Abstract
We have examined several emerging brominated flame retardants (BFRs) including 2-ethyl-1-hexyl-2,3,4,5-tetrabromobenzoate (TBB), bis(2-ethylhexyl) tetrabromophthalate (TBPH), 1,2-bis(2,4,6-tribromophenoxy) ethane (BTBPE), 4,5,6,7-tetrabromo-1,1,3-trimethyl-3-(2,3,4,5-tetrabromophenyl)-indane (OBIND), and decabromodiphenyl ethane (DBDPE) in paired human maternal serum (n = 102) and breast milk (n = 105) collected in 2008–2009 in the Sherbrooke region in Canada. Three legacy BFRs were also included in the study for comparison: decabromobiphenyl (BB-209), 2,2′,4,4′,5,5′-hexabromobiphenyl (BB-153), and 2,2′,4,4′,5,5′-hexabromodiphenyl ethers (BDE-153). TBB, BB-153, and BDE-153 had detection frequencies greater than 55% in both serum and milk samples. Their lipid weight (lw) adjusted median concentrations (ng g–1 lw) in serum and milk were 1.6 and 0.41 for TBB, 0.48 and 0.31 for BB-153, and 1.5 and 4.4 for BDE-153, respectively. The detection frequencies for the other BFRs measured in serum and milk were 16.7% and 32.4% for TBPH, 3.9% and 0.0% for BTBPE, 2.0% and 0.0% for BB-209, 9.8% and 1.0% for OBIND, and 5.9% and 8.6% for DBDPE. The ratio of TBB over the sum of TBB and TBPH (fTBB) in serum (0.23) was lower than that in milk (0.46), indicating TBB has a larger tendency than TBPH to be redistributed from blood to milk. Overall, these data confirm the presence of non-PBDE BFRs in humans, and the need to better understand their sources, routes of exposure, and potential human health effects.
Introduction
Flame retardants are chemicals used to inhibit or resist the spread of fire. They are widely used in different consumer products such as upholstered furniture, curtains, carpeting, textiles, plastics, and electronic devices to meet flammability standards in minimizing fire-related damage and death.1 Brominated flame retardants (BFRs) are one of the major groups of flame retardants. Polybrominated diphenyl ethers (PBDEs) are a well-known, major class of BFRs. Some PBDEs have been recognized within the international community to be persistent, bioaccumulative, and to have potential endocrine disrupting effects and developmental neurotoxicity.2
Regulations on the elimination or restriction of production and use of PBDEs have been implemented in many jurisdictions around the world. Alternative flame retardants have been developed as substitutes. As with PBDEs, many of these are additive, which means that they are not chemically bonded to the treated materials and have the potential to be released in the environment. For example, 2-ethyl-1-hexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) tetrabromophthalate (TBPH) (Figure 1) are the two major additive BFRs in Firemaster 550, which is used as a replacement for PentaBDE.1 TBB and TBPH have been reported in the environment, such as the Great Lakes atmosphere,3 wastewater,4 fish,5 and gulls.6 They have also recently been found in household dust in Canada7 and the U.S.8−10 where concentrations are typically an order of magnitude higher than those observed in Europe11−13 and elsewhere.14,15
