if the women are under additional stress from having children with FXS and they need support and behavioral management guidance.69
SMALL CGG EXPANSION ALLELES AS THE SOURCE OF THE FULL MUTATION ALLELES
Apart from the flurry of studies on psychological and clinical manifestations of FXS, a major interest was in the origin of the large expansions leading to this severe developmental abnormality. According to the earliest reports, the mothers of the affected offspring did not manifest any obvious developmental anomalies reminiscent of those seen in FXS, but had a potential to generate offspring with this syndrome. Hence the term premutation (PM) was coined to reflect this potential, as well as the apparently unaffected status of the carriers of these PM alleles.70
The reason for, and the pattern of, these intergenerational differences were explained by the molecular data from large pedigrees which showed that these high risk parents carried small-size expansions of CGG repeat in the FMR1 gene, which did not cause gene silencing, but further expanded if transmitted from mothers to offspring into the full mutation range, with the rates of expansion increasing proportionally to the size of repeat.71-77 The detailed risk, as up-dated by Nolin and colleagues78 in a large multicentre study of female carriers (with correction for ascertainment) were as follows. For PM carriers: 3.7% for the repeat range of 55-59, 5.3% for the range of 60-69, 31.1% for the range of 70-79, 57.8% for the range of 80-89, 80.1% for the range of 90-99, between 94.4 and 100% for the range of 100-139 repeats, and 100% for repeat sizes >139 repeats. It was established that the PM alleles carried by the fathers were usually stable, and on occasions they might even be reduced in size on transmission to their daughters.
The introduction of PCR techniques79 allowed for the recognition of the potential for expansions of the alleles below the PM range (~35 to 54), and the risks for expansion of these small size (intermediate size, or GZ) alleles of 49-54 range were established.78,80-87 Instability in maternal transmission of GZ alleles over one generation has been reported as 2%-4% on average, with the possibility of expanding to the full mutation within two generations. The lowest small expansion allele reported to expand to the full mutation had 56 CGG repeats.88
Unlike for the premutation (PM) alleles, grey zone (GZ) alleles have a much higher instability rate (~16%) if transmitted through the male than the female carriers.89 It is the potential for expansion that determined the latest guidelines for an overall classification of FMR1 alleles.90 The upper bound of normal alleles (that is, showing no meiotic or mitotic instability) was set at ~44 CGG repeats; the range of intermediate size or GZ alleles (showing only minor increase or decrease in repeat number, but never expanding to full mutation) was set between 45 and 54 CGG repeats; and the alleles in the PM range (set between 55 and 200 repeats), if carried by females, showed a gradually increasing risk of expansion to the full mutation. The relevance of this classification in the light of the most recent molecular and clinical findings in carriers of small expansions will be discussed in the next sections.
The above figures represent an average risk for different categories of FMR1 alleles, but the individual risks may vary widely between different carriers, and between different families. In some families small expansions may be transmitted unchanged throughout several generations, and in others the risk of expansion from PM in the mother to full mutation in the offspring may be high or very high. This variation prompted a search for those molecular and other factors which might predispose to, or protect from, further expansions of the small CGG repeat size alleles. The major modifying factor is the sex of a parent, where, as described above, the risk of expansion of PM alleles is limited to the mothers but for the GZ alleles, it is relatively higher for the fathers than from the mothers.89 In addition, the data from large pedigrees suggested that the sex of offspring might be another modifying factor, with male offspring manifesting higher rate of large expansions from the parent than female offspring;91,92 however this concept still remains controversial.78,93
Some other molecular factors associated with FMR1 gene have been found to be influential in expansion of small size CGG repeats. Earlier hypothesis implicated a role for the number and position of AGG triplets. It has been argued that the AGG triplets interspersed within the CGG repeat tract stabilize this repeat, and in their absence increased length of ‘pure’ CGG are generated, which are more prone to replication slippage.80,94-98 However, more recent data suggested that the role of AGG is a late event in progressive CGG expansion83 although definitely related to the risk for expansion in the next generation.99
The role of molecular markers flanking the CGG repeat in the expansion process has been well established. In earlier studies, these markers comprised dinucleotide (CA) microsatellites which, in combination, generated polymorphic haplotypes.94,100 The frequencies of some of these haplotypes differed significantly between normal as opposed to fragile X both full mutation and PM alleles in all populations tested.97,101,102 The fact that the haplotype associations in the smallest expansion (GZ) alleles showed a pattern similar to this seen in fragile X alleles indicated that these alleles evolved from among GZ alleles.94,103,104
Currently, single nucleotide polymorphisms, SNPs, are more widely used and are now recognized as important markers in studies of fragile X, because of greater discriminatory power of large number of SNPs that can be tested to identify haplotypes that segregate with different CGG repeat size allele categories.104,105 The finding that the haplotypes combining the two SNPs, ATL1-alleles A and G104,106 and FMRb-alleles A and G103,106 exhibit strong associations with PM/full mutation, as well as with GZ alleles provided further evidence for the role of GZ alleles along the pathway leading from the normal to the fragile X full mutation alleles.
PHENOTYPIC FEATURES IN PM CARRIERS
The effect of small expansions on the phenotypes is an important problem considering their high population prevalence. The PM (using 55 repeats as the lower bound) is relatively common in the general population affecting 1 in 113 to 259 females and 1 in 260 to 813 males.83,107-111 Population prevalence of intermediate (GZ) size alleles is spectacularly higher reaching 1 in ~ 30 males;81,83,84 and may be at least twice as common in females.112,113 Therefore, any evidence for clinical and neuropsychological involvement in carriers of these alleles would have a significant bearing on the causes of health problems in a previously unrecognized but sizeable group of individuals.
Phenotypic effects of small expansions has taken a long time to be appreciated since the term ‘PM’ was first coined by Pembrey and colleagues in 198570 who stated that it ‘causes no harm other then predisposing to the final event’, with ‘final event’ being the full mutation associated with the FXS. It took more than a decade to change the belief in a clear-cut division of carriers of fragile X into two distinct categories: ‘affected’ with FXS, as opposed to unaffected ‘carrier females’ and ‘transmitting males’. Other than the finding of FXPOI in 1991,3 the earliest studies of small samples of male PM carriers,114,115 and both male and female carriers116 demonstrated minor yet significant physical and intellectual impairments. Physical problems found in approximately 25% of PM carriers included prominent ears and hyperextensible finger joints. 117,118 More stringent analysis of cognitive status used genetic statistical models based on pedigree data, which allowed for adjusting the measures obtained from the carriers for these measures in other relatives. This analysis provided evidence for a significance effect of PM on standard cognitive (Wechsler) measures, including performance IQ, Block Design, Perceptual Organization, and Object Assembly, in the adult male carriers and Symbol Search, in the adult female carriers,119 as illustrated in Figure 1.
Figure 1.
Standardized percent deviation of FSIQ-adjusted summary and subtest (Wechsler) scores from the normal mean due to the effect of premutation (upper figure), and full mutation (lower figure) separately for males and females, based on pedigree models. The baseline represents the FSIQ of a 100.119 Reproduced from Loesch DZ et al. Am J Med Genet A 2003; 122A(1):13-23.119
A significant effect of PM irrespective of age was alos established, using pedigree models, on some FSIQ-adjusted executive function test scores.120 These included impairment of the motor planning, inhibition and working memory measured by the Behavioural Dyscontrol Scale (BDS),121 in the male carriers, and of visuospatial memory and visuospatial constructional ability, assessed by Rey Complex Figure Design and Recognition Test (RCFT),122 in the female carriers. Similar findings of significant impairments on tests of executive function (Verbal Fluency, Trail Making Test and Tower of London) and memory were reported in a sample of 20 unrelated male PM carriers.123 This data may indicate that the CGG expansion alleles within the PM range may affect specific neuronal circuits manifesting as relevant neuropsychological deficits. These deficits may also include, specifically for the female sex, impairment in arithmetics as reported in 39 PM women based on performance on the Wide Range Achievement Test-3,124 visual pathway deficits involving low social/high temporal frequency contrast sensitivity and frequency-doubling veneer,125 and motion perception deficits.126 That the PM alleles affect brain functioning has been also shown in CGG knock-in (KI) female mice where, apart from deficits in processing special information, poor performance on tests of temporal order of presenting two objects, specifically related to the upper-end of PM range (150-200 CGGs) was encountered.127 This latter finding is reminiscent of the results of a recent study based on 60 female PM carriers, where a (significant) decrease in IQ (Wechsler Scales) verbal and performance scores was more evident for the females in the upper PM range (CGG>100), and with a 10% decrement of FMRP expression.128
Although these findings have been largely based on adult males and females, the overlap of the observed cognitive deficits with some of those seen in FXS and their relevance to minor FMRP deficits and the upper end of PM range, indicated a significant neurodevelopmental component in the origin of these changes. The presence of the associated behavioural deficits, including autism spectrum, and attention deficit hyperactivity disorder (ADHD), in boys carrying the PM allele, support this notion.14,16,129 Another supportive piece of evidence has been provided by demonstration of several minor physical anomalies in unrelated PM male and female carriers, which are typical of the FXS phenotype,117,118 and in families,130 as well as of the distinct abnormalities in brain structure on MR images.131-134 Notably, reduced hippocampal volume was found to be associated with memory deficits in both male and female carriers,131 or with anxiety-related psychological symptoms in female carriers.134 In another study, the wide range of structural brain anomalies, including reduced volume of the whole brain, caudate and thalamic nuclei bilaterally were found to be associated with changes in metabolic rates in several brain areas in female carriers.132 The results of the study, which used fMRI to measure brain responses to fearful faces and fearful social images, demonstrated diminished activation of amygdala and several other brain areas that mediate social cognition in the group of PM adult male carriers compared with age and IQ-matched controls.135 This reduction was also significantly associated with self-report of psychological symptoms on the Symptom Checklist-90-revised (SCL-90-R). A significant association between reduced activity and volume of the hippocampus during a memory recall task, and psychological symptom severity, was also reported in two other studies based on male136 and female134 PM carriers.
It is clear from these and other reports (to be discussed below) that it is difficult to draw the clear demarcation line between the phenotypic abnormalities which can be related to neurodevelopmental changes, and those determined by the late onset progressive brain pathology. While the first category of changes appears to represent a mild version of physical and neuropsychological anomalies linked to the full mutation that may be related mainly to subtle deficits of FMRP at the upper end of the PM range, 31,137 evidence for the late onset age-dependent changes suggest alternative or additional major pathogenic effects, related to the elevated levels of expanded FMR1 transcript, which may account for the late neurodegenerative changes, or for both neurodegenerative and neurodevelopmental abnormalities (as detailed in the later sections of this chapter).
The finding of age-dependent deficit in the ability to inhibit prepotent responses,138 and in working memory tasks, particularly executive control of memory,139,140 in a sample of 40 adult PM male carriers, may additionally illustrate an overlap between these 2 dimensions. The progress in severity of cognitive decline was shown to be more striking in the older age group of carrier males aged >50 years, with 33.3% penetrance of dementia for the average age of 63.4 years, which represented sixfold increase compared with noncarriers, and further increasing with age and allele size.141 However, a critical review of neuropsychological status of male and female PM carriers142 recommends caution in the interpretation of these data and the use of larger samples obtained from several sources.
Although no psychiatric pathology has been found in the earliest study of female carriers,143 it has been widely reported among numerous subsequent studies of adult carrier males and females. In a large comprehensive study by Franke et al in 1998144 an overall rate of a lifetime diagnosis of affective disorders was found as high as 55.7%; of anxiety disorders, 41%; of panic disorder, 11.5%; and 18% was a lifetime risk of social phobia, which was independent on the effect of having children with FXS. Although distinct schizoaffective disorders have only been reported in rare individuals,145 schizotypal features and avoidant personality disorder occurred in respectively 8.2% and 4.9% of carriers.144 Later studies provided confirmatory evidence for the high prevalence of mood, especially depressive disorders, in female PM carriers. The Spanish study based on a small sample of PM carriers and mothers of FXS children showed higher scores for depression (based on SCL-90-R and Beck Depression Inventory) compared with noncarrier mothers of mentally retarded children.146 In the much larger study by Roberts et al in 2009,147 93 PM females were compared to 2,159 controls from the National Comorbidity Survey Replication (NCS-R) US dataset. The results based on Structured Clinical Interview for the DSM-IV (SCID-1) showed a significant increase in the risk of lifetime major depressive disorder (43% in carriers compared with 31.9% controls), which is close to the earlier estimate of nearly 60% risk of all affective disorders by Franke et al.144 The risk of lifetime panic disorder and current agoraphobia were not high (8.6% and 3.2%, respectively), but they were significantly elevated compared with control results. Interestingly, the risks were higher in the lower end of PM range, which suggested that the link of the affective disorders as seen in the carriers with minor FMRP depletion is unlikely.
Significantly higher levels of obsessive-compulsive disorder were reported in the later study of 122 female and 26 male carriers of PM alleles.20 Increase in depression was reported in 34 PM carrier mothers of FXS children who were compared, using SCL-90 and Beck Depression Inventory, with 39 noncarrier mothers of mentally retarded child and 39 mothers from the general (Spanish) population.146 Notably, the results of a survey of 199 female and 57 male PM carriers from North Carolina enrolling in a national survey of families of children with FXS showed that the range of problems such as anxiety, depression or learning difficulties in females, and attention problems, aggression and anxiety in males, tend to cluster in the same individuals.148 These confounding effects may be additional reasons for difficulties in the phenotypic risk estimates for the carriers, and thus for conflicting results from different studies, which may have already been affected by small sample sizes, inadequate psychometric measures, and biases related to preselection of the tested or control samples. In the large study by Hunter and colleagues in 2008,149 the effect of small CGG expansions on mood and anxiety was investigated using regression models (adjusting for potential confounders) in a sample of 119 male and 446 female PM carriers aged 18-50 ascertained from either fragile X families or the general population. The results have shown that repeat length was (marginally) associated with depression and negative affect in males, and with negative affect in females, which suggested that, although PM carriers may be at risk of emotional morbidity, the effect of repeat size appears subtle. However, considering the later finding by Roberts et al19 that the lower end of the range of PM alleles may have the largest effect on the risk of mood disorder, the linear model149 analysis may not have been the most appropriate one for probing this kind of a relationship.
TOXICITY OF THE ELEVATED FMR1 TRANSCRIPT CONTRIBUTES TO ABNORMAL PHENOTYPES
Search for possible pathophysiological mechanisms involved in abnormal phenotypes or specific disorders related to PM alleles resulted in a discovery of an elevated level of FMR1 transcript (mRNA) in peripheral blood lymphocytes of PM carriers,11 which was up to 10-fold higher than normal in carriers of the higher end (>100) alleles, and 2-4 times higher than normal in carriers of the lower end (55-100) alleles. The original finding was confirmed and expanded in several later studies.150-155 This elevation results from increased transcriptional activity rather than an increased mRNA stability,11,151,156 and direct evidence for an increased transcription rate has later been obtained using a nuclear run-on experiment.157 The elevated FMR1 transcript levels were found to be associated with the size of CGG repeat expansion within the PM-size range.11,150,152,153,155 The data obtained from 63 male and 61 female carriers (with the effect of inactivation accounted for in the females) showed that this relationship was linear and consistent in both sexes.150 However, in the more recent study of 90 PM females using piecewise regression model, the relationship between mRNA levels and CGG repeat was shown to be much stronger above the threshold of 90-100 repeats after correction for the activation ratio (AR); whereas without the correction, a significant regression was only limited to the lower portion (<100) of PM range.158 These findings have also provided evidence for skewed inactivation in females carrying PM alleles with >100 repeats, and these data emphasize the need for applying the relevant correction in correlation analyses involving molecular and phenotypic changes. A recently proposed statistical method of covariate adjusted correlation analysis between CGG repeat number and FMR1 mRNA, accommodating both additive and multiplicative effects of activation ratio nonparametrically,159 will allow the more accurate evaluation of the effect of this ratio on molecular measures in females.
FRAGILE X-ASSOCIATED TREMOR/ATAXIA SYNDROME (FXTAS)
Clinical Manifestations and Prevalence in Males
The fragile X-associated tremor/ataxia syndrome (FXTAS), a recently identified form of late-onset (>50 years) neurodegenerative disorder, was first described in a sample of five older PM carrier males who were the grandfathers of children with FXS.7 The major clinical features of this progressive disorder comprise intention or postural/ action tremor, cerebellar gait and limb ataxia, and parkinsonism.7-9,160-164 Although severe rigidity typical of Parkinson's disease is uncommon, mild rigidity in the upper limbs was reported in 71% of FXTAS patients, 40% out of this group manifested mild resting tremor, and 57% showed bradykinesia.165 The three scales recognized as the measures of tremor (Clinical Rating Scale for Tremor, CRST), ataxia (International Cerebellar Ataxia Scale, ICARS), and parkinsonism (Unified Parkinson's Disease Rating Scale, UPDRS) were used in several different studies of male PM carriers, and these scores were found significantly higher compared with age and sex-matched controls.162,163,165,166 Gait ataxia usually starts from balance problems manifesting as difficulty with tandem walking and progresses till the person is unable to walk without support or is wheel-chair bound. The tremor most often involves upper extremities, with postural/action tremor initially, and resting tremor at the later stages. Autonomic dysfunction and neuropathy have also been reported,7,160,165,167 and the latter has been confirmed by the results of a tibial nerve conduction study showing significantly slower velocity and prolonged F-wave latency in typical FXTAS patients compared with controls and unaffected PM carriers.168 The analysis of the progression of the major motor signs conducted in 55 patients showed that tremor was usually the first sign of the disorder occurring at the median age of 60 years; median delay of onset of ataxia was 2 years; onset of falls, 6 years; dependence on walking aid, 15 years; and death, 21 years.163 Approximately 60% of male PM carriers manifesting tremor/ataxia also show increased T2 signal intensity in white matter of the MCP on MR images.169 Other MR imaging findings include widespread cerebral, cerebellar and brainstem atrophy, as well as patchy or confluent areas of hyperintensity on T2-weighted images in periventricular and deep white matter of the cerebral hemispheres and corpus callosum.8,165,166,170 Patchy or confluent T2 hyperintensities were recently reported in the basis pontis of a female affected with FXTAS,171 and our own observations in the affected male carriers suggest that these changes may have been underreported.172 The pattern of white matter pathology as seen on MR images and histological preparations in FXTAS is distinct from that associated with hypertensive vascular disease,173 with the changes being in the form of various degrees of spongiosis and loss of axons and myelin in the areas shown as having T2 hyperintensities. An example of the typical white matter and other changes as recorded on MR images of a PM carrier affected with FXTAS is given in Figure 2.
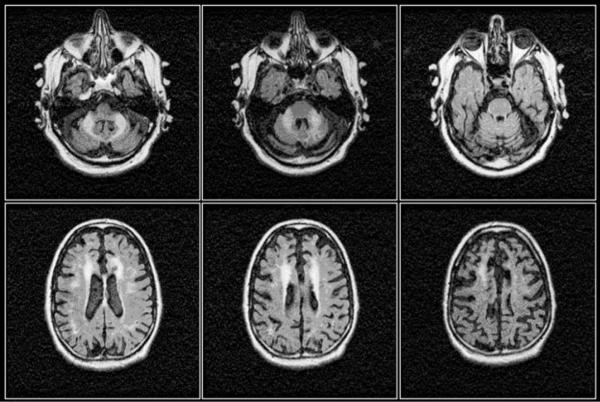
Figure 2.
Axial FLAIR images of the brain of the 64 year old male carrier of 80 CGG repeats with typical manifestations of FXTAS comprising marked cerebellar ataxia, intention tremor, autonomic failure (originally diagnosed with multiple system atrophy, MSA). Marked bilateral T2 hyperintense signals within the middle cerebellar peduncles (MCP sign), combined with symmetrical T2 hyperintense signals within the corona radiate and considerable cerebral and cerebellar atrophy are evident from the images.166 Reproduced from Loesch DZ et al. Clinical Genetics 2005; 67(5):412-417.
The frequencies of these characteristic abnormalities are significantly higher in the affected carriers than in age and sex matched controls.169,170,174 Confirmatory evidence for the relevance of these changes to the effect of PM alleles have been provided by the MRI volumetric studies, showing significant volume loss ofthe cerebellum, brainstem, thalamus, hippocampus and cerebral cortex in the affected carriers compared with age-matched normal controls.133,169,173,174
The characteristic neurohistological changes of FXTAS are ubiquitin-positive intranuclear inclusions abundant in both neuronal and astrocytic nuclei throughout the cerebrum, cerebellum (apart from Purkinje cells) and brainstem, being most numerous in the hippocampal formation (~50%, contrasting with only 5%-10% in cerebral cortex); the inclusions were also found in autonomic neurons and astrocytes of the spinal cord.176 Although they contain about 20 different proteins, they are negative for PAS, tau, silver, polyglutamine and alpha synuclein,173,176 which distinguishes them from the intranuclear inclusions of the CAG repeat (polyglutamine) disorders, and obviously from cytoplasmic inclusions associated with multiple system atrophy or Lewy body dementia (Fig. 3). The same inclusions were also found in neuronal nuclei of a KI mice carrying expanded (~100 CGG) repeat in the mouse gene.177 Although the inclusions were not originally seen in the astrocytes in the KI mouse they have subsequently been documented in astrocytes in addition to neurons as in PM carriers.178
Figure 3.

Eosinophilic inclusions in the nucleus of both neurons (left panel) and astrocytes (right panel) from a patient with FXTAS. Picture courtesy of Claudia Greco MD and Paul Hagerman MD, PhD.
Cognitive Deficits and Psychiatric Symptoms
The earlier studies of cognitive functioning of men with FXTAS showed marked impairment of executive cognitive abilities, with only slightly lowered performance on standard cognitive IQ scores.179,180 Moreover, significantly higher scores on the Neuropsychiatric Inventory (NPI) scales (total, aggression, depression, apathy and irritability) in the smaller sample of FXTAS males compared with normal controls were reported and interpreted as indicative of fronto-subcortical dementia.181 In another study of 15 patients with FXTAS, twelve were diagnosed with dementia, and seven others with mood and anxiety disorders.182 A larger study of 68 adults with FXTAS, including 50 males and 18 females, found dementia in approximately 50% of men that was similar in severity to Alzheimer's dementia.183 However, none of the 18 females withFXTAS demonstrated dementia or significant cognitive impairment.183 These data, considered in context with disease duration, indicated that deficits in executive cognitive functioning (ECF), working memory and speed, as well as capacity of information processing, combined with obvious personality changes, typically represent the early stages of FXTAS. Amongst the most affected aspects of executive functioning is the initiation of purposeful goal directed activity, and the inhibition of inappropriate or irrelevant behaviour. Standard cognitive measures using Wechsler scale usually reveals deficits of working memory represented by Arithmetic and Digit Span scores, whereas other verbal IQ subtests, especially language and verbal comprehension, are relatively unaffected. The Folstein Mini Mental State Examination (MMSE) usually remains within normal limits until the late stages of a disorder because it does not include the assessment of executive functioning. In the latest stages of the disorder, however, severity of dementia is fast progressing as the result of diffuse deficits in other cognitive functions. That these deficits are related to FXTAS has been shown by the results of a study based on 109 men divided into FXTAS+ carriers, FXTAS- carriers, and noncarriers. This data showed that, while FXTAS- carriers performed worse than controls on some aspects of ECF, FXTAS+ carriers performed worse than controls on working memory, recall of information, declarative learning and memory, information processing speed and temporal sequencing, as well as on ECF tests.25 On the other hand, there is obvious overlap, especially in ECF impairments, between FXTAS and asymptomatic carriers, with the former showing more severe and widespread deficits. This overlap may be further illustrated by the latest study based on analysis of functional magnetic resonance imaging (fMRI) BOLD signals obtained during the performance of verbal working memory from 15 FXTAS+, 15 FXTAS− and age matched normal control individuals.26 The results have shown that activation of prefrontal cortex, which may underlie executive and memory deficits, was reduced in both carrier samples compared to normal controls; in addition, FXTAS+ group showed reduced activation of the right ventral inferior frontal cortex.
A large scale comprehensive longitudinal study is still required to establish the sequence of different aspects of cognitive and neuropsychiatric changes in carriers.9 This is because their relevance to FXTAS has not yet been clearly established, considering that, although neuropsychiatric manifestations often follow the establishment of motor disability, some, such as anxiety or depression, also occur in carriers not affected with FXTAS, or prior to the onset of neurological changes.
Prevalence and Atypical Manifestations
The initial clinical and genetic descriptions of male PM carriers preselected for tremor and ataxia was shortly followed by estimates of prevalence of this syndrome by screening of unselected population of PM carriers over 50 years of age ascertained through fragile X families with at least one child diagnosed with the FXS. The results of two parallel studies conducted in the US165 and Australia166 provided compelling evidence for the association between the PM carrier status and neurological involvement. The US study showed age-related increase in prevalence of tremor/ataxia, ranging from 17% to 75% between the age of 51 and 85, with the overall prevalence of 37% for this age range. A similar study based on Australian families generated an overall prevalence of 41.7% for the comparable age range. In both studies, an excess of manifestations of tremor/ataxia in the sample of PM carriers was significant compared to the sample of noncarriers matched for age. In a more recent study of 44 male PM carriers aged >50 years and ascertained through 151 fragile X Spanish families from Barcelona 45.5% of respondents surveyed over the phone reported tremor, balance problems, falls and/or gait ataxia.22 However, the extensive variability of neurological manifestations needs to be considered while interpreting the prevalence results, since these estimates were biased towards considering syndromic manifestations rather than any less specific clinical changes indicating a significant neurological involvement. Shortly after the first description of FXTAS stringent diagnostic criteria were recommended, which included intention tremor or gait ataxia, and MCP sign (‘definite’ FXTAS), with less stringent criteria including problems of executive function deficits and brain atrophy incorporated into possible or probable FXTAS.8 Thus, the combination of intention tremor and gait ataxia, and parkinsonism, was classified as ‘probable’ FXTAS and the combination of intention tremor or gait ataxia and cerebral white matter lesion and atrophy, as ‘possible’ FXTAS. Modifications of the FXTAS diagnostic criteria was made with addition of the FXTAS inclusions inthe definite criteria.12
However, it appears that neurological involvement in PM carriers does not always meet those criteria, as illustrated by a number of published cases,166,184-186 and those known through personal communications or our unpublished observations. Some carriers manifested typical MCP change with minimal or no clinical signs of tremor/ataxia, and others showed cerebral white matter lesions without the MCP sign, combined with some atypical neurological manifestations, psychiatric manifestations or autoimmune disease such as fibromyalgia and/or hypothyroidism22,27 or multiple sclerosis.187,188 Severe and fast progressing dementia was a major feature in a 62-year-old PM carrier, who at the later stages of his condition manifested typical FXTAS signs including gait ataxia and white matter degeneration including MCP sign.189 A more recent study in France demonstrated a high rate of cognitive changes even before the onset of tremor or ataxia in carriers compared to controls.141
Another atypical form reported in a 58-year-old female PM carrier presented with dementia and parkinsonism, and typical ubiquitin positive intranuclear inclusions in neurons and astrocytes, but no MCP sign or ataxia.190 The occurrence of low symptomatic or atypical manifestations determined by the same major etiological factor as a typically diagnosed case of FXTAS warrants the term ‘FXTAS spectrum’, which better reflects the fact that neurological, cognitive and psychiatric involvements associated with neurodegenerative changes in PM carriers extend beyond the classical definition of FXTAS. This new definitionalso covers the cases of co-occurrence ofsome of the clinical features ofFXTAS with other neurodegenerative disorders, such as Parkinson's disease with Lewy bodies and Alzheimers disease, which will cause a more rapid decline in function than is seen in FXTAS without other neurodegenerative disorders. However, there are still clinical problems associated with the PM that may not be on the FXTAS spectrum, such as the autoimmune disease, but do seem to be related to the RNA toxicity and as the molecular mechanisms are clarified, a deeper understanding of these effects will be known.
The observed variability of manifestations is predictable considering complex mechanisms which might be involved in the origin of late onset neurological involvement, where the toxicity of the expanded FMR1 transcript may interact with the number of other genetic or environmental effects throughout the lifespan (as detailed in Pathology section below), all contributing to the late-onset neurodegenerative changes, with FXTAS representing the most severe form of involvement. The occurrence of features of both FXTAS and Alzheimer's disease in a carrier of PM189 is a good illustration of such combined effects towards more severe and complex manifestations; on the other hand, some other factors may be protective in nature thus determining less severe or vestigial manifestations, as well as the fact that at least 30% of individuals carrying PM alleles do not show any neurological involvement, even at advanced age. FXTAS appears to be clustered in families and a family without any neurological manifestations appears to have a lower incidence of involvement in subsequent members. Although the rates of FXTAS are low in daughters of men who have had FXTAS, other symptoms such as sleep disturbances, psychopathology and intermittent tremor are increased in these women as they age compared to women who do not have a father with FXTAS.191
Genotype-Phenotype Relationships
Confirmatory evidence for the link between the effect of FMR1 small expansion alleles and specific neurodegerative processes has been provided by the results of genotype-phenotype correlations. The earliest data showed that FMR1 CGG repeat length was significantly associated with the total brain volume loss in a sample of male PM carriers aged >50 years.175 This association was also reported in a sample of male carriers affected with FXTAS.169 A strong significant association was foundbetween CGG repeat number and the percentage of neurons and astrocytes with intranuclear inclusions in the cortical grey matter and hippocampus;173 a similar association was found between the number of repeats and the proportion of inclusion-bearing neurones in dentate gyrus and inferior colliculus in the expanded (CGG)n mice.192 Some important relationships have also been reported between the number of CGG repeats and clinical outcomes. A negative correlation was found between CGG repeat number and the age of death173 and age of onset of motor signs in FXTAS.193 In a sample of male PM carriers who were not preselected for FXTAS diagnosis, CGG repeat size was found associated with the degree of motor impairment represented by all three motor rating scales,194 and both neuropathic features195 and abnormal nerve conduction.168 Only a few of those relationships were also significant in female carriers.
Relationships between the phenotypic changes and elevated FMR1 mRNA levels in PM carriers are less obvious than those with the size of CGG repeat expansion. This is not unpredictable since, while there is close correspondence between the size of CGG expansion betweenblood and brain cells,196 there are blood-brain discrepancies between the levels of mRNA transcript.154 Nevertheless, significant relationships between the elevated FMR1 mRNA and psychological symptoms measured by SCL-90-R Global Severity Index was reported in 68 male and 144 female PM carriers both with and without a diagnosis of FXTAS,20 with the relationships predominantly concerning obsessive-compulsive disorder and psychoticism. Moreover, in another study, a (negative) correlation was found between the levels of FMR1 mRNA and the right ventral inferior frontal activity on fMRI during performance on working verbal memory test in 30 female PM carriers (15 with and 15 without FXTAS) compared with 12 matched healthy controls.26 However, since the studies concerned with such relationships have been scarce, global interpretation of the findings so far is limited.