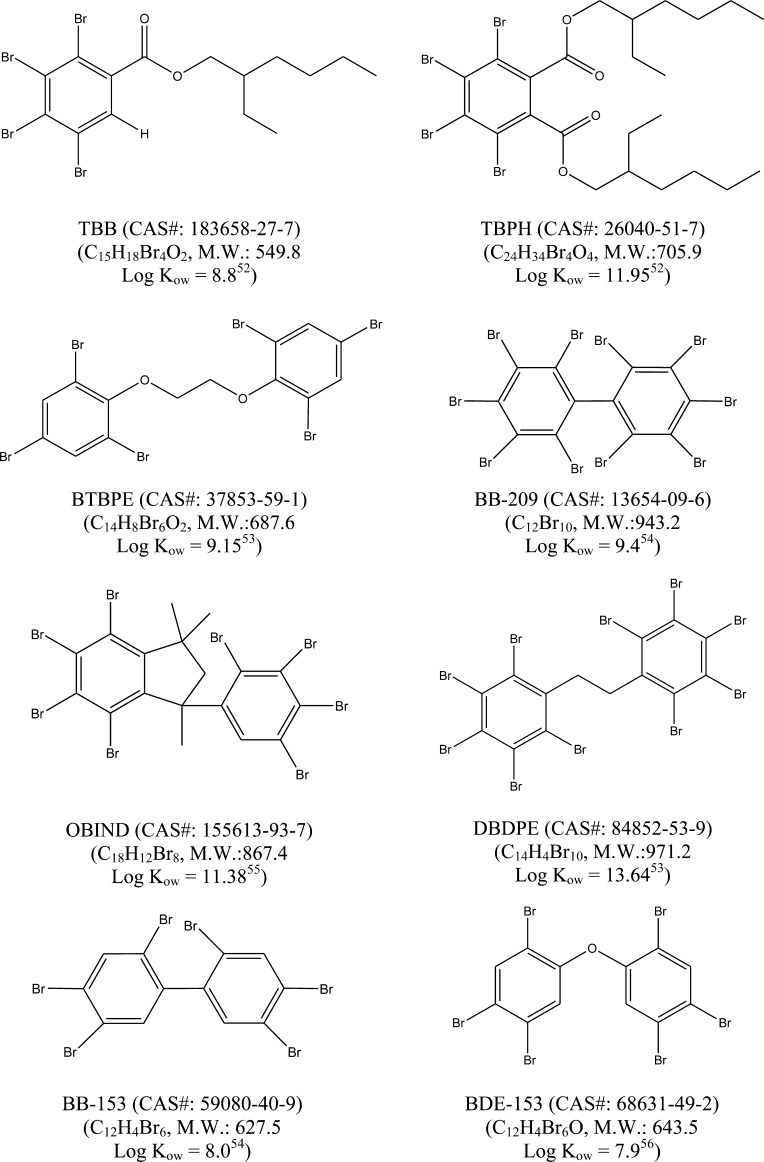
Figure 1.
Molecular structures, formulas, molecular weight (M.W.) and octanol–water partition coefficients (Kow) of brominated flame retardants measured in this study. TBB = 2-ethyl-1-hexyl 2,3,4,5-tetrabromobenzoate, TBPH = bis(2-ethylhexyl)tetrabromophthalate, BTBPE = 1,2-bis(2,4,6-tribromophenoxy) ethane (BTBPE), BB-209 = decabromobiphenyl, OBIND = 4,5,6,7-tetrabromo-1,1,3-trimethyl-3-(2,3,4,5-tetrabromophenyl)-indane, DBDPE = decabromodiphenyl ethane, BB-153 = 2,2′,4,4′,5,5′-hexabromobiphenyl, and BDE-153 = 2,2′,4,4′,5,5′-hexabromodiphenyl ethers.
1,2-Bis(2,4,6-tribromophenoxy) ethane (BTBPE) (Figure 1) is another additive BFR and is used to substitute OctaBDE to meet market demand. It is a major BFR component in Firemaster 680.16 BTBPE has been detected in several environmental compartments including ambient air,17 sediments,18,19 and bird eggs,20 as well as in household dust collected in the U.S.,8 Europe,12,13 and New Zealand.15
Decabromodiphenyl ethane (DBDPE) has a similar structure as the decabrominateddiphenyl ether (BDE-209) and has been marketed as an alternative to the DecaBDE commercial mixture.21 DBDPE has been manufactured for over 20 years and is still a High Production Volume (HPV) chemical in the U.S. today.19,21 DBDPE was found in air,22 wastewater,4 sediments,19 and herring gull eggs,20 and was detected in household dust in the U.S.,8 Europe,11,12 and elsewhere.14,15,23
Although the production information on 4,5,6,7-Tetrabromo-1,1,3-trimethyl-3-(2,3,4,5-tetrabromophenyl)-Indane (OBIND), which is used primarily in polyamides and polystyrene, is very limited, this chemical has been detected in peregrine falcon eggs6,24 and ring-billed gulls6 in Canada. It has been detected in household dust in Canada with a low detection frequency,7 and was below the detection limit in household dust samples from both the U.S.8 and the Czech Republic.25
While there is a body of evidence indicating the presence of two major non-PBDE BFRs, namely tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCD) in humans,26−29 data describing emerging non-PBDE BFRs described above in humans are very limited. There is so far no data on the presence of TBB and OBIND in humans. Human data on TBPH, DBDPE, and BTBPE are also scarce. The objective of this study was to investigate the possible presence of these emerging non-PBDE BFRs in paired human maternal serum and milk samples in an effort to investigate the extent of internal human exposure to these chemicals. Two legacy non-PBDE BFRs, BB-209, and BB-153 as well as a major PBDE congener, BDE-153, were included in this study for comparative purposes relative to the emerging BFRs.
Experimental Section
Eight target chemicals, namely 2-ethyl-1-hexyl-2,3,4,5-tetrabromobenzoate(TBB), bis(2-ethylhexyl) tetrabromophthalate (TBPH), 1,2-bis(2,4,6-tribromophenoxy) ethane (BTBPE), 4,5,6,7-tetrabromo-1,1,3-trimethyl-3-(2,3,4,5-tetrabromophenyl)-indane (OBIND), decabromodiphenyl ethane (DBDPE), decabromobiphenyl (BB-209), 2,2′,4,4′,5,5′-hexabromobiphenyl (BB-153) and 2,2′,4,4′,5,5′-hexabromodiphenyl ethers (BDE-153), as well as labeled chemicals, 13C14-DBDPE and 13C6-BTBPE were purchased from Wellington Laboratories (Guelph, Canada). The other labeled chemicals, 13C12-BDE-153, 13C10-syn-Dechlorane Plus (13C10-syn-DP), 13C10-anti-Dechlorane Plus (13C10-anti-DP), and 13C12-BDE-209 were purchased from Cambridge Isotope Laboratories Inc. (Massachusetts, U.S.A.).
Sample collection and experimental procedures for the sample preparation were described elsewhere.30 A total of 102 human serum and 105 breast milk samples, of which 100 serum samples paired with milk samples (i.e., from the same mother), were randomly selected from the biobank of a cohort study on nursing women in Sherbrooke, Québec, Canada. Samples in the biobank were collected in 2008–2009. Samples (5 g of milk or 2 g of serum) were mixed with 5 mL of water, 1 mL of 2% potassium oxalate, 10 mL of ethanol and 5 mL of diethyl ether after being spiked with several isotope labeled standards serving as recovery surrogates. This mixture was solvent-extracted three times, each with 10 mL of pentane. Fat content in the extract was removed using gel permeation chromatography. Further sample cleanup was performed on an aluminum oxide mini-column built inside a Pasteur pipet. The final solution was reduced to a volume of 0.2 mL and an internal standard, 13C10-syn-DP, was added prior to GC/MS analysis.
An Agilent 7890A gas chromatograph/7000A Triple Quad mass spectrometer (GC/MS) (Agilent Technologies, California, U.S.A.) instrumental system equipped with a DB-1MS column (0.25 mm i.d. × 0.25 μm film thickness, J&W Scientific, California, U.S.A.) was employed for instrumental analysis. The MS was operated in electron capture negative chemical ionization (ECNCI) mode. Two columns with different lengths were used. TBB, TBPH, BTBPE, BB-153, and BDE-153 were analyzed using a 30-m long column, while BB-209, OBIND, and DBDPE were analyzed using a 5-m long column. GC oven temperature for the 30-m column started at 120 °C for 1 min, was ramped to 300 °C at 10 °C min–1, kept for 8 min, ramped to 310 °C at 10 °C min–1, and held for 12 min. Oven temperature for the 5-m column was set at 150 °C, held for 1 min, and then was ramped to 310 °C at 8 °C min–1, and held for 15 min.
The temperature remained constant at 270 and 300 °C for the injection port and the transfer line, respectively. Selected ion monitoring (SIM) was employed to determine individual peak. The following pair of ions were monitoring for each target compound with the first one being used as quantifying ion and the second one for peak identification: m/z 80.9 and m/z 78.9 for TBB, BB-153, TBPH, and OBIND, m/z 563.7 and m/z 561.7 for BDE-153, m/z 251.8 and m/z 250.8 for BTBPE, m/z 943.5 and m/z 945.5 for BB-209, and m/z 890.5 and m/z 892.6 for DBDPE. The ions selected for the quantification of labeled standards were m/z 575.7 for 13C12–BDE-153, m/z 258 for 13C6-BTBPE, m/z 663.8 for 13C10-anti-DP and 13C10-syn-DP, m/z 494.8 for 13C12-BDE-209, and m/z 906.5 for 13C14-DBDPE. Peak areas were normalized for all detected GC/MS peaks against the peak area of spiked internal standard 13C10-syn-DP.
Detection censoring criteria for the positive identification of peaks were as follows: (a) instrument signal-to-noise (s/n) ratio of at least 3:1; (b) ratio of the two monitored ions within the range of 70 to 130% of that of the standards; and (c) the match of retention times of the standards within ± 0.01 min.
A procedural blank of distilled water was added to each batch of seven serum or milk samples; the amounts of the chemical measured in the samples were subtracted by the level found in the blank. Eighteen blanks were performed for milk samples, and the median and mean values, adjusted to a nominal sample size of 5 g and a mean lipid value of 2%, were 0.15 and 0.23, 0.00 and 0.05, 0.02 and 0.03, 0.14 and 0.26, and 0.00 and 0.09 (ng g–1 lw) for TBB, TBPH, BB-153, BDE-153, and DBDPE, respectively. Fifteen blanks were analyzed for serum samples, and the blank values, adjusted to a nominal sample size of 2 g and a mean lipid value of 0.69%, were 0.87 and 2.12, 0.00 and 0.09, 0.12 and 0.23, 0.80 and 0.99, and 0.00 and 3.3 (ng g–1 lw) for TBB, TBPH, BB-153, BDE-153, and DBDPE, respectively. Levels of OBIND, BB-209 and BTBPE in blanks were largely nondetectable.