Since the discovery of FXTAS, there have been several studies from different locations assessing the prevalence of this syndrome in neurological disorders including essential tremor, cerebellar ataxia, Parkinson's disease, and multiple system atrophy (MSA).197-210 Overall, the prevalence of PM alleles amongst groups of patients with late onset movement disorders has been lower than expected.211 The diagnostic groups with the highest prevalence of PM carriers were males over 50 with cerebellar ataxia, with the overall prevalence of 17/872, 2%, ranging from 0%-7%197-199,201,208 and individuals with multiple system atrophy-cerebellar type (MSA-C), with an overall prevalence of 2% with a range of 0% to 3.95%.204,208 Surveys of male patients originally diagnosed with atypical,200 or typical203,205,212 Parkinson's disease (PD) have failed to show a statistically significant excess of PM carriers. Three carriers were found among 776 patients with idiopathic PD213 and, although this was at least a three-fold greater rate than the normal population prevalence, it was not statistically significant. However, a significant excess of PM male carriers in the sample comprising typical and atypical idiopathic PD was reported in the latest screening study.214
Clinical Manifestations and Prevalence of FXTAS in Females
FXTAS has also been reported in female PM carriers.22,206,215-220 However, it occurs at a much lower rate (~8% to 16.5%) than in male carriers, and clinical manifestations and cognitive decline tend to be milder and occur at a later age than in men; this is largely because ofthe protective role ofthe second X chromosome.211,216-218 In another study22 based on a sample of 85 Spanish females aged >50 years, FXTAS symptoms were encountered in 14 (16.5%) individuals using less stringent diagnostic criteria.
Brain changes as seen on MR images are also less severe in FXTAS females than in their male counterparts. Although a significant reduction in brain volumes and increased severity of white matter disease were found in a FXTAS group of 15 females compared with 20 age-matched controls,169 these changes were less pronounced than in a FXTAS group of 36 males; the MCP sign occurred in only 13% of affected females compared with 58%, in males, and there was no significant association between the volumetric measures and either the severity of FXTAS manifestations, or CGG repeat expansion. However, females presenting with typical symptoms of FXTAS had greater medical comorbidity than males.27 In this comprehensive study of 146 PM female carriers aged 20-75 years and 69 age-matched controls, 18 carriers (12.3%), who met the criteria of FXTAS diagnosis, had significantly higher prevalence by history of thyroid disorders (50%), hypertension (61.1%), fibromyalgia (43.8%), diagnosed peripheral neuropathy (52.9%), and persistent muscular pain (76.5%) compared to controls. In non-FXTAS group of carriers, the frequencies of chronic muscle pain, paraesthesias in extremities and history of tremor, were significantly elevated compared with normal controls. The size of CGG repeat and the levels of mRNA were both significantly higher in the FXTAS than in non-FXTAS groups, but the ARs were surprisingly not different. The more recent similar study based on a larger sample of 280 female PM carriers from Spain (with 195 between 4-50 years of age and 85 older than 50 years) found lower cormorbidity than reported in the American sample with thyroid disease (15.9%), and muscle pain (24.4%).22 However females, just as their male counterparts, may present with atypical clinical manifestations of FXTAS, such as in the case of a 58-year-old PM female recently described,190 who developed progressive dementia and parkinsonian signs, but neither action-intention tremor nor ataxia or MCP sign. Magnetic resonance imaging, however, showed extensive subcortical wmd, consistent with the distribution of wmd on post-morten examination, which showed white matter spongiosis in this region, as well as widespread ubiquitin-positive intranuclear inclusions in both neurons and astrocytes.
To date there has been no systematic study of cognitive status of females affected with FXTAS. But the analysis ofpsychological symptoms obtained from the SCL-90-R showed a significantly elevated somatization, obssessive-compulsive symptoms, depression, and overall symptom severity in a sample of 22 women affected with FXTAS.20
Pathogenesis of FXTAS
The associations of FXTAS and of the other related neurological manifestations with CGG repeat expansion within the PM range, the elevation of the expanded FMR1 mRNA, as well as the presence of this mRNA in the intranuclear inclusions, gave rise to the hypothesis of a toxic ‘gain-of-function’ effect of excessive FMR1 mRNA on brain tissue.7,151,173,176 This concept originated from pathogenesis of myotonic dystrophy,221 where another (CUG) repeat expansion in the gene's promoter leads to sequestration of Muscleblind-like 1 (MBNL1) protein found in the intranuclear inclusions, leading to dysregulation and splicing of several other mRNAs and thus clinical involvement through deficit of the relevant proteins.222,223 According to this model hypothesised for small CGG expansions in FMR1 mRNA, the ‘toxic’ effect of elevated transcript leads to dysregulation of specific candidate proteins, such as purine-rich element binding protein (Pur α), or nuclear ribonucleoprotein A2/B1 (hnRNP A2/B1), which are sequestrated from their normal function and deposited in cells’ nuclei causing their premature death. In support of this claim, the lack of Pur α protein in knock-out (KO) mice resulted in severe loss of neurones in the hippocampus and cerebellum, and development of tremor and seizures at two weeks of age,224 whereas over-expression of this protein alleviated manifestations of neurodegeneration in a fly FXTAS model.225 However, it has also been speculated that the elevated toxic RNA may not be directly involved in the sequestration of these proteins, but may act as a trigger of stress responses leading to over-expression of candidate proteins involved in neuroprotection.13,178,225 Increased stress responses to toxic gain of function of FMR1 mRNA with expanded CGG repeat have most recently been shown in cultured human fibroblasts and CNS tissue obtained from PM carriers.226 These data provide evidence for a somewhat modified model of a toxicity of the expanded mRNA, in which increased expression of this transcript results in altered expression and disruption of the nuclear Lamin A/C architecture and induction of the expression of stress response genes. Since this type of cellular pathology may be induced within a very short time frame, the authors suggest that FMR1 mRNA-induced abnormal skeletal organization in neural cells may take place in early development, thus underlying both neurodevelopmental and late-onset clinical conditions relevant to the PM carrier status. The risk and the type of manifestations of the latter may be determined by the presence and the nature of additional genetic effects varying between individuals, as well as some environmental factors such as pollutants.227
Identification of the contents of intranuclear inclusions has been an important step towards better understanding of the mechanisms leading to FXTAS.151,228,229 Unlike in many other disorders associated with unstable repeat expansions, there was lack of a principal protein, and ubiquinated proteins represented only a minor component in the inclusions’ deposits, which indicated that their formation is unlikely to result from accretion of specific abnormal proteins. The minor proteins deposited included lamin A/C, two RNA directly binding proteins: Pur α, hnRNP A2/B1 and MBL1, along with the FMR1 mRNA. Recent evidence of the sequestration of Sam 68, an important RNA splicing protein,230 and the recent evidence of DROSHA sequestration in the inclusions suggests that dysregulation of miRNAs is involved in the toxicity of the premutation.231
Although precise mechanisms leading from the elevated RNA to neurodegeneration still remain speculative, sufficient evidence fordirect RNA toxicity hasbeenprovidedby the results ofanimal and cell based studies (reviewed in: Garcia-Arocena and Hagerman 201013). The earliest findings in Drosophila melanogaster showed that human PM-size CGG repeat transcripts ectopically expressed in neural eye tissue caused neurodegeneration in a dosage- and repeat length-dependent manner.232 Furthermore, the ubiquitin-positive intranuclear neuronal inclusions and neurodegeneration similar to that in human PM carriers affected with FXTAS were observed in the FMR1 KI mice;177 these mice also manifested cognitive decline, neuromotor, and behavioural disturbances associated with the elevated mRNA.233 Further evidence for the RNA with the expanded CGG repeats to cause neurodegeneration in FXTAS was provided by the findings showing that this RNA expressed in Purkinje neurons of experimental mice outside the context of FMR1 mRNA resulted in neuronal pathology presenting as intranuclear inclusions, neuronal death and behavioural changes.234 In the most recent study of PM KI mice the presence of ubiquitin-positive intranuclear inclusions were found in neurons throughout the brain in cortical and subcortical regions increasing in number and size with advanced age;127 importantly, these inclusions were also present in protoplasmic astrocytes including Bergman glia in the cerebellum, which might have some relevance to a particularly severe white matter neurodegeneration in cerebellar peduncles.
The typical inclusions have also been found in the anterior and posterior pituitary gland in humans as well as in KI PM mice and throughout the limbic system including the amygdala235-237 which imply the possibility of a dysfunction of hypothalamus-pituitary-adrenal gland axis, and may explain some of neuroendocrine and psychiatric problems seen in carriers with and without obvious FXTAS. The inclusions have also been located in the Leydig and mucoid cells in the testicles obtained from human PM carriers which may explain the low testosterone levels and frequent onset of impotence before the tremor and ataxia of FXTAS.235
Another important step towards understanding of pathophysiological mechanisms of broader neuropathology linked to the RNA with expanded CGG repeats has been made in the latest study based on female KI mice heterozygous for the PM allele, where cultured neurones manifested dendritic changes and lower cell viability.238 This finding, combined with the recent report of abnormal cellular distribution of lamin A/C isoforms in embryonic fibroblasts of KI mice with the expanded CGG repeat in the murine FMR1 gene226 point to both neurodegenerative and neurodevelopmental effects of the expanded-repeat mRNA; notably, in the same study, altered lamin A/C expression/organization and increased stress response were also found in cultured skin fibroblasts and CNS tissue from male PM carriers either with or without FXTAS. These data provide strong evidence for biological processes underlying the postulated link between early onset or mid-life pathologies associated with elevated levels of FMR1 transcript, such as behavioural and cognitive deficits, psychiatric symptoms or FXPOI (described later in this chapter), on the one hand, and late-onset neurodegenerative disorders, on the other.
Additional difficulty in understanding the individual steps leading from the toxic RNA to changes at the cellular and clinical level may be related to the likely involvement of other factors and processes interacting with, and modifying the effects of the expanded toxic RNA. After all, only certain proportions of PM carriers are affected with either neurodevelopmental or neurodegenerative conditions, or present the symptoms ofboth of them. Clearly, individual genetic make-up, including background genes of minor effects, contributes to the variability in penetrance at the individual as well as the family level. The role of environmental exposure, especially to neurotoxic agents, postulated in context with the severity of expression ofFXTAS,166 and recently demonstrated by several clinical examples227 should not be underestimated. This aspect is especially relevant considering the latest evidence suggesting the involvement of mitochondrial dysfunction in pathogenesis of neurological involvement in PM carriers based on fibroblast and brain samples,239 and on blood leucocytes from PM and GZ allele carriers.240 Finally, the contribution of the recently identified antisense FMR1 mRNA, which is also elevated in PM carriers241,242 to neurotoxicity, needs to be further investigated. Our preliminary data240 have shown that the elevated levels of this transcript may play a pivotal role in the severity of neurological involvement in the carriers of small expansion FMR1 alleles.
FRAGILE X-ASSOCIATED PRIMARY OVARIAN INSUFFICIENCY
Another distinct disorder linked to the FMR1 PM alleles is Primary Ovarian Insufficiency (POI). This term was proposed243 to reflect, apart from the cessation of menstrual periods before age 40, a spectrum of diminished ovarian functions occurring in female carriers of the FMR1 PM alleles. The more specific and currently used term of ‘Fragile X-associated Primary Ovarian Insufficiency’ (FXPOI) was introduced to describe any degree of POI attributable to the FMR1 aetiology.244 FXPOI affects approximately 20% of PM carriers, consistently in many population/ethnic groups,245-249 representing 20-fold increase compared with the risk of 1% seen in the general population. Estimated frequencies of PM carriers among females reporting to infertility clinics with familial or sporadic FXPOI were: 11.5% with 95% CI: 5.4%-20.8%, for familiar sample; and 3.2% with 95% CI: 1.4%-6.2%, in sporadic sample, compared with the population frequency of these carriers of ~0.4%, as reviewed by De Caro et al.244
Notably, data obtained4 from over 500 women with a wide range of CGG repeats from the normal range into the high end of the PM range demonstrated a significant association between CGG repeat number and prevalence of FXPOI that was nonlinear. For those with a repeat <40 the prevalence of POI was 0.9%, for those with 41-58 repeats it increased to 2.2%, for those with 59-79 repeats it was 5.9%, for those with 80-99 repeats it reached 18.6%, but for those with ≥100 repeats the prevalence decreased to 12.5%. These authors suggested that perhaps those with a high CGG repeat number may have some ovarian target cells with a full mutation, or some cells have early methylation at a lower CGG repeat number that protects them from the toxicity of the PM. An overall effect of the CGG repeat size on age of menopause has been reported,4 with low end CGG repeats demonstrating menopause 2.5 years earlier than controls, and medium to high end PM carriers demonstrating menopause 4 years earlier than low- end carriers. Nonlinear association between the CGG repeat number and the risk of FXPOI and age of menopause was later confirmed in different samples.250-252 In the 2007 study based on data from 948 women with the wide range of repeats sizes, the mean age of menopause for carriers of 59-79 repeats and >100 repeats was similar (48.5 and 47.5, respectively), but was ~3 years lower (44.9) in carriers with 80-100 repeats. Although the highest decrease in menopausal age was in carriers of CGG expansions with the 80-100 range, it was reduced across the total PM range compared with 52.3 + 0.5 years found in the sample of noncarriers.
Although the FMR1 PM represents a common major gene affecting the age of menopause in particular, and the female reproductive system in general, other factors modifying this effect should be considered. Smoking has been shown to aggravate this major effect in carriers of PM by 1 year. The identity of other major genes possibly interacting with the effect of FMR1 has not yet been established. However, the effect of the background genes (residual genetic variance) in the presence of the major effect of the FMR1 PM, was recently estimated in a study based on 230 fragile X families and 219 families from the general population.253 The analysis used a random effect version of the Cox proportional hazards model allowing for shared polygenic (additive) effects and the effects of confounders.254 The results confirmed significant major effect of FMR1 PM, with the greatest effect of mid-size group, and provided evidence of a substantial additive (background) genetic component even after adjusting for the major effect; estimated additive genetic variance ranged from 0.55 to 0.96 (depending on different model assumptions), with P-values ranging from 0.0002 to 0.0027. Examples from single families further illustrate a significance of shared genetic effect on the risk of menopausal anomalies. In one family255 premature menopause occurred in all six sisters carrying PM; in another family256 two sisters, one PM carrier and one noncarrier, presented with POI. The reported examples of menopausal problems co-existing with the other FMR1 PM- associated conditions such as FXTAS or psychiatric involvement in one and the same carrier,220 or between two generations of carriers191 are also relevant to a significance of shared family effect, which should be considered in individual risk estimates and counselling.