The concentrations of target BFRs were further corrected with the recoveries of the following surrogates that were spiked in the same samples: 13C12-BDE-153 for TBB, BB-153, and BDE-153, 13C6-BTBPE for BTBPE, 13C10-anti-DP for TBPH, 13C12-BDE-209 for BB-209 and OBIND, and 13C14-DBDPE for DBDPE. Average percent recoveries and standard deviation (s.d.) of the spiked surrogates in serum and milk samples were 49 ± 16 and 50 ± 18 for 13C12-BDE-153, 90 ± 51 and 77 ± 49 for 13C6-BTBPE, 46 ± 8 and 42 ± 12 for 13C10-anti-DP, 42 ± 20 and 33 ± 22 for 13C12-BDE-209, and 30 ± 15 and 15 ± 11 for 13C14-DBDPE, respectively.
Levels of target chemicals were reported on a lipid weight adjusted basis. Methods for the determination of lipid content in serum and milk were described elsewhere.33 The lowest detected, lipid-adjusted concentration in the data set was used as the estimated limit of detection (LOD, see Table 1). Half the value of the LOD was used to replace nondetect data points for statistical analysis of the data. Eighteen pairs of duplicates of milk samples were included in the study. Due to limited volume available for serum samples, no duplicate analysis was conducted for serum samples. The mean percent differences from 18 pairs of duplicates were 27% for TBB (n = 14, mean = 1.0 ng g–1 lw), 13% for BB-153 (n = 14, mean = 0.65 ng g–1 lw), and 9% for BDE-153 (n = 16, mean = 21 ng g–1 lw), respectively. Values below the detection limit were not included.
Table 1. Concentrations (ng g–1 lw) of Measured Brominated Flame Retardants in Maternal Serum and Milk Samples Collected from Mothers in Sherbrooke, Québec, Canadaa.
| %lipid | TBB | TBPH | BB-153 | BDE-153 | DBDPE | OBIND | BB-209 | BTBPE | |
|---|---|---|---|---|---|---|---|---|---|
| serum (n = 102) | |||||||||
| %DF | 56.9 | 16.7 | 71.6 | 58.8 | 5.9 | 9.8 | 2.0 | 3.9 | |
| min | 0.35 | ND | ND | ND | ND | ND | ND | ND | ND |
| 10%ile | 0.53 | ND | ND | ND | ND | ND | ND | ND | ND |
| 25%ile | 0.61 | ND | ND | ND | ND | ND | ND | ND | ND |
| 50%ile | 0.67 | 1.6 | ND | 0.48 | 1.5 | ND | ND | ND | ND |
| 75%ile | 0.76 | 5.5 | ND | 0.91 | 6.1 | ND | ND | ND | ND |
| 90%ile | 0.85 | 12 | 11 | 3.2 | 48 | ND | ND | ND | ND |
| 95%ile | 0.93 | 22 | 33 | 6.8 | 108 | 3.4 | 2.9 | ND | ND |
| max | 1.0 | 68 | 164 | 166 | 308 | 123 | 90 | 72 | 16 |
| mean | 0.69 | 5.4 | 2.8 | 18 | |||||
| s.d. | 0.13 | 10 | 16 | 47 | |||||
| geomean | 0.67 | 1.3 | 0.20 | 1.9 | |||||
| LOD | 0.38 | 7.3 | 0.01 | 0.52 | 3.5 | 1.5 | 0.67b | 3.2b | |
| milk (n = 105) | |||||||||
| %DF | 78.1 | 32.4 | 75.2 | 80.0 | 8.6 | 1.0 | 0 | 0 | |
| min | 0.54 | ND | ND | ND | ND | ND | ND | ND | ND |
| 10%ile | 0.85 | ND | ND | ND | ND | ND | ND | ND | ND |
| 25%ile | 1.2 | 0.07 | ND | 0.08 | 1.5 | ND | ND | ND | ND |
| 50%ile | 1.8 | 0.41 | ND | 0.31 | 4.4 | ND | ND | ND | ND |
| 75%ile | 2.6 | 1.0 | 0.80 | 0.51 | 11 | ND | ND | ND | ND |
| 90%ile | 3.3 | 2.7 | 3.0 | 0.99 | 39 | ND | ND | ND | ND |
| 95%ile | 3.7 | 5.3 | 4.0 | 1.5 | 93 | 3.3 | ND | ND | ND |
| max | 9.2 | 24 | 6.6 | 19 | 180 | 25 | 1.3 | ND | ND |
| mean | 2.0 | 1.3 | 0.62 | 16 | |||||
| s.d. | 1.1 | 3.2 | 1.9 | 31 | |||||
| geomean | 1.8 | 0.29 | 0.14 | 4.1 | |||||
| LOD | 0.03 | 0.15 | 0.01 | 0.66 | 1.7 | 0.20b | 0.49b | 0.86b | |
ND = not detected; LOD = limit of detection, defined as signal/noise >3 in real samples.