The features reflecting pathology of the reproductive system in PM females extend beyond, though are not irrelevant to, the earlier menopausal age. The earliest and most comprehensive endocrine study of the hormonal profile of female PM carriers demonstrated that those carriers that were cycling normally had endocrine dysfunction.243 These authors studied 11 normally ovulating PM carriers (ages 23 to 41 years) and demonstrated a significantly shortened cycle, elevated follicle stimulating hormone (FSH) throughout the cycle (91% with elevations >2 SD above the mean), elevated Inhibin -B in the follicular phase, and elevated Inhibin- A and progesterone in the luteal phase compared to controls. These findings suggested a decreased number of follicles and granulosa cell dysfunction or decreased cell number in the corpus luteum compared to controls. In addition, 45% (5 of 11) of these carriers had a history of infertility as defined by 1 year of unprotected intercourse without a pregnancy. This study demonstrated sub-clinical ovarian dysfunction in PM females who do not have POI. For women at the premenopausal stage there was a CGG repeat, and AR effect on the FSH level, but only when controls and carriers were included together. Consistent finding of an increase in FSH serum levels in PM carrier who were still cycling was reported in the other studies.257,258 This effect remained after adjusting for smoking and use of contraceptives257 or age and shared family effects.258 However, significantly increased FSH levels were limited only to women aged 30-39, which suggested late onset of ovarian dysfunction.4 But the results of the later study showed that this dysfunction may occur at an earlier age. Using another biomarker of ovarian reserve, mullerian-inhibiting substance (AMH), which, unlike FSH, is only expressed in growing follicles, a significant reduction of the AMH levels was shown for all age groups within the 18-50 years range in the higher (>70 repeats) versus lower (<70 repeats) risk PM carriers.244 Some other abnormalities within the FXPOI spectrum include irregular menses,245,252 shorter cycles,243,252 and irregular length and skipped cycles in the mid-size repeat PM carriers;252 in the same group of carriers these authors also found increased twinning. Moreover, the findings of significant decrease of mineral bone density259 inPM carriers compared with normal controls, and a significant increase in osteoporosis, with the highest increase of 11.9% in the mid-range PM carriers based on large samples,260 are of particular concern.
The mechanisms involved in ovarian insufficiency in small expansion carriers are still unknown. The fundamental question to be answered is what stage of individual growth/development is most relevant to the damaging effect of the processes related to these expansions. Do these events lead to a smaller ovarian endowment already present at birth? Do they lead to alteration of recruitment of follicles later in life? Or do they lead to follicular atresia and thus premature degeneration? It is also unknown if these processes directly influence the ovarian tissue, or act via an altered hypothalamo-pituitary-gonadal axis, especially considering that the typical intranuclear inclusion formation has been found in neurons of the anterior and posterior pituitary gland in a male PM carrier.235 Although these changes have not yet been investigated in female carriers, it has been postulated that these degenerative changes in gonadotropic cells lead to dysfunction of hypothalamic-pituitary-gonadal axis and thus to abnormal follicular recruitment.244 Alternatively, FXPOI may result from direct damage to the ovaries either by increased FMRP levels during specific stages of development, or by the ‘gain of function’ toxic effect of elevated FMR1 mRNA leading to premature ovarian degeneration later in life.244 The recent important finding of an overproduction of FMRP among carriers of 80-89 repeat alleles261 may give some support to the hypothesis of this protein's related damage to ovaries, since most clinical manifestations of FXPOI have been most prominent in carriers of medium size repeat PM alleles.
The lack of convincing evidence for any anomalies in the age of menarche or around menarcheal age in PM carriers259,262 suggest that the changes related to the effect of this PM occur at later stages of reproductive life. However, one study252 found a small but significant difference in the age of menarche only in a subgroup of low repeat PM carriers (12.18 ± 1.38 in the carriers compared with 12.44 ± 1.51 in noncarriers), although overall the age of menarche was not different from controls. Considering scarcity and inconsistency of the data concerning adolescent period in female carriers, more studies are required to identify the onset and the progress of FXPOI, which might be related to the specific effect of FMR1 PM. Evidence that it may extend over to the adolescence period has been provided by data showing that the onset of menopause was below the age 30 in 30% of PM carriers,247,263 and in rare cases, it was at the age of 18,256,264 and the lowest reported ages were between 13 and 17 years in three carriers.263
DO THE INTERMEDIATE SIZE (GZ) ALLELES CAUSE ABNORMAL PHENOTYPES?
Understanding of phenotypic effects of GZ alleles is important considering their bridging position between the common and fragile X (PM and full mutation) allele categories, as indicated by haplotype associations (discussed above). Most importantly, given their high population frequency of~3-4 per 100 males,83,84,97,112,265 even a small effect contributing to the developmental or late-onset conditions, if substantiated, is likely to be borne by a considerable number of individuals.
The GZ alleles, with the originally recommended range of 45-54 CGGs,90 but varying between different studies (reviewed by Mitchell et al84) have, until recently, been included in the normal category with regard to phenotypic expression. However, the breakthrough has come with a demonstration of the elevated FMR1 transcript in a sample of 32 Australian GZ male carriers identified in the SEN (special educational needs) population.266
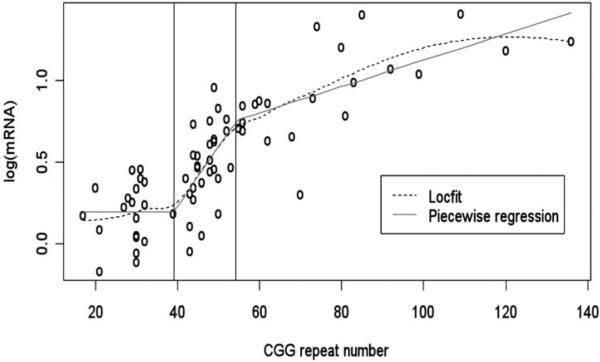
This study also showed that, if the regression is applied across a wider range of FMR1 alleles, including both GZ and PM allele categories, the sharp increase in mRNA levels that is proportional to the number of CGG repeats seen in the GZ range, slows down in the PM range (Fig. 4). The onset of this disturbance indicated by this study's data is at approximately 39 repeats; however more observations, especially at the upper end of the ‘normal’ alleles, are required to estimate this threshold more precisely. In the earliest study of small CGG expansions that included only two GZ carriers, increased transcription and a slight decrease in translation of the gene was also reported.155
Figure 4.
Nonparametric local fit (Locfit) and piecewise regression fit to the log-transformed mRNA levels versus CGG repeat numbers for data from all three allelic categories: Normal, GZ and PM.266 Reproduced from Loesch DZ et al. J Med Genet 2007; 44(3):198-204.266
Findings from another study, based on human cell lines transfected with the FMR1 5′-UTR containing CGG repeat lengths ranging in size from 0 to 99 repeats and a downstream reporter gene, confirmed a transcription defect in GZ class of alleles.156 Although a different reporter gene (luciferase and not FMR1) gene was used, the results demonstrated an increase in transcription levels for constructs possessing either PM or GZ alleles, further supporting the later findings based on human subjects.266 If, indeed, the elevated levels of FMR1 mRNA cause neurodevelopmental and neurodegenerative changes in PM carriers, one can postulate that the elevated levels observed in carriers of GZ alleles, even if more moderate, could cause conditions reminiscent of those seen in a significant proportion of PM carriers.
However, in contrast with the PM alleles, establishing the pathogenic role of the GZ allele is not straightforward. First, these alleles have been relatively poorly defined with respect to repeat size, and the range of this category has differed between different studies (reviewed in refs. 83,84,97,112,267). Second, measurement of CGG repeat number is not consistently performed across all laboratories, and comparisons between studies may not be valid. Third, unlike for PM alleles, GZ alleles cannot, except for rare cases, be identified through cascade testing of fragile X families, so that the prevalence and the type of phenotypic involvements cannot be determined in an unbiased manner. On the other hand, the results obtained from screening population groups with specific disorder or developmental delay would be biased and thus inconclusive. Therefore, the most stringent approach would be to test an entirely unbiased population, such as a series of consecutive newborns initially screened for FMR1 CGG size, and their phenotypes subsequently assessed. Such an undertaking, while ideal, would be logistically daunting. The next best is to study a well defined population of affected individuals, and to compare the CGGn profile with that of an appropriate control group.
The earliest systematic study based on screening of a well defined population preselected for significant learning problems (SEN) suggested phenotypic (neurodevelopmental) effects of GZ alleles.267 The results provided evidence for an increased frequency of these alleles in this tested population compared with that in control X chromosomes. However, the studies have notbeen consistent, with some confirming this association,82,89 and others failing to do so.96,268,270 One study82 found that 4.4% of 3,732 UK SEN males had GZ alleles, which represented a significant increase compared with non-SEN samples. Our own study conducted in the population of 1253 SEN male students from Tasmanian schools also found increased frequency of GZ alleles (3.45%) compared with 2.43% in 578 consecutive male births in the same state.84 Although the frequency figures are similar to those obtained in the earlier studies, the SEN-control difference was insignificant. The possibility of an increased risk of learning orbehavioural problems in carriers of GZ alleles has also been suggested by the fact these carriers are sometimes identified among children with learning or behavioural deficits attending genetic or psychiatric clinics. A summary of physical and neuropsychological test results from a sample of such children, combined with the carriers identified through comprehensive testing of fragile X families (the total of 10 boys aged 4-15 years, including 6 PM and 4 GZ carriers), was presented.15 A majority of participants manifested psychiatric (autism spectrum and/or hyperactivity), psychological (expressive language and/or other minor cognitive impairments), and several physical characteristics (all normally associated with the full mutation). However, no relationship was found between clinical affectedness and either CGG repeat size or FMRP levels. Although descriptions of this kind are informative, such data are not amenable to statistical comparison with normal samples, because of ascertainment bias caused by preselection of participants for the traits examined in the study. Nevertheless, the results of a screening of a small but well defined sample of children diagnosed with autism spectrum disorders (ASDs) suggested a significant role of GZ alleles in the riskforthis condition, and also suggested possible mechanisms involved in their effect.271 It is clear from these results that the question whether or not GZ alleles are associated with neurodevelopmental problems is still unresolved and requires further population-based study, proper controls and more sensitive and specialized assessment tools.
The stronger evidence for phenotypic effects of GZ alleles has been provided by the data showing a significant association of these alleles with FXPOI spectrum of abnormalities.272,273 Grouping the alleles into sizes of 35-54 and 55+ repeats, of the women having suffered POI, 14.2% (15 of 106) had an allele in the range of 35-54, whereas in the general population the figure was 6.5% (21 of 322).272 The increase in the risks is significant, and the data speak for an effect of the CGG repeat length, whether GZ or PM, upon ovarian function.249 In the later study of small samples (27 POI and 32 control women), six POI (22%) women and one control woman (3%) carrying FMR1 alleles of >40 repeats, were identified, and this represented a significant increase in the POI sample.274 An interesting new perspective on this issue has been provided by the latest finding, though based on a small samples of 23 tested and 11 control individuals that the fall in ovarian reserve represented by the FSH and AMH markers is already apparent in carriers of repeats >30.275 In another study, the rise in serum concentration of FSH was associated with CGG repeat size within the normal range but exceeding 30 CGGs, both in 80 females with POI and 70 controls.276
Since FXTAS has been another late-onset condition found associated with a toxicity of elevated RNA in PM carriers, a similar, though less apparent, association may be expected for GZ carriers with elevated expanded FMR1 mRNA. In rare studies, the small samples of patients with Parkinson's disease spectrum were screened for the GZ alleles and, although there was a trend towards the increased prevalence (reaching 7%), no significant excess of these alleles relative to their population prevalence has been reported.205,212,213 However, a significant relationship between the size of CGG repeat within the GZ range and cognitive decline and hallucinations was reported in one of those studies.213 Among 507 patients (253 male, 254 female) with MSA, 3.6% of males and 3.9% of females had a GZ of size 40-54, comparing with 3.2% and 4.2%, respectively, in controls;204 in a smaller French series of 77 patients, five had a GZ of size 41-54, for a fraction of 6.5%, this being somewhat higher, although not significantly so, than the control figure of 3.4% (4/117).208
In the latest study,214 228 Australian males affected with parkinsonism were screened for GZ, as well as PM alleles. Importantly, this sample included broader diagnostic group of the primary parkinsonian syndromes according to the classification in Schapira et al.277 The frequencies of either of these alleles were compared with the frequencies in a population-based sample of 578 Guthrie spots from consecutive Tasmanian male newborns (controls). Apart from a significant excess of PM, there was also a more than 2-fold increase in GZ carriers, with odds ratio (OR) = 2.36, and 95%confidence intervals (CI): 1.20-4.63, compared with controls.
These results have suggested that both PM and GZ alleles may contribute to the risk of parkinsonism, and that the ‘toxic’ effect of expanded mRNA linked to the PM alleles may also occur when the expansions are smaller. It is possible that the modest elevation of ‘toxic’ RNA, such as is the case in the GZ and lower-end PM carriers, may present an additional risk for neurodegeneration by synergizing with other susceptibility genes for parkinsonism. This notion is supported by common occurrence of parkinsonian manifestations amongst patients primarily diagnosed as FXTAS (as detailed in previous section).
Detailed clinical and genetic testing was performed in 14 carriers of the small expansion FMR1 alleles encompassing the GZ and the lower-end PM range.240 This was in order to determine whether these alleles present a significant modifying factor for the manifestations of parkinsonian disorders. The results were compared with the same data obtained from 24 noncarriers. Both GZ and PM carriers presented with more severe symptoms than a sample of noncarriers matched for age, diagnosis, and treatment. The Parkinson's (UPDRS) motor score and the measures of cognitive decline (MMSE and/or ACE-R scores) were significantly correlated with the size of the CGG repeat, and the (elevated) levels of antisense FMR1 and Cytochrome C1 mRNAs in blood leucocytes. In addition, the carriers showed a significant depletion of NADH dehydrogenase (ND) subunit 1 (ND1) gene mitochondrial DNA in whole blood. These data have shown that both small CGG expansion FMR1 alleles may play a significant role in the acquisition of the parkinsonian phenotype, possibly through the cytotoxic effect of elevated sense and/or antisense FMR1 transcripts involving mitochondrial dysfunction and leading to progressive neurodegeneration. Notably, evidence for mitochondrial involvement was also presented in another study based on fibroblasts and brain samples from PM carriers affected with FXTAS.239
In conclusion, although the results on phenotypic involvements of GZ alleles are still sketchy, there is sufficient evidence to postulate their role in the aetiology of certain human conditions affecting the brain and reproductive system. Since the expansion size and thus the elevation of the FMR1 transcripts is less than in the PM carriers, the effect may be more subtle and more difficult to detect, especially as the carriers cannot be, as is the case with PM carriers, ascertained through fragile X families. Moreover, if the effect of FMR1 mRNA toxicity in GZ carriers is not sufficient to be the sole determinant of a disease such as FXTAS, it may increase their risk of other disorders that are likely to involve mitochondrial dysfunction, such as Parkinson's disease or multiple sclerosis. This hypothesis requires testing via further comprehensive studies based on large samples.
Apart from the possible effect of small CGG expansions/elevation of FMR1 mRNA on the phenotype, the results of the latest studies239,240,278 have thrown new light on the ambiguity currently associated with the definition of GZ alleles. We determined a threshold of ~39 CGGs for the onset of a progressive increase in FMR1 transcription.266 Although more data are required to narrow the error margins, it appears that the lower bound for the GZ currently defined as 45CGGs90 should be revisited in the light of these findings. Moreover, these data also suggested a continuous scale of involvement, especially at the lower end of PM range, rather than the clear-cut separation between GZ and PM categories recommended90 solely on the basis of the potential for CGG expansion. It appears both from the elevation of the FMR1 transcript in GZ alleles, and the observed effects of these alleles on the phenotype that the difference between these two categories is quantitative in nature rather than qualitative. The phenotypic effects reported so far in GZ carriers, including POI, late onset neurological involvement, autism, and possibly other behavioural problems and learning problems are similar to these seen in PM carriers. The elevation of the FMR1 transcript, as well as the linear relationship between the levels of mRNA and the number of CGGs, are other common features of both the GZ and PM FMR1 alleles and thus the mechanism of this elevation must also be similar.