LOD derived from lowest standard concentration that produced signal/noise >3.
Statistical analysis was performed for the data sets using SigmaStat for Windows Version 3.11 (Systat Software, Inc., Chicago, IL). Correlation was tested by Pearson Product Moment for normally distributed data, and by Spearman Rank Order if data were not normally distributed.
Results
The lipid content in serum samples ranged from 0.35% to 1.0%, with a mean value of 0.69% and a standard deviation (s.d.) of 0.13%, while for milk samples the lipid content ranged from 0.54% to 9.2% with a mean ± s.d. of 2.0 ± 1.1% (Table 1). However, if the value of 9.2% was removed, the maximal lipid content in milk was 4.8%.
Among the eight BFRs (Figure 1) measured in this study, TBB, BB-153, and BDE-153, were detected in more than half (50%) of the samples. In serum, BB-153 was the most frequently detected BFR (71.6% detection frequency), followed by BDE-153 (58.8%) and TBB (56.9%), while in milk, BDE-153 was most frequently detected (80.0%) among the three, followed by TBB (78.1%), and BB-153 (75.2%). The levels of these three BFRs in humans followed the general trend of BDE-153 > TBB > BB-153—a trend more apparent when comparing higher percentiles such as 75%ile, 90%ile, and 95%ile (Table 1).
Correlations among BB-153, BDE-153, and TBB were evaluated. Levels of BB-153 and BDE-153 were found to be strongly correlated in serum samples (p < 0.001, Table 2A). They were also significantly correlated in milk samples, but the correlation was much weaker (p = 0.046, Table 2B). Among these three BFRs, only BDE-153 showed a significant correlation between the levels in serum and milk (p < 0.001). The correlation of BDE-153 levels in serum and milk samples for each individual sample was plotted in Figure 2.
Table 2. Pearson Product Moment Correlation Test of TBB, BB-153, and BDE-153 in Serum (Table 2A) and Milk (Table 2B) Samples (r = Correlation Coefficient, p = p Value).
| A | serum (n = 102) |
||
|---|---|---|---|
| BB-153 | BDE-153 | ||
| TBB | r | –0.026 | –0.031 |
| p | 0.795 | 0.756 | |
| BB-153 | r | 0.628 | |
| p | <0.001 | ||
| B | milk (n = 105) |
||
|---|---|---|---|
| BB-153 | BDE-153 | ||
| TBB | r | 0.145 | –0.034 |
| p | 0.141 | 0.728 | |
| BB-153 | r | 0.196 | |
| p | 0.046 | ||
Figure 2.
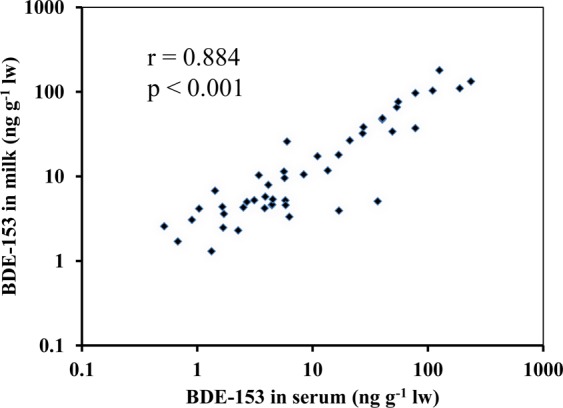
Scatter plot of BDE-153 levels in 46 paired serum and milk samples in which BDE-153 was detected in both matrices.
TBPH was detected in 16.7% and 32.4% of serum and milk samples, respectively. Levels of TBPH were in general lower than TBB levels, except for the levels at 95%ile and maximum in serum samples (Table 1). The fraction of TBB (fTBB), which is defined as the ratio of the amount of TBB to the sum of the TBB and TBPH amount in a sample, ranged from 0.03 to 0.50 with a mean ± s.d. value of 0.23 ± 0.14 in serum (n = 16), and from 0.01 to 0.92 with a mean ± s.d. value of 0.46 ± 0.24 in milk (n = 26), respectively. The fTBB values were calculated where both compounds were detected above the LOD for the same individual.
The detection frequencies of the remaining four BFRs, namely DBDPE, OBIND, BTBPE, and BB-209, were all below 10%; among them BTBPE and BB-209 were not detected in milk samples (Table 1).
Discussion
TBB and TBPH Data
TBB and TBPH are the two major BFR components in the Firemaster 550 additive flame retardant mixture. Although the presence of TBB in the environment has been reported, this study is the first to report its presence in humans. As a result, no direct comparison of our results to other human data was possible. There was no human data for TBPH either, except for one recent report in which TBPH was detected in only one of the 10 pooled human serum samples collected from people residing within 10 km of a production site of halogenated flame retardants in Laizhou Bay.31 The single value of 260 ng g–1 lw in the Chinese sample was roughly double the maximum value of 164 ng g–1 lw measured in our study. TBB, however, was not measured in the Chinese study.
The levels of TBB and TBPH in the human serum samples analyzed in the current study were found to be in the same order of magnitude as some legacy BFRs such as BB-153 and BDE-153. This and the fact that both TBB and TBPH were detected in breast milk indicated possible exposure of general populations and nursing infants to these emerging chemicals. This is particularly important considering their possible toxicological effects, which are currently being evaluated by a number of regulatory agencies including the U.S. Environmental Protection Agency32 and Health Canada.33
TBB fraction (fTBB) in the technical mixture of Firemaster 550 has been reported to be in the range of 0.70–0.80.3 The fraction in indoor dust in North America was reported to be 0.5,7,9,10 with one exception (0.27).8 In comparison, fTBB in human serum samples determined in this study was smaller at 0.23. Therefore, a general trend of decrease in fTBB values from products to indoor environment and to humans can be seen. Interestingly, the fTBB value in milk (0.46) was higher than that in serum, indicative of possible TBB preferential partitioning from blood to milk in humans relative to TBPH. This is likely driven by their respective lipophilic properties; TBPH is a highly hydrophobic substance with an estimated Kow value (log Kow of 12) that is more than 3 orders of magnitude greater than TBB (log Kow of 8.8)34 (Figure 1). It is known that persistent organic pollutants with a log Kow greater than 8 tend to have a lower bioaccumulation potential from the environment to biota.35,36
BB-153 and BDE-153 Data
BB-153 and BDE-153 (both hexa-brominated congeners) were also dominant BFRs among the target analytes in our study (Table 1). BB-153 was the congener of highest concentration in the commercial mixture of polybrominated biphenyls (PBBs).37 Rawn et al. recently reported levels of BDE-153 in 59 pooled serum samples collected from 4583 individuals between 2007 and 2009 within the Canadian Health Measures Survey (CHMS).27 The mean and geometric mean of BDE-153 levels in the 20–39 year old age group in CHMS were 12 ng g–1 lw and 11 ng g–1 lw, respectively. In comparison, the mean BDE-153 concentration observed in our study (18 ng g–1 lw) was higher, while the geometric mean (1.9 ng g–1 lw) was lower than that in CHMS. It is difficult to elucidate reasons for the differences in burdens observed between our study and that of the CHMS. Variability may be due to several study-specific characteristics including timing of sampling, cohort composition, sample size, and analytical methods (e.g., lipid content estimation, detection limits, and recoveries). The median value of BDE-153 (1.5 ng g–1 lw) in sera of our study, however, was closer to the median value in the plasma of Danish nursing women (1.1 ng g–1 lw).38
Both BDE-153 and BB-153 were also measured in 2062 serum samples from the general population in the U.S. under the NHANES (National Health and Nutrition Examination Survey) program from 2003 to 2004.39 The median value of BDE-153 and BB-153 among the 20 to 39 year old age group in the NHANES data set was 5.4 ng g–1 lw and 1.6 ng g–1 lw, respectively. These values were three to four times higher than the values (1.5 ng g–1 lw and 0.48 ng g–1 lw, respectively) found in our study. The ratio of the median values of BDE-153 and BB-153 in both studies, however, was similar at about 3. A strong correlation between BB-153 and BDE-153 (Table 2A) in serum samples was observed in our study but not in the NHANES data set.39
With regard to breast milk, the detection frequency of BB-153 in our study (75.2%) was lower than that of milk samples collected from Danish (100%) and Finnish (97%) participants.40 The median concentration of BB-153 in milk samples (0.31 ng g–1 lw) in our study, however, were higher than those measured in milk samples of Danish (0.20 ng g–1 lw) and Finnish (0.13 ng g–1 lw) participants.40 The mean values of BDE-153 and BB-153 in human milk from 39 first time mothers in New Zealand were 0.72 and 0.15 ng g–1 lw, respectively.41 The median value of BDE-153 in milk in our study (4.4 ng g–1 lw) was double that (2.0 ng g–1 lw) reported in a previous study of 48 milk samples from the same cohort;42 however, the difference in BDE-153 concentration between both studies was not significantly different (p = 0.06).