PRESENT STATUS OF TREATMENT OF FRAGILE X-RELATED DISORDERS
Over the years a variety of pharmacological interventions has been developed for treatment of FXS related to the full mutation68 and also for PM associated disorders including FXTAS.10,279 Typically treatment ofboth FXS and FXTAS involves multiple professionals and multimodality intervention that have been described at length elsewhere.10,280
For the psychopharmacology interventions in those with FXS both stimulants and alpha agonists, such as guanfacine and clonidine, can be helpful for treatment of ADHD.68 However, the stimulants are usually not started until after 5 years of age because of frequent side effects in those under 5 years. Selective serotonin reuptake inhibitors (SSRIs) are commonly used to treat anxiety and hyperarousal and early treatment may help with language and socialization benefits.68 In addition, the atypical antipsychotic aripiprasole (Abilify) has been frequently used with generally good success for anxiety and aggression.281 Aripiprazole will also stabilize mood in addition to treating ADHD symptoms, but often a lower dose is better tolerated without activation.68
The most exciting development in the treatment ofFXS is the introduction of targeted treatments that can reverse some of the neurobiological abnormalities that have been identified in the animal models of FXS. The up-regulation of the metabotropic glutamate receptor 5 system (mGluR5) leading to long term depression (LTD) described by Huber et al282 and Bear et al283 has lead to the use of mGluR5 antagonists that have been shown to be helpful in the animal models of FXS40,284,285 and are now demonstrating benefit in individuals with FXS.286 At least three mGluR5 antagonists have been developed and 2 are undergoing assessments in adults with FXS in phase II trials with plans for broad phase III trials in the near future. Another way of decreasing the level of glutamate at the synapse is with the use of a GABAB agonist, specifically Arbaclofen, the right isomer of baclofen. Studies in mice and a phase II trial in children and adults aged 6 to 40 years suggest benefit in those with FXS plus autism and FXS with low sociability.287 These promising results in FXS and preliminary results in those with autism without FXS have lead to phase III trials in both disorders.
The use of minocycline for 1 month after birth in the KO mouse led to benefits in behaviour and cognition in addition to improvements in the immature dendritic structure that is typical for FXS.288 Minocycline appears to work by lowering matrix metalloproteinase 9 (MMP9) levels in the KO mouse which are elevated compared to wild type mice. The results of the Bilousova trial stimulated use of minocycline by families of children with FXS and a subsequent survey of parents regarding the benefits and side effects of minocycline suggested that about 70% of children improved on minocycline particularly in language and behaviour.289 These preliminary results are being followed by a controlled trial of minocycline in children with FXS between the ages of 3.5 years and 16 years. Side effects of minocycline include graying of the tooth enamel when used under 8 years old although occasional occurrence of graying of skin and nails has been reported in older individuals. Minocycline can rarely cause pseudotumor cerebri, a lupus-like rash and seropositivity of the antinuclear antibody (ANA), so regular follow-up of these patients is necessary.
Lastly the use of nutritional supplements including antioxidants have demonstrated benefit in the treatment of the KO mouse with improvements in behaviour and testicular size and this is related to the findings of oxidative stress in the KO mouse.290 Melatonin which can be helpful for the sleep disturbances in FXS291 also seems to have an antioxidant effect in the mouse.292
The targeted treatments will hopefully strengthen synaptic connections in FXS, but these patients will also need to use educational interventions before the intellectual disabilities of FXS can be reversed.293 However, the future looks bright for the treatment of FXS and further studies have been planned. The treatment of PM disorders is currently symptomatic, including the treatment of FXTAS10,279 although neuroprotective agents such as memantine are currently being studied. Further research into the molecular mechanisms leading to RNA toxicity will certainly lead to more trials in both animal models and humans.
CONCLUSION
The fragile X field has changed remarkably since the discovery of the FMR1 gene in 1991. The nonpenetrant status of the premutation carrier has expanded into an array of clinical involvement influencing psychiatric, endocrinologic, neurocognitive and neurologic health. The clinician must be alert to clinical involvement in all generations of a family identified with a proband. Screening studies of high risk populations will identify many more individuals affected by the mutations in FMR1 and the premutation will be an important additive component to many common problems of aging including motor and cognitive decline. We are living in an age of targeted treatments and the portal disorder of FXS will open the doors to new treatment endeavors in autism and other disorders that have overlapping molecular involvement because of FMRP's ubiquitous presence and influence. This is an exciting time for clinicians focused on targeted treatments and FXS is likely to be the first neurodevelopmental disorder whose phenotype will be reversed.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Child Health and Human Development Grant HD 036071 to Dr RJ Hagerman and Dr DZ Loesch, NINDS RL1AG032115 to Dr Hagerman and NHMRC project grant No 330400, to Dr DZ Loesch. We thank Jacky Au for his excellent work regarding the preparation of this manuscript.
REFERENCES
- 1.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 2.Lubs HA. A marker X chromosome. American Journal of Human Genetics. 1969;21(3):231–244. [PMC free article] [PubMed] [Google Scholar]
- 3.Cronister A, Schreiner R, Wittenberger M, et al. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991;38(2-3):269–274. doi: 10.1002/ajmg.1320380221. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan AK, Marcus M, Epstein MP, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20(2):402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 5.Wittenberger MD, Hagerman RJ, Sherman SL, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87(3):456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 7.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 8.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. American Journal of Human Genetics. 2003;72(4):869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry-Kravis E, Abrams L, Coffey SM, et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics and testing guidelines. Mov Disord. 2007;22(14):2018–2030. doi: 10.1002/mds.21493. [DOI] [PubMed] [Google Scholar]
- 10.Tassone F, Berry-Kravis E, editors. Fragile X-associated Tremor Ataxia Syndrome (FXTAS) Springer-Verlag New York Inc.; New York, NY: 2011. [Google Scholar]
- 11.Tassone F, Hagerman RJ, Taylor AK, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. American Journal of Human Genetics. 2000;66(1):6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagerman PJ, Hagerman RJ. The fragile-X premutation: a maturing perspective. American Journal of Human Genetics. 2004;74(5):805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19(R1):R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27(2 Suppl):S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 15.Aziz M, Stathopulu E, Callias M, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet. 2003;121B(1):119–127. doi: 10.1002/ajmg.b.20030. in press. [DOI] [PubMed] [Google Scholar]
- 16.Clifford S, Dissanayake C, Bui QM, et al. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37(4):738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 17.Goodlin-Jones B, Tassone F, Gane LW, et al. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25(6):392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Bailey DB, Jr., Raspa M, Olmsted M, et al. Co-occurring conditions associated with FMR1 gene variations: findings from national parent survey. American Journal of Medical Genetics. 2008;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JE, Bailey DB, Jr., Mankowski J, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(1):130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- 20.Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B(1):115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 21.Cornish K, Kogan C, Turk J, et al. The emerging fragile X premutation phenotype: evidence from the domain of social cognition. Brain and cognition. 2005;57(1):53–60. doi: 10.1016/j.bandc.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009;17(10):1359–1362. doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourgeois JA, Coffey SM, Rivera SM, et al. A review of fragile X premutation disorders: expanding the psychiatric perspective. J Clin Psychiatry. 2009;70(6):852–862. doi: 10.4088/JCP.08m04476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois J, Seritan A, Casillas E, et al. Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. J Clin Psychiatry. 2010 doi: 10.4088/JCP.09m05407blu. doi: 10.4088/JCP.4009m05407blu, Published online by Physicians Postgraduate Press, Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22(1):48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto RI, Backer KC, Tassone F, et al. An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS). Journal of Psychiatric Research. 2010 doi: 10.1016/j.jpsychires.2010.04.030. doi:10.1016/j.jpsychires.2010.1004.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. American Journal of Medical Genetics. 2008;146A(8):1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagerman RJ. Physical and behavioral phenotype. In: Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, Treatment and Research. 3rd edition The Johns Hopkins University Press; Baltimore: 2002. pp. 3–109. [Google Scholar]
- 29.Pieretti M, Zhang FP, Fu YH, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 30.Tassone F, Hagerman RJ, Ikle DN, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84(3):250–261. [PubMed] [Google Scholar]
- 31.Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10(1):31–41. doi: 10.1002/mrdd.20006. [DOI] [PubMed] [Google Scholar]
- 32.Comery TA, Harris JB, Willems PJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Prof Natl Acad Sci USA. 1997;94(10):5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devys D, Lutz Y, Rouyer N, et al. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nature genetics. 1993;4(4):335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- 34.Hinds HL, Ashley CT, Sutcliffe JS, et al. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993;3(1):36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- 35.Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10(10):1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 36.Witt R, Kaspar B, Brazelton AD, et al. Developmental localization of fragile X mRNA in rat brain. Soc Neurosci. 1995;21(1):293–296. [Google Scholar]
- 37.Weiler IJ, Greenough WT. Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination. Am J Med Genet. 1999;83(4):248–252. doi: 10.1002/(sici)1096-8628(19990402)83:4<248::aid-ajmg3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Weiler IJ, Irwin SA, Klintsova AY, et al. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. PNAS. 1997;94(10):5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolen G, Carpenter RL, Ocain TD, et al. Mechanism-based approaches to treating fragile X. Pharmacol Ther. 2010;127(1):78–93. doi: 10.1016/j.pharmthera.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Oostra BA, Willemsen R. FMR1: a gene with three faces. Biochim Biophys Acta. 2009;1790(6):467–477. doi: 10.1016/j.bbagen.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin M, Kang J, Burlin TV, et al. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25(20):5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darnell JC, van Dreische S, Zhand C, et al. HITS-CLIP Identifies Specific Neuronal mRNA Targets of Translational Repression by the Fragile X Mental Retardation Protein, FMRP [abstract].. Paper presented at: Keystone Symposia; Snowbird, UT. 2010. [Google Scholar]
- 44.Hagerman R, Hoem G, Hagerman P. Fragile X and autism: intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1(1):12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zalfa F, Eleuteri B, Dickson KS, et al. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10(5):578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahlhaus R, El-Husseini A. Altered neuroligin expression is involved in social deficits in a mouse model of the fragile X syndrome. Behavioural Brain Research. 2010;208(1):96–105. doi: 10.1016/j.bbr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Miyashiro KY, Beckel-Mitchener A, Purk TP, et al. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37(3):417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 48.Darnell JC, Mostovetsky O, Darnell RB. FMRP RNA targets: identification and validation. Genes, Brain and Behavior. 2005;4(6):341–349. doi: 10.1111/j.1601-183X.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- 49.Sharma A, Hoeffer CA, Takayasu Y, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30(2):694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tassone F. mTOR up-regulation in patients with FXS [abstract].. Paper presented at: FRAXA Investigators meeting; Durham, NH. 2010. [Google Scholar]
- 51.de Vries PJ. Targeted treatments for cognitive and neurodevelopmental disorders in tuberous sclerosis complex. Neurotherapeutics. 2010;7(3):275–282. doi: 10.1016/j.nurt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris SW, Hessl D, Goodlin-Jones B, et al. Autism profiles of males with fragile X syndrome. Am J Ment Retard. 2008;113(6):427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatton DD, Sideris J, Skinner M, et al. Autistic behavior in children with fragile X syndrome: prevalence, stability and the impact of FMRP. American Journal of Medical Genetics. 2006;140(17):1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann WE, Cortell R, Kau AS, et al. Autism spectrum disorder in fragile X syndrome: communication, social interaction and specific behaviors. Am J Med Genet. 2004;129A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- 55.Cordeiro L, Ballinger E, Hagerman R, et al. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders. doi: 10.1007/s11689-010-9067-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munir F, Cornish KM, Wilding J. A neuropsychological profile of attention deficits in young males with fragile X syndrome. Neuropsychologia. 2000;38(9):1261–1270. doi: 10.1016/s0028-3932(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan K, Hatton D, Hammer J, et al. ADHD symptoms in children with FXS. American Journal of Medical Genetics. 2006;140(21):2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- 58.Cornish KM, Turk J, Hagerman R. The fragile X continuum: new advances and perspectives. J Intellect Disabil Res. 2008;52(Pt 6):469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 59.Butler MG, Pratesi R, Watson MS, et al. Anthropometric and craniofacial patterns in mentally retarded males with emphasis on the fragile X syndrome. Clinical genetics. 1993;44(3):129–138. doi: 10.1111/j.1399-0004.1993.tb03863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagerman RJ, Hull CE, Safanda JF, et al. High functioning fragile X males: demonstration ofan unmethylated fully expanded FMR-1 mutation associated with protein expression. Am J Med Genet. 1994;51(4):298–308. doi: 10.1002/ajmg.1320510404. [DOI] [PubMed] [Google Scholar]
- 61.Wright-Talamante C, Cheema A, Riddle JE, et al. A controlled study of longitudinal IQ changes in females and males with fragile X syndrome. Am J Med Genet. 1996;64(2):350–355. doi: 10.1002/(SICI)1096-8628(19960809)64:2<350::AID-AJMG23>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 62.Tassone F, Hagerman RJ, Loesch DZ, et al. Fragile X males with unmethylated, full mutation trinucleotide repeat expansions have elevated levels of FMR1 messenger RNA. Am J Med Genet. 2000;94(3):232–236. doi: 10.1002/1096-8628(20000918)94:3<232::aid-ajmg9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 63.Hall D, Pickler L, Riley K, et al. Parkinsonism and cognitive decline in a fragile X mosaic male. Mov Disord. 2010;25(10):1523–1524. doi: 10.1002/mds.23150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Utari A, Adams E, Berry-Kravis E, et al. Aging in fragile X syndrome. Journal of Neurodevelopmental Disorders. 2010;2(2):70–76. doi: 10.1007/s11689-010-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Vries BB, Wiegers AM, Smits AP, et al. Mental status of females with an FMR1 gene full mutation. American Journal of Human Genetics. 1996;58(5):1025–1032. [PMC free article] [PubMed] [Google Scholar]