Despite the fact that information about the time lapse between the collection of serum and milk samples from the same individual could not be determined in our study,30 BDE-153 concentrations showed a statistically significant correlation of its levels in serum and milk (Figure 2), although such a correlation was not observed for other measured BFRs. A similar association between sample media has also been observed in maternal and umbilical plasma collected from a Danish cohort.38 A statistically significant correlation of Dechlorane Plus levels between milk and blood that had been collected 2 to 7 days following delivery when maternal blood had also been collected was reported.43
BTBPE, BB-209, OBIND, and DPDPE Data
The low detection frequency for these four BFRs may relate to a combination of factors such as low prevalence in the environment and their physical-chemical properties that may influence the uptake and bioaccumulation of these BFRs. These BFRs all exhibit a combination of high molecular weights and high log Kow values (Figure 1), relative to other chemicals measured in this study such as TBB, BB-153, and BDE-153.
Only very limited human biomonitoring data are available in the literature for comparison of our results for these emerging BFRs. DBDPE was detected in only 3% of the human milk samples collected from New Zealand women in 2008.41 BTBPE and DBDPE were not detected above the limit of detection of 1.31 ng g–1 lw and 1.03 ng g–1 lw, respectively, in blood plasma samples from five study participants from Sweden.44 No other studies on BTBPE and DBDPE biomonitoring in the literature were found. As previously mentioned, no reports of OBIND and BB-209 in human samples were identified. BB-209 was not detected in human samples; it was detected in one of the pooled cat sera in Sweden at 450 ng g–1 lw in one pooled sample, thus highly limiting comparability to our study.45
Implications for Human Exposure
Dietary exposure has been found to be an important source of PBDE exposure for the general population,46 and breast milk has been found to be an important source of exposure for nursing infants, who obtain most, if not all, of their dietary intake from human milk. However, little to no information on the dietary exposure to emerging non-PBDE BFRs could be identified.47 One study reported the presence of TBB and TBPH in 32 fish collected in lakes in Ontario, Canada.48 Another source of BFR exposure is house dust. Several studies have linked body burdens of PBDEs to indoor dust concentrations. For example, serum PentaBDEs of a toddler cohort were significantly correlated with both hand-wipe and house dust samples.49 Significant and positive associations were observed between PBDE concentrations (excluding BDE-209) in breast milk of first-time mothers and house dust (p = 0.003).50 Breast milk concentrations were also positively correlated (p < 0.05) with mattress and floor dust concentrations for select PBDE congeners, including BDE-209.51 The relative contribution of each source to the total intake of a given substance may vary based on diet, age and activity patterns.52−56
Many of the BFRs measured in our study have been reported to be present in indoor dust. For example, several BFRs in our study were measured in dust samples (n = 116) collected in 2007 and 2008 from homes in Vancouver, Canada, where median concentrations in dust were found in the following order: TBB (120 ng g–1) > TBPH (99 ng g–1) > BDE-153 (42 ng g–1) > BTBPE (30 ng g–1) > OBIND (13 ng g–1).7 These concentrations, however, were considerably lower than BDE-209 (1300 ng g–1) from the same dust samples. The emerging BFRs in our study were also detected in several U.S. household dust studies. In samples (n = 38) collected from unspecified location in the U.S.A. in 2002 and 2003, median concentrations of BFRs were found to be in the following order: TBPH (435 ng g–1) > TBB (68.4 ng g–1) > BTBPE (18.2 ng g–1).9 Similar patterns were observed for samples (n = 20) collected from Boston in 2006: TBPH (142 ng g–1) > TBB (133 ng g–1) > BTBPE (30 ng g–1).10 Although detection frequencies of BTBPE in house dust from Vancouver, Canada (100%)7 and Boston, U.S.A. (>70%)10 were relatively high, concentrations of BTBPE were approximately 4 times lower than TBB in both studies.
Median concentrations of DBDPE were higher than TBB in two separate dust studies in the United States.8,10 In Europe, DBDPE was monitored extensively in household dust, and has generally been measured in higher concentrations relative to other BFRs measured in our study. For example, in a Belgium house dust study, DBDPE was measured at a median concentration of 153 ng g–1 (n = 39) while TBB was measured at 1 ng g–1.13 In dust samples (n = 47) collected in 2010 from households in Romania, DBDPE median concentrations (170 ng g–1) exceeded those of TBPH (10 ng g–1), BTBPE (4 ng g–1), and TBB (<2 ng g–1).11
Despite their detection in dust, both BTBPE and DBDPE were not detected in plasma from the inhabitants of the households in Sweden,44 and DBDPE was measured in New Zealand human milk in a range of 0.015–0.33 ng g–1 lw41 with very limited detectability (3% detection frequency), similar to our study where low concentrations (ND–25 ng g–1 lw) and low detection frequencies (8.6%) were also observed. While DBDPE may be present in house dust, toxicokinetics and analytical methods are likely playing a role in their low detection frequency in human samples.