- 66.Bennetto L, Pennington BF. Neuropsychology. In: Hagerman RJ, Hagerman PJ, editors. Fragile X Syndrome: Diagnosis, Treatment and Research. 3rd edition Johns Hopkins University Press; Baltimore: 2002. pp. 206–248. [Google Scholar]
- 67.Angkustsiri K, Wirojanan J, Deprey LJ, et al. Fragile X syndrome with anxiety disorder and exceptional verbal intelligence. American Journal of Medical Genetics. 2008;146(3):376–379. doi: 10.1002/ajmg.a.32118. [DOI] [PubMed] [Google Scholar]
- 68.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123(1):378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Epstein J, Riley K, Sobesky W. The treatment of emotional and behavioral problems. In: Hagerman R, Hagerman P, editors. Fragile X Syndrome: Diagnosis, Treatment and Research. 3rd edition The Johns Hopkins University Press; Baltimore: 2002. pp. 339–362. [Google Scholar]
- 70.Pembrey ME, Winter RM, Davies KE. A premutation that generates a defect at crossing over explains the inheritance of fragile X mental retardation. Am J Med Genet. 1985;21:709–717. doi: 10.1002/ajmg.1320210413. [DOI] [PubMed] [Google Scholar]
- 71.Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 72.Yu S, Mulley J, Loesch D, et al. Fragile-X syndrome: unique genetics of the heritable unstable element. American Journal of Human Genetics. 1992;50(5):968–980. [PMC free article] [PubMed] [Google Scholar]
- 73.Heitz D, Devys D, Imbert G, et al. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant ofthe transition to full mutation. Journal of Medical Genetics. 1992;29(11):794–801. doi: 10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snow K, Doud LK, Hagerman R, et al. Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. Am J Hum Genet. 1993;53(6):1217–1228. [PMC free article] [PubMed] [Google Scholar]
- 75.Vaisanen ML, Kahkonen M, Leisti J. Diagnosis of fragile X syndrome by direct mutation analysis. Hum Genet. 1994;93(2):143–147. doi: 10.1007/BF00210599. [DOI] [PubMed] [Google Scholar]
- 76.Nolin SL, Lewis FA, 3rd, Ye LL, et al. Familial transmission of the FMR1 CGG repeat. American Journal of Human Genetics. 1996;59(6):1252–1261. [PMC free article] [PubMed] [Google Scholar]
- 77.Ashley-Koch AE, Robinson H, Glicksman AE, et al. Examination of factors associated with instability of the FMR1 CGG repeat. Am J Hum Genet. 1998;63(3):776–785. doi: 10.1086/302018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nolin SL, Brown WT, Glicksman A, et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. American Journal of Human Genetics. 2003;72:454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown WT, Houck GE, Jr., Jeziorowska A, et al. Rapid Fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 1993;270(13):1569–1575. [PubMed] [Google Scholar]
- 80.Snow K, Tester DJ, Kruckeberg KE, et al. Sequence analysis ofthe fragile X trinucleotide repeat: implications for the origin of the fragile X mutation. Hum Mol Genet. 1994;3(9):1543–1551. doi: 10.1093/hmg/3.9.1543. [DOI] [PubMed] [Google Scholar]
- 81.Brown WT, Nolin S, Houck G, Jr, et al. Prenatal diagnosis and carrier screening for fragile X by PCR. Am J Med Genet. 1996;64(1):191–195. doi: 10.1002/(SICI)1096-8628(19960712)64:1<191::AID-AJMG34>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 82.Youings SA, Murray A, Dennis N, et al. FRAXA and FRAXE: the results of a five year survey. J Med Genet. 2000;37(6):415–421. doi: 10.1136/jmg.37.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dombrowski C, Levesque S, Morel ML, et al. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation offragile X syndrome alleles. Hum Mol Genet. 2002;11(4):371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell RJ, Holden JJ, Zhang C, et al. FMR1 alleles in Tasmania: a screening study of the special educational needs population. Clinical genetics. 2005;67(1):38–46. doi: 10.1111/j.1399-0004.2004.00344.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhou Y, Lum JM, Yeo GH, et al. Simplified molecular diagnosis of fragile X syndrome by fluorescent methylation-specific PCR and GeneScan analysis. Clin Chem. 2006;52(8):1492–1500. doi: 10.1373/clinchem.2006.068593. [DOI] [PubMed] [Google Scholar]
- 86.Khaniani MS, Kalitsis P, Burgess T, et al. An improved diagnostic PCR assay for identification of cryptic heterozygosity for CGG triplet repeat alleles in the Fragile X Gene (FMR1). Mol Cytogenet. 2008;1(1):5. doi: 10.1186/1755-8166-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Filipovic-Sadic S, Sah S, Chen L, et al. A novel FMR1 PCR method for the routine detection oflow-abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56(3):399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fernandez-Carvajal I, Lopez Posadas B, Pan R, et al. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diagn. 2009;11(4):306–310. doi: 10.2353/jmoldx.2009.080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan AK, Crawford DC, Scott EH, et al. Paternally transmitted FMR1 alleles are less stable than maternally transmitted alleles in the common and intermediate size range. Am J Hum Genet. 2002;70(6):1532–1544. doi: 10.1086/340846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maddalena A, Richards CS, McGinniss MJ, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3(3):200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loesch DZ, Huggins R, Petrovic V, et al. Expansion of the CGG repeat in fragile X in the FMR1 gene depends on the sex of the offspring. American Journal of Human Genetics. 1995;57(6):1408–1413. [PMC free article] [PubMed] [Google Scholar]
- 92.Huggins RM, Loesch DZ, Qian GQ, et al. Hierarchical Bayes model for random haplotype and family effects in the transmission of fragile-X. Genet Epidemiol. 2004;26(4):294–304. doi: 10.1002/gepi.10316. [DOI] [PubMed] [Google Scholar]
- 93.Ashley AE, Sherman SL. Population dynamics of a meiotic/mitotic expansion model for the fragile X syndrome. Am J Hum Genet. 1995;57(6):1414–1425. [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong N, Ju W, Pietrofesa J, et al. Fragile X “gray zone” alleles: AGG patterns, expansion risks and associated haplotypes. Am J Med Genet. 1996;64(2):261–265. doi: 10.1002/(SICI)1096-8628(19960809)64:2<261::AID-AJMG5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 95.Eichler EE, Holden JJ, Popovich BW, et al. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nature genetics. 1994;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- 96.Eichler EE, Macpherson JN, Murray A, et al. Haplotype and interspersion analysis of the FMR1 CGG repeat identifies two different mutational pathways for the origin of the fragile X syndrome. Hum Mol Genet. 1996;5(3):319–330. doi: 10.1093/hmg/5.3.319. [DOI] [PubMed] [Google Scholar]
- 97.Eichler EE, Nelson DL. Genetic variation and evolutionary stability of the FMR1 CGG repeat in six closed human populations. Am J Med Genet. 1996;64(1):220–225. doi: 10.1002/(SICI)1096-8628(19960712)64:1<220::AID-AJMG40>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 98.Zhong N, Yang W, Dobkin C, et al. Fragile X gene instability: anchoring AGGs and linked microsatellites. Am J Hum Genet. 1995;57(2):351–361. [PMC free article] [PubMed] [Google Scholar]
- 99.Yrigollen C, Long K, Tong T, et al. The role of AGG interruptions in the stability of FMR1 gene.. Paper presented at: 12th International Fragile X Conference; Detroit, MI. 2010. [Google Scholar]
- 100.Richards RI, Holman K, Kozman H, et al. Fragile X syndrome: genetic localisation by linkage mapping of two microsatellite repeats FRAXAC1 and FRAXAC2 which immediately flank the fragile site. J Med Genet. 1991;28(12):818–823. doi: 10.1136/jmg.28.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhong N, Kajanoja E, Smits B, et al. Fragile X founder effects and new mutations in Finland. Am J Med Genet. 1996a;64(1):226–233. doi: 10.1002/(SICI)1096-8628(19960712)64:1<226::AID-AJMG41>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 102.Faradz SMH, Pattiiha MZ, Leigh DA, et al. Genetic diversity at the FMR1 locus in the Indonesian population. Annals of Human Genetics. 2000;64(4):329–339. doi: 10.1017/S0003480000008204. [DOI] [PubMed] [Google Scholar]
- 103.Kunst CB, Warren ST. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994;77(6):853–861. doi: 10.1016/0092-8674(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 104.Gunter C, Paradee W, Crawford DC, et al. Re-examination of factors associated with expansion of CGG repeats using a single nucleotide polymorphism in FMR1. Hum Mol Genet. 1998;7(12):1935–1946. doi: 10.1093/hmg/7.12.1935. [DOI] [PubMed] [Google Scholar]
- 105.Brightwell G, Wycherley R, Waghorn A. SNP genotypingusing a simple and rapid single-tube modification of ARMS illustrated by analysis of 6 SNPs in a population of males with FRAXA repeat expansions. Molecular and Cellular Probes. 2002;16(4):297–305. doi: 10.1006/mcpr.2002.0424. [DOI] [PubMed] [Google Scholar]
- 106.Curlis Y, Zhang C, Holden JJ, et al. Haplotype study of intermediate-length alleles at the fragile X (FMR1) gene: ATL1, FMRb and microsatellite haplotypes differ from those found in common-size FMR1 alleles. Hum Biol. 2005;77(1):137–151. doi: 10.1353/hub.2005.0029. [DOI] [PubMed] [Google Scholar]
- 107.Toledano-Alhadef H, Basel-Vanagaite L, Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69(2):351–360. doi: 10.1086/321974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dawson AJ, Chodirker BN, Chudley AE. Frequency of FMR1 premutations in a consecutive newborn population by PCR screening of Guthrie blood spots. Biochem Mol Med. 1995;56(1):63–69. doi: 10.1006/bmme.1995.1057. [DOI] [PubMed] [Google Scholar]
- 109.Rousseau F, Rouillard P, Morel ML, et al. Prevalence of carriers of premutation-size alleles of the FMRI gene—and implications for the population genetics of the fragile X syndrome. American Journal of Human Genetics. 1995;57(5):1006–1018. [PMC free article] [PubMed] [Google Scholar]
- 110.Hagerman PJ. The fragile X prevalence paradox. Journal of Medical Genetics. 2008;45(8):498–499. doi: 10.1136/jmg.2008.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, et al. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn. 2009;11(4):324–329. doi: 10.2353/jmoldx.2009.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song FJ, Barton P, Sleightholme V, et al. Screening for fragile X syndrome: a literature review and modelling study. Health Technol Assess. 2003;7(16):1–106. doi: 10.3310/hta7160. [DOI] [PubMed] [Google Scholar]
- 113.Otsuka S, Sakamoto Y, Siomi H, et al. Fragile X carrier screening and FMR1 allele distribution in the Japanese population. Brain and Development. 2010;32(2):110–114. doi: 10.1016/j.braindev.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 114.Loesch DZ, Huggins R, Hay DA, et al. Genotype-phenotype relationships in fragile X syndrome: a family study. American Journal of Human Genetics. 1993;53(5):1064–1073. [PMC free article] [PubMed] [Google Scholar]
- 115.Hagerman RJ, Staley LW, O'Conner R, et al. Learning-disabled males with a fragile X CGG expansion in the upper premutation size range. Pediatrics. 1996;97(1):122–126. [PubMed] [Google Scholar]
- 116.Loesch D, Hay D, Mulley J. Transmitting males and carrier females in fragile X-revisited. Am J Med Genet. 1994;51(4):392–399. doi: 10.1002/ajmg.1320510418. [DOI] [PubMed] [Google Scholar]
- 117.Hull C, Hagerman RJ. A study ofthe physical, behavioral and medical phenotype, including anthropometric measures, of females with fragile X syndrome. Am J Dis Child. 1993;147(11):1236–1241. doi: 10.1001/archpedi.1993.02160350110017. [DOI] [PubMed] [Google Scholar]
- 118.Riddle JE, Cheema A, Sobesky WE, et al. Phenotypic involvement in females with the FMR1 gene mutation. Am J Ment Retard. 1998;102(6):590–601. doi: 10.1352/0895-8017(1998)102<0590:piifwt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 119.Loesch DZ, Huggins RM, Bui QM, et al. Effect of fragile X status categories and FMRP deficits on cognitive profiles estimated by robust pedigree analysis. Am J Med Genet A. 2003;122A(l):13–23. doi: 10.1002/ajmg.a.20214. [DOI] [PubMed] [Google Scholar]
- 120.Loesch DZ, Bui QM, Grigsby J, et al. Effect of the fragile X status categories and the fragile X mental retardation protein levels on executive functioning in males and females with fragile X. Neuropsychology. 2003;17(4):646–657. doi: 10.1037/0894-4105.17.4.646. [DOI] [PubMed] [Google Scholar]
- 121.Grigsby J, Kaye K. Behavioral Dyscontrol Scale: Manual. 2nd edition 1996. [Google Scholar]
- 122.Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial Manual. Psychological Assessment Resources, Inc.; Odessa FI: 1995. [Google Scholar]
- 123.Moore CJ, Daly EM, Schmitz N, et al. A neuropsychological investigation of male premutation carriers of fragile X syndrome. Neuropsychologia. 2004;42(14):1934–1947. doi: 10.1016/j.neuropsychologia.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 124.Lachiewicz AM, Dawson DV, Spiridigliozzi GA, et al. Arithmetic difficulties in females with the fragile X premutation. American Journal of Medical Genetics. 2006;140(7):665–672. doi: 10.1002/ajmg.a.31082. [DOI] [PubMed] [Google Scholar]
- 125.Keri S, Benedek G. Visualpathway deficit infernale fragile Xpremutationcarriers: apotential endophenotype. Brain Cogn. 2009;69(2):291–295. doi: 10.1016/j.bandc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 126.Keri S, Benedek G. The perception of biological and mechanical motion in female fragile X premutation carriers. Brain Cogn. 2010;72(2):197–201. doi: 10.1016/j.bandc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 127.Hunsaker MR, Goodrich-Hunsaker NJ, Willemsen R, et al. Temporal ordering deficits in female CGG KI mice heterozygous for the fragile X premutation. Behavioural Brain Research. 2010;213(2):263–268. doi: 10.1016/j.bbr.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Minguez M, Ibáñez B, Ribate MP, et al. Risk of cognitive impairment in female premutation carriers of fragile X premutation: analysis by means of robust segmented linear regression models. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(2):262–270. doi: 10.1002/ajmg.b.30803. [DOI] [PubMed] [Google Scholar]
- 129.Goodlin-Jones BL, Tassone F, Gane LW, et al. Autistic spectrum disorder and the fragile X premutation. J Dev Behav Pediatr. 2004;25(6):392–398. doi: 10.1097/00004703-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 130.Loesch DZ, Huggins RM, Bui QM, et al. Relationship of deficits of FMR1 gene specific protein with physical phenotype of fragile X males and females in pedigrees: a new perspective. Am J Med Genet A. 2003;118A(2):127–134. doi: 10.1002/ajmg.a.10099. [DOI] [PubMed] [Google Scholar]
- 131.Jakala P, Hanninen T, Ryynanen M, et al. Fragile-X: neuropsychological test performance, CGG triplet repeat lengths and hippocampal volumes. J Clin Invest. 1997;100(2):331–338. doi: 10.1172/JCI119538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murphy DGM, Mentis MJ, Pietrini P, et al. Premutation female carriers of fragile X syndrome: a pilot study on brain anatomy and metabolism. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(10):1294–1301. doi: 10.1097/00004583-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 133.Moore CJ, Daly EM, Tassone F, et al. The effect of premutation of X chromosome CGG trinucleotide repeats on brain anatomy. Brain. 2004;127(Pt 12):2672–2681. doi: 10.1093/brain/awh256. [DOI] [PubMed] [Google Scholar]
- 134.Adams PE, Adams JS, Nguyen DV, et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(3):775–78. doi: 10.1002/ajmg.b.31046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hessl D, Rivera S, Koldewyn K, et al. Amygdala dysfunction in men with the fragile X premutation. Brain. 2007;130(2):404–416. doi: 10.1093/brain/awl338. [DOI] [PubMed] [Google Scholar]
- 136.Koldewyn K, Hessl D, Adams J, et al. Reduced hippocampal activation during recall is associated with elevated FMR1 mRNA and psychiatric symptoms in men with the fragile X premutation. Brain Imaging Behav. 2008;2(2):105–116. doi: 10.1007/s11682-008-9020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hagerman RJ, Hagerman PJ. The fragile X premutation: into the phenotypic fold. Current Opinion in Genetics and Development. 2002;12:278–283. doi: 10.1016/s0959-437x(02)00299-x. [DOI] [PubMed] [Google Scholar]
- 138.Cornish KM, Li L, Kogan CS, et al. Age-dependent cognitive changes in carriers of the fragile X syndrome. Cortex. 2008;44(6):628–636. doi: 10.1016/j.cortex.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kogan C, Turk J, Hagerman RJ, et al. Impact ofthe fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated tremor/ataxia syndrome: a controlled study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):859–872. doi: 10.1002/ajmg.b.30685. [DOI] [PubMed] [Google Scholar]
- 140.Cornish KM, Kogan CS, Li L, et al. Lifespan changes in working memory in fragile X premutation males. Brain and Cognition. 2009;69(3):551–558. doi: 10.1016/j.bandc.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sevin M, Kutalik Z, Bergman S, et al. Penetrance of marked cognitive impairment in older male carriers of the FMR1 gene premutation. Journal of Medical Genetics. 2009;46(12):818–824. doi: 10.1136/jmg.2008.065953. [DOI] [PubMed] [Google Scholar]
- 142.Hunter J, Abramowitz A, Rusin M, et al. Is there evidence for neuropsychological and neurobehavioral phenotypes among adults without FXTAS who carry the FMR1 premutation? A review of current literature. Genet Med. 2009;11(2):79–89. doi: 10.1097/GIM.0b013e31818de6ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Reiss AL, Freund L, Abrams MT, et al. Neurobehavioral effects of the fragile X premutation in adult women: a controlled study. American journal of human genetics. 1993;52(5):884–894. [PMC free article] [PubMed] [Google Scholar]
- 144.Franke P, Leboyer M, Gänsicke M, et al. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Research. 1998;80(2):113–127. doi: 10.1016/s0165-1781(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 145.Al-Semaan Y, Malla AK, Lazosky A. Schizoaffective disorder in a fragile-X carrier. Australian and New Zealand Journal of Psychiatry. 1999;33(3):436–440. doi: 10.1046/j.1440-1614.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 146.Rodriguez-Revenga L, Madrigal I, Alegret M, et al. Evidence of depressive symptoms in fragile-X syndrome premutated females. Psychiatr Genet. 2008;18(4):153–155. doi: 10.1097/YPG.0b013e3282f97e0b. [DOI] [PubMed] [Google Scholar]
- 147.Roberts J, Bailey D, Jr., Mankowski J, et al. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(1):130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- 148.Bailey DJ, Raspa M, Olmsted M, et al. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008;146A(16):2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- 149.Hunter J, Allen E, Abramowitz A, et al. Investigation of phenotypes associated with mood and anxiety among male and female fragile X premutation carriers. Behavior Genetics. 2008;38(5):493–502. doi: 10.1007/s10519-008-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Allen EG, He W, Yadav-Shah M, et al. A study of the distributional characteristics of FMR1 transcript levels in 238 individuals. Hum Genet. 2004;114(5):439–447. doi: 10.1007/s00439-004-1086-x. [DOI] [PubMed] [Google Scholar]
- 151.Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions offragile X-associated tremor/ataxia syndrome (FXTAS). RNA Biol. 2004;1(2):103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 152.Tassone F, Hagerman RJ, Chamberlain WD, et al. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am J Med Genet Fall. 2000;97(3):195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 153.Tassone F, Hagerman R, Taylor A, et al. Clinical involvement and protein expression in individuals with the FMR1 premutation. American Journal of Medical Genetics. 2000;91(2):144–152. doi: 10.1002/(sici)1096-8628(20000313)91:2<144::aid-ajmg14>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 154.Tassone F, Hagerman RJ, Garcia-Arocena D, et al. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. Journal of Medical Genetics. 2004;41(4):e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kenneson A, Zhang F, Hagedorn CH, et al. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10(14):1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 156.Chen LS, Tassone F, Sahota P, et al. The (CGG)n repeat element within the 5' untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Hum Mol Genet. 2003;12(23):3067–3074. doi: 10.1093/hmg/ddg331. [DOI] [PubMed] [Google Scholar]
- 157.Tassone F, Beilina A, Carosi C, et al. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13(4):555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.García-Alegría E, Berta Ibáñez, Mónica Mínguez, et al. Analysis of FMR1 gene expression in female premutation carriers using robust segmented linear regression models. RNA. 2007;13:756–762. doi: 10.1261/rna.206307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Şentürk D, Nguyen DV, Tassone F, et al. Covariate adjusted correlation analysis with application to FMR1 premutation female carrier data. Biometrics. 2009;65(3):781–792. doi: 10.1111/j.1541-0420.2008.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hagerman R, Greco CM, Chudley AE, et al. Neuropathology and neurodegenerative features in some older male premutation carriers of fragile X syndrome. Am J Hum Genet. 2001;69(177) [Google Scholar]
- 161.Hagerman RJ, Hagerman PJ. Fragile X Syndrome and fragile X-associated tremor/ataxia syndrome. In: Robert D, Wells, Ashizawa T, editors. Genetic Instabilities and Neurological Diseases. 2nd edition Academic Press; Houston, USA: 2006. pp. 165–174. [Google Scholar]
- 162.Berry-Kravis E, Lewin F, Wuu J, et al. Tremor and ataxia in fragile X premutation carriers: blinded videotape study. Ann Neurol. 2003;53(5):616–623. doi: 10.1002/ana.10522. [DOI] [PubMed] [Google Scholar]
- 163.Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22(2):203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 164.Leehey MA, Hagerman RJ, Landau WM, et al. Tremor/ataxia syndrome in fragile X carrier males. Movement Disorders. 2002;17(4):744–745. [Google Scholar]
- 165.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 166.Loesch DZ, Churchyard A, Brotchie P, et al. Evidence for and a spectrum of, neurological involvement in carriers of the fragile X premutation: FXTAS and beyond. Clinical Genetics. 2005;67(5):412–417. doi: 10.1111/j.1399-0004.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- 167.Pugliese P, Annesi G, Cutuli N, et al. The fragile X premutation presenting as postprandial hypotension. Neurology. 2004;63(11):2188–2189. doi: 10.1212/01.wnl.0000145709.61117.08. [DOI] [PubMed] [Google Scholar]
- 168.Soontarapornchai K, Maselli R, Fenton-Farrell G, et al. Abnormal nerve conduction features in fragile X premutation carriers. Archives of Neurology. 2008;65(4):495–498. doi: 10.1001/archneur.65.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Adams JS, Adams PE, Nguyen D, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology. 2007;69(9):851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- 170.Brunberg JA, Jacquemont S, Hagerman RJ, et al. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. Am J Neuroradiol. 2002;23(10):1757–1766. [PMC free article] [PubMed] [Google Scholar]
- 171.Moris G, Arias M, Lopez MV, Alvarez V. Hyperintensity in the basis pontis: atypical neuroradiological findings in a woman with FXTAS. Movement Disorders. 2010;25(5):649–650. doi: 10.1002/mds.22811. [DOI] [PubMed] [Google Scholar]
- 172.Loesch DZ, Kotschet K, Trost NC, et al. White matter changes in basic pontis in small expansion FMR1 allele carriers with parkinsonism. Neuropsychiatric Genetics. 2011 doi: 10.1002/ajmg.b.31189. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain. 2006;129(Pt 1):243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 174.Cohen S, Masyn K, Adams J, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67(8):1426–1431. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- 175.Loesch DZ, Litewka L, Brotchie P, et al. Magnetic resonance imaging study in older fragile X premutation male carriers. Annals of Neurology. 2005;58(2):326–330. doi: 10.1002/ana.20542. [DOI] [PubMed] [Google Scholar]
- 176.Greco CM, Hagerman RJ, Tassone F, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125(8):1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 177.Willemsen R, Hoogeveen-Westerveld M, Reis S, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12(9):949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 178.Wenzel HJ, Hunsaker MR, Greco CM, et al. Ubiquitin-positive intranuclear inclusions in neuronal and glial cells in a mouse model of the fragile X premutation. Brain Res. 2010;1318:155–166. doi: 10.1016/j.brainres.2009.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grigsby J, Brega AG, Jacquemont S, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS). J Neurol Sci. 2006;248(1-2):227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 180.Grigsby J, Brega AG, Leehey MA, et al. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Mov Disord. 2007;22(5):645–650. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- 181.Bacalman S, Farzin F, Bourgeois JA, et al. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. J Clin Psychiatry. 2006;67(1):87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- 182.Bourgeois JA, Cogswell JB, Hessl D, et al. Cognitive, anxiety and mood disorders in the fragile X-associated tremor/ataxia syndrome. Gen Hosp Psychiatry. 2007;29(4):349–356. doi: 10.1016/j.genhosppsych.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Seritan AL, Nguyen DV, Farias ST, et al. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): comparison with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(7):1138–1144. doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Loesch DZ, Cook M, Litewka L, et al. A low symptomatic form of neurodegeneration in younger carriers of the FMR1 premutation, manifesting typical radiological changes. Journal of Medical Genetics. 2008;45(3):179–181. doi: 10.1136/jmg.2007.054171. [DOI] [PubMed] [Google Scholar]
- 185.Loesch DZ, Litewka L, Churchyard A, et al. Tremor/ataxia syndrome and fragile X premutation: diagnostic caveats. J Clin Neurosci. 2007;14(3):245–248. doi: 10.1016/j.jocn.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 186.Capelli LP, Goncalves MR, Kok F, et al. Fragile X-associated tremor/ataxia syndrome: intrafamilial variability and the size of the FMR1 premutation CGG repeat. Mov Disord. 2007;22(6):866–870. doi: 10.1002/mds.21347. [DOI] [PubMed] [Google Scholar]
- 187.Greco CM, Tassone F, Garcia-Arocena D, et al. Clinical and neuropathologic findings in a woman with the FMR1 premutation and multiple sclerosis. Archives of Neurology. 2008;65(8):1114–1116. doi: 10.1001/archneur.65.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang L, Coffey S, Lua LL, et al. FMR1 premutation in females diagnosed with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(7):812–814. doi: 10.1136/jnnp.2008.160960. [DOI] [PubMed] [Google Scholar]
- 189.Mothersead PK, Conrad K, Hagerman RJ, et al. GRAND ROUNDS: an atypical progressive dementia in a male carrier of the fragile X premutation: an example of fragile X-associated tremor/ataxia syndrome. Appl Neuropsychol. 2005;12(3):169–178. doi: 10.1207/S15324826AN1203_7. [DOI] [PubMed] [Google Scholar]
- 190.Yachnis AT, Roth HL, Heilman KM. Fragile X Dementia Parkinsonism Syndrome (FXDPS). Cognitive and Behavioral Neurology. 2010;23(1):39–43. doi: 10.1097/WNN.0b013e3181b6e1b9. doi: 10.1097/WNN.1090b1013e3181b1096e1091b1099. [DOI] [PubMed] [Google Scholar]
- 191.Chonchaiya W, Nguyen DV, Au J, et al. Clinical involvement in daughters of men with fragile X-associated tremor ataxia syndrome. Clinical Genetics. 2010;78(1):39–46. doi: 10.1111/j.1399-0004.2010.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brouwer JR, Huizer K, Severijnen LA, et al. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008;107(6):1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tassone F, Adams J, Berry-Kravis EM, et al. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS). Am J Med Genet B Neuropsychiatr Genet. 2007;144(4):566–569. doi: 10.1002/ajmg.b.30482. [DOI] [PubMed] [Google Scholar]
- 194.Leehey MA, Berry-Kravis E, Goetz CG, et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70(16 Pt 2):1397–1402. doi: 10.1212/01.wnl.0000281692.98200.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Berry-Kravis E, Goetz CG, Leehey MA, et al. Neuropathic features in fragile X premutation carriers. Am J Med Genet A. 2007;143(1):19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- 196.Tassone F, Hagerman RJ, Gane LW, et al. Strong similarities of the FMR1 mutation in multiple tissues: postmortem studies of a male with a full mutation and a male carrier of a premutation. American Journal of Medical Genetics. 1999;84(3):240–244. [PubMed] [Google Scholar]
- 197.Milunsky JM, Maher TA. Fragile X carrier screening and spinocerebellar ataxia in older males. American Journal of Medical Genetics. 2004;125(3):320. doi: 10.1002/ajmg.a.20465. [DOI] [PubMed] [Google Scholar]
- 198.Macpherson J, Waghorn A, Hammans S, et al. Observation of an excess of fragile-X premutations in a population of males referred with spinocerebellar ataxia. Hum Genet. 2003;112(5-6):619–620. doi: 10.1007/s00439-003-0939-z. [DOI] [PubMed] [Google Scholar]
- 199.Van Esch H, Dom R, Bex D, et al. Screening for FMR-1 premutations in 122 older Flemish males presenting with ataxia. Eur J Hum Genet. 2005;13(1):121–123. doi: 10.1038/sj.ejhg.5201312. [DOI] [PubMed] [Google Scholar]
- 200.Tan EK, Zhao Y, Puong KY, et al. Fragile X premutation alleles in SCA, ET and parkinsonism in an Asian cohort. Neurology. 2004;63(2):362–363. doi: 10.1212/01.wnl.0000130199.57181.7b. [DOI] [PubMed] [Google Scholar]
- 201.Brussino A, Gellera C, Saluto A, et al. FMR1 gene premutation is a frequent genetic cause of late-onset sporadic cerebellar ataxia. Neurology. 2005;64(1):145–147. doi: 10.1212/01.WNL.0000148723.37489.3F. [DOI] [PubMed] [Google Scholar]
- 202.Garcia Arocena D, Louis ED, Tassone F, et al. Screen for expanded FMR1 alleles in patients with essential tremor. Mov Disord. 2004;19(8):930–933. doi: 10.1002/mds.20043. [DOI] [PubMed] [Google Scholar]
- 203.Deng H, Le W, Jankovic J. Premutation alleles associated with Parkinson disease and essential tremor. Jama. 2004;292(14):1685–1686. doi: 10.1001/jama.292.14.1685-b. [DOI] [PubMed] [Google Scholar]
- 204.Kamm C, Healy DG, Quinn NP, et al. The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group. Brain. 2005;128(Pt 8):1855–1860. doi: 10.1093/brain/awh535. [DOI] [PubMed] [Google Scholar]
- 205.Toft M, Aasly J, Bisceglio G, et al. Parkinsonism, FXTAS and FMR1 premutations. Mov Disord. 2005;20(2):230–233. doi: 10.1002/mds.20297. [DOI] [PubMed] [Google Scholar]
- 206.Zuhlke C, Budnik A, Gehlken U, et al. FMR1 premutation as a rare cause of late onset ataxia—evidence for FXTAS in female carriers. J Neurol. 2004;251(11):1418–1419. doi: 10.1007/s00415-004-0558-1. [DOI] [PubMed] [Google Scholar]
- 207.Garland EM, Vnencak-Jones CL, Biaggioni I, et al. Fragile X gene premutation in multiple system atrophy. J Neurol Sci. 2004;227(1):115–118. doi: 10.1016/j.jns.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 208.Biancalana V, Toft M, Le Ber I, et al. FMR1 premutations associated with fragile X-associated tremor/ ataxia syndrome in multiple system atrophy. Archives of Neurology. 2005;62(6):962–966. doi: 10.1001/archneur.62.6.962. [DOI] [PubMed] [Google Scholar]
- 209.Seixas AI, Maurer MH, Lin M, et al. FXTAS, SCA10 and SCA17 in American patients with movement disorders. American Journal of Medical Genetics. 2005;136(1):87–89. doi: 10.1002/ajmg.a.30761. [DOI] [PubMed] [Google Scholar]
- 210.Tan EK, Zhao Y, Puong KY, et al. Expanded FMR1 alleles are rare in idiopathic Parkinson's disease. Neurogenetics. 2005;6(1):51–52. doi: 10.1007/s10048-004-0200-5. [DOI] [PubMed] [Google Scholar]
- 211.Jacquemont S, Farzin F, Hall D, et al. Aging in individuals with the FMR1 mutation. Am J Ment Retard. 2004;109(2):154–164. doi: 10.1352/0895-8017(2004)109<154:AIIWTF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kurz MW, Schlitter AM, Klenk Y, et al. FMR1 alleles in Parkinson's disease: relation to cognitive decline and hallucinations, a longitudinal study. J Geriatr Psychiatry Neurol. 2007;20(2):89–92. doi: 10.1177/0891988706297737. [DOI] [PubMed] [Google Scholar]
- 213.Kraff J, Tang HT, Cilia R, et al. Screen for excess FMR1 premutation alleles among males with parkinsonism. Arch Neurol. 2007;64(7):1002–1006. doi: 10.1001/archneur.64.7.1002. [DOI] [PubMed] [Google Scholar]
- 214.Loesch DZ, Khaniani MS, Slater HR, et al. Small CGG repeat expansion alleles ofFMR1 gene are associated with parkinsonism. Clinical Genetics. 2009;76(5):471–476. doi: 10.1111/j.1399-0004.2009.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Karmon Y, Gadoth N. Fragile X associated tremor/ataxia syndrome (FXTAS) with dementia in a female harbouring FMR1 premutation. J Neurol Neurosurg Psychiatry. 2008;79(6):738–739. doi: 10.1136/jnnp.2007.139642. [DOI] [PubMed] [Google Scholar]
- 216.Hagerman RJ, Leavitt BR, Farzin F, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. American Journal of Human Genetics. 2004;74(5):1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Berry-Kravis E, Potanos K, Weinberg D, et al. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Annals of Neurology. 2005;57(1):144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- 218.Jacquemont S, Orrico A, Galli L, et al. Spastic paraparesis, cerebellar ataxia and intention tremor: a severe variant of FXTAS? Journal of Medical Genetics. 2005;42(2):e14. doi: 10.1136/jmg.2004.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.O'Dwyer JP, Clabby C, Crown J, et al. Fragile X-associated tremor/ataxia syndrome presenting in a woman after chemotherapy. Neurology. 2005;65(2):331–332. doi: 10.1212/01.wnl.0000168865.36352.53. [DOI] [PubMed] [Google Scholar]
- 220.Al-Hinti JT, Nagan N, Harik SI. Fragile X premutation in a woman with cognitive impairment, tremor and history of premature ovarian failure. Alzheimer Dis Assoc Disord. 2007;21(3):262–264. doi: 10.1097/WAD.0b013e31811ec130. [DOI] [PubMed] [Google Scholar]
- 221.Galvao R, Mendes-Soares L, Camara J, et al. Triplet repeats, RNA secondary structure and toxic gain-of-function models for pathogenesis. Brain Res Bull. 2001;56(3-4):191–201. doi: 10.1016/s0361-9230(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 222.Finsterer J. Myotonic dystrophy type 2. European Journal of Neurology. 2002;9(5):441–447. doi: 10.1046/j.1468-1331.2002.00453.x. [DOI] [PubMed] [Google Scholar]
- 223.Mankodi A, Thornton CA. Myotonic syndromes. Current Opinion in Neurology. 2002;15(5):545–552. doi: 10.1097/00019052-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 224.Khalili K, Del Valle L, Muralidharan V, et al. Puralpha is essential for postnatal brain development and developmentally coupled cellular proliferation as revealed by genetic inactivation in the mouse. Mol Cell Biol. 2003;23(19):6857–6875. doi: 10.1128/MCB.23.19.6857-6875.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Jin P, Duan R, Qurashi A, et al. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model offragile X tremor/ataxia syndrome. Neuron. 2007;55(4):556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Garcia-Arocena D, Yang JE, Brouwer JR, et al. Fibroblast phenotype in male carriers of FMR1 premutation alleles. Hum Mol Genet. 2010;19(2):299–312. doi: 10.1093/hmg/ddp497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Paul R, Pessah IN, Gane L, et al. Early onset of neurological symptoms in fragile X premutation carriers exposed to neurotoxins. Neurotoxicology. 2010;31(4):399–402. doi: 10.1016/j.neuro.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Iwahashi CK, Yasui DH, An HJ, et al. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129(Pt 1):256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- 229.Arocena DG, Iwahashi CK, Won N, et al. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet. 2005;14(23):3661–3671. doi: 10.1093/hmg/ddi394. [DOI] [PubMed] [Google Scholar]
- 230.Sellier C, Rau F, Liu Y, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. The EMBO Journal. 2010;29(7):1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sellier C, Hagerman P, Willemsen R, et al. DROSHA/DGCR8 sequestration by expanded CGG repeats leads to global micro-RNA processing alteration in FXTAS patients [abstract].. Paper presented at: 12th International Fragile X Conference; Detroit, MI. 2010. [Google Scholar]
- 232.Jin P, Zarnescu DC, Zhang F, et al. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39(5):739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 233.Van Dam D, Errijgers V, Kooy RF, et al. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for fragile-X-associated tremor/ataxia syndrome (FXTAS). Behavioural Brain Research. 2005;162(2):233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 234.Hashem V, Galloway JN, Mori M, et al. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum Mol Genet. 2009;18(13):2443–2451. doi: 10.1093/hmg/ddp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Greco CM, Soontrapornchai K, Wirojanan J, et al. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. The Journal of Urology. 2007;177(4):1434–1437. doi: 10.1016/j.juro.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 236.Brouwer JR, Severijnen E, de Jong FH, et al. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008;33(6):863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hunsaker MR, Greco C, Tassone F, et al. Rare intranuclear inclusions in the brains of three older adult males with fragile X syndrome: implications for the spectrum of fragile X-associated disorders. JNEN. doi: 10.1097/NEN.0b013e31821d3194. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19(l):196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ross-Inta C, Omanska-Klusek A, Wong S, et al. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429(3):545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Loesch DZ, Godler DE, Evans A, et al. Evidence for the toxicity of bidirectional transcripts and mitochondrial dysfunction in blood associated with small CGG expansions in the FMR1 gene in patients with parkinsonism. Genet Med. 2011 Jan 25; doi: 10.1097/GIM.0b013e3182064362. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ladd PD, Smith LE, Rabaia NA, et al. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007;16(24):3174–3187. doi: 10.1093/hmg/ddm293. [DOI] [PubMed] [Google Scholar]
- 242.Khalil AM, Faghihi MA, Modarresi F, et al. A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS ONE. 2008;3(1):e1486. doi: 10.1371/journal.pone.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89(9):4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 244.De Caro JJ, Dominguez C, Sherman SL. Reproductive health of adolescent girls who carry the FMR1 premutation: expected phenotype based on current knowledge of fragile x-associated primary ovarian insufficiency. Ann N Y Acad Sei. 2008;1135:99–111. doi: 10.1196/annals.1429.029. [DOI] [PubMed] [Google Scholar]
- 245.Schwartz CE, Dean J, Howard-Peebles PN, et al. Obstetrical and gynecological complications in fragile X carriers: a multicenter study. Am J Med Genet. 1994;51(4):400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- 246.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the international collaborative POF in fragile X study- preliminary data. Am J Med Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- 247.Murray A, Ennis S, MacSwiney F, et al. Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet. 2000;8(4):247–252. doi: 10.1038/sj.ejhg.5200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vianna-Morgante AM, Costa SS. Premature ovarian failure is associated with maternally and paternally inherited premutation in Brazilian families with fragile X [see comments] [letter]. American Journal of Human Genetics. 2000;67(1):254–255. doi: 10.1086/302976. discussion 256-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet (Semin Med Genet) 2000;97(3):189–194. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 250.Tejada MI, Garcia-Alegria E, Bilbao A, et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause. 2008;15(5):945–949. doi: 10.1097/gme.0b013e3181647762. [DOI] [PubMed] [Google Scholar]
- 251.Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14(2):253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Allen EG, Sullivan AK, Marcus M, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007:dem148. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- 253.Hunter JE, Epstein MP, Tinker SW, et al. Fragile X-associated primary ovarian insufficiency: evidence for additional genetic contributions to severity. Genetic Epidemiology. 2008;32(6):553–559. doi: 10.1002/gepi.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pankratz VS, de Andrade M, Therneau TM. Random-effects Cox proportional hazards model: general variance components methods for time-to-event data. Genetic Epidemiology. 2005;28(2):97–109. doi: 10.1002/gepi.20043. [DOI] [PubMed] [Google Scholar]
- 255.Lin Y-S, Yang M-L. Familial premature ovarian failure in female premutated carriers of fragile X syndrome: a Case Report and Literature Review. Taiwanese Journal of Obstetrics and Gynecology. 2006;45(1):60–63. doi: 10.1016/S1028-4559(09)60193-5. [DOI] [PubMed] [Google Scholar]
- 256.Miano M, Laperuta C, Chiurazzi P, et al. Ovarian dysfunction and FMR1 alleles in a large Italian family with POF and FRAXA disorders: case report. BMC Medical Genetics. 2007;8(1):18. doi: 10.1186/1471-2350-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hundscheid RDL, Braat DDM, Kiemeney LALM, et al. Increased serum FSH in female fragile X premutation carriers with either regular menstrual cycles or on oral contraceptives. Hum Reprod. 2001;16(3):457–462. doi: 10.1093/humrep/16.3.457. [DOI] [PubMed] [Google Scholar]
- 258.Murray A, Webb J, MacSwiney F, et al. Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod. 1999;14(5):1217–1218. doi: 10.1093/humrep/14.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hundscheid RD, Smits AP, Thomas CM, et al. Female carriers of fragile X premutations have no increased risk for additional diseases other than premature ovarian failure. American Journal of Medical Genetics. 2003;117(1):6–9. doi: 10.1002/ajmg.a.10862. [DOI] [PubMed] [Google Scholar]
- 260.Allen EG, Sullivan AK, Marcus M, et al. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22(8):2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- 261.Peprah E, He W, Allen E, et al. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J Hum Genet. 2010;55(1):66–68. doi: 10.1038/jhg.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Burgess B, Partington M, Turner G, et al. Normal age of menarche in fragile X syndrome. American Journal of Medical Genetics. 1996;64(2):376–376. doi: 10.1002/ajmg.1320640203. [DOI] [PubMed] [Google Scholar]
- 263.Vianna-Morgante AM. Twinning and premature ovarian failure in premutation fragile X carriers. Am J Med Genet. 1999;83(4):326. doi: 10.1002/(sici)1096-8628(19990402)83:4<326::aid-ajmg18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 264.Van Esch H, Buekenhout L, Race V, et al. Very early premature ovarian failure in two sisters compound heterozygous for the FMR1 premutation. Eur J Med Genet. 2009;52(1):37–40. doi: 10.1016/j.ejmg.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 265.Patsalis PC, Sismani C, Hettinger JA, et al. Frequencies of “grey-zone” and premutation-size FMR1 CGG-repeat alleles in patients with developmental disability in Cyprus and Canada. Am J Med Genet. 1999;84(3):195–197. [PubMed] [Google Scholar]
- 266.Loesch DZ, Bui QM, Huggins RM, et al. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised and correlate with the number of CGG repeats. J Med Genet. 2007;44(3):198–204. doi: 10.1136/jmg.2006.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Murray A, Youings S, Dennis N, et al. Population screening at the FRAXA and FRAXE loci: molecular analyses ofboys with learning difficulties and their mothers. Hum Mol Genet. 1996;5(6):727–735. doi: 10.1093/hmg/5.6.727. [DOI] [PubMed] [Google Scholar]
- 268.Mazzocco MM, Sonna NL, Teisl JT, et al. The FMR1 and FMR2 mutations are not common etiologies of academic difficulty among school-age children. J Dev Behav Pediatr. 1997;18(6):392–398. doi: 10.1097/00004703-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 269.Mazzocco MMM, Myers GF, Hamner JL, et al. The prevalence of the FMR1 and FMR2 mutations among preschool children with language delay. The Journal of Pediatrics. 1998;132(5):795–801. doi: 10.1016/s0022-3476(98)70306-3. [DOI] [PubMed] [Google Scholar]
- 270.Haddad LA, Aguiar MJ, Costa SS, et al. Fully mutated and gray-zone FRAXA alleles in Brazilian mentally retarded boys. Am J Med Genet. 1999;84(3):198–201. doi: 10.1002/(sici)1096-8628(19990528)84:3<198::aid-ajmg5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 271.Loesch DZ, Godler DE, Khaniani M, et al. Linking the FMR1 alleles with small CGG expansions with neurodevelopmental disorders: preliminary data suggest an involvement of epigenetic mechanisms. American Journal of Medical Genetics. 2009;149A(10):2306–2310. doi: 10.1002/ajmg.a.32990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117(4):376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 273.Bodega B, Bione S, Dalpra L, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21(4):952–957. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- 274.Streuli I, Fraisse T, Ibecheole V, et al. Intermediate and premutation FMR1 alleles in women with occult primary ovarian insufficiency. Fertil Steril. 2009;92(2):464–470. doi: 10.1016/j.fertnstert.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 275.Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Mullerian hormone. Fertil Steril. 2009;91(5):1700–1706. doi: 10.1016/j.fertnstert.2008.01.098. [DOI] [PubMed] [Google Scholar]
- 276.Chatterjee S, Maitra A, Kadam S, et al. CGG repeat sizing in the FMR1 gene in Indian women with premature ovarian failure. Reprod Biomed Online. 2009;19(2):281–286. doi: 10.1016/s1472-6483(10)60086-7. [DOI] [PubMed] [Google Scholar]
- 277.Schapira A, Hartmann A, Agid Y. Parkinsonian Disorders in Clinical Practice. Wiley-Blackwell; Oxford, UK: 2009. [Google Scholar]
- 278.Loesch DZ, Bui QM, Dissanayake C, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev. 2007;31(3):315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hagerman RJ, Hall DA, Coffey S, et al. Treatment offragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3(2):251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Hagerman RJ, Hagerman PJ. Fragile X Syndrome: Diagnosis, Treatment and Research. 3rd edition The Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- 281.Erickson CA, Stigler KA, Posey DJ, et al. Aripiprazole in autism spectrum disorders and fragile X syndrome. Neurotherapeutics. 2010;7(3):258–263. doi: 10.1016/j.nurt.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Huber KM, Gallagher SM, Warren ST, et al. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99(11):7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bear MF, Huber KM, Warren ST. The mGluR theory offragile X mental retardation. Trends in Neurosciences. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 284.Yan QJ, Rammal M, Tranfaglia M, et al. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49(7):1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 285.de Vrij FM, Levenga J, van der Linde HC, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiology of Disease. 2008;31(1):127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Berry-Kravis E, Hessl D, Coffey S, et al. A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. Journal of Medical Genetics. 2009;46(4):266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Berry-Kravis E, Cherubini M, Zarevics P, et al. Arbaclofen for the Treatment of Children and Adults with Fragile X Syndrome: Results of a Phase 2, Randomized, Double-Blind, Placebo-Controlled, Crossover Study [Abstract].. Paper presented at: International Meeting for Autism Research; Philadelphia, PA. 2010. [Google Scholar]
- 288.Bilousova TV, Dansie L, Ngo M, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. Journal of Medical Genetics. 2009;46(2):94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 289.Utari A, Chonchaiya W, Rivera SM, et al. Side effects of minocycline treatment in patients with fragile x syndrome and exploration of outcome measures. Am J Intellect Dev Disabil. 2010;115(5):433–443. doi: 10.1352/1944-7558-115.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.de Diego-Otero Y, Romero-Zerbo Y, el Bekay R, et al. Alpha-tocopherol protects against oxidative stress in the fragile X knockout mouse: an experimental therapeutic approach for the Fmrl deficiency. Neuropsychopharmacology. 2009;34(4):1011–1026. doi: 10.1038/npp.2008.152. [DOI] [PubMed] [Google Scholar]
- 291.Wirojanan J, Jacquemont S, Diaz R, et al. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J Clin Sleep Med. 2009;5(2):145–150. [PMC free article] [PubMed] [Google Scholar]
- 292.Romero-Zerbo Y, Decara J, el Bekay R, et al. Protective effects of melatonin against oxidative stress in Fmr1 knockout mice: a therapeutic research model for the fragile X syndrome. J Pineal Res. 2009;46(2):224–234. doi: 10.1111/j.1600-079X.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 293.Wang LW, Berry-Kravis E, Hagerman RJ. Fragile X: leading the way for targeted treatments in autism. Neurotherapeutics. 2010;7(3):264–274. doi: 10.1016/j.nurt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]