Additional sources of exposure may include mouthing and hand-to-mouth exposure from products containing BFRs, such as electronics and other polymer-based consumer goods. A recent study showed that exposure to PentaBDE in an office environment contributed most to PentaBDE body burden of participating office occupants, with exposure likely linked to PBDE residues on hands.49 Overall, the association between the amounts of emerging BFRs in humans and potential sources remains to be fully elucidated and requires further investigation.
Acknowledgments
We acknowledge the financial support from the Canadian government’s Chemicals Management Plan, the Fonds de Recherche du Québec–Santé (Grant No. 12397) and the Canadian Institutes of Health Research (CIHR) (Grant No. MOP-84551). We thank the personnel of the maternity service unit at the Centre Hospitalier Universitaire de Sherbrooke (CHUS) for their active participation in the collection of samples at delivery, and Dr. Donna Cherniak and her team at CHUS in organizing the recruitment of pregnant women. We also acknowledge valuable comments from colleagues at Existing Substances Risk Assessment Bureau of Health Canada, and Dr. Thea Rawn and Dr. Rocio Aranda-Rodriguez for reviewing the manuscript. Dr. Abdelouahab is supported by a Banting Postdoctoral Fellowship. Dr. Takser is supported by a CIHR New Investigator Award. Dr. Zhou and Dr. Siddique are supported by the Canadian Government Laboratory Visiting Fellow program.
The authors declare no competing financial interest.
References
- Shaw S. D.; Blum A.; Weber R.; Kannan K.; Rich D.; Lucas D.; Koshland C. P.; Dobraca D.; Hanson S.; Birnbaum L. S. Halogenated flame retardants: Do the fire safety benefits justify the risks?. Rev. Environ. Health 2010, 254261–305. [DOI] [PubMed] [Google Scholar]
- Costa L. G.; Giordano G.; Tagliaferri S.; Caglieri A.; Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: Environmental contamination, human body burden and potential adverse health effects. Acta Bio Med. Atenei Parmensis 2008, 793172–183. [PubMed] [Google Scholar]
- Ma Y.; Venier M.; Hites R. A. 2-Ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate flame retardants in the Great Lakes atmosphere. Environ. Sci. Technol. 2012, 461204–208. [DOI] [PubMed] [Google Scholar]
- Zhou S. N.; Reiner E. J.; Marvin C.; Helm P.; Riddell N.; Dorman F.; Misselwitz M.; Shen L.; Crozier P.; MacPherson K.; Brindle I. D. Development of liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry for analysis of halogenated flame retardants in wastewater. Anal. Bioanal. Chem. 2010, 39631311–1320. [DOI] [PubMed] [Google Scholar]
- Zhou S. N.; Reiner E. J.; Marvin C. H.; Helm P. A.; Shen L.; Brindle I. D. Liquid chromatography/atmospheric pressure photoionization tandem mass spectrometry for analysis of Dechloranes. Rapid Commun. Mass Spectrom.. 2011, 253436–442. [DOI] [PubMed] [Google Scholar]
- Gentes M. L.; Letcher R. J.; Caron-Beaudoin E.; Verreault J. Novel flame retardants in urban-feeding Ring-Billed Gulls from the St. Lawrence River, Canada. Environ. Sci. Technol. 2012, 46179735–9744. [DOI] [PubMed] [Google Scholar]
- Shoeib M.; Harner T.; Webster G. M.; Sverko E.; Cheng Y. Legacy and current-use flame retardants in house dust from Vancouver, Canada. Environ. Pollut. 2012, 169, 175–182. [DOI] [PubMed] [Google Scholar]
- Dodson R. E.; Perovich L. J.; Covaci A.; Van den Eede N.; Ionas A. C.; Dirtu A. C.; Brody J. G.; Rudel R. A. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012, 462413056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. I.; Stapleton H. M.; Mukherjee B.; Hauser R.; Meeker J. D. Associations between brominated flame retardants in house dust and hormone levels in men. Sci. Total Environ. 2013, 445, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M.; Allen J. G.; Kelly S. M.; Konstantinov A.; Klosterhaus S.; Watkins D.; McClean M. D.; Webster T. F. Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 2008, 42186910–6916. [DOI] [PubMed] [Google Scholar]
- Dirtu A. C.; Ali N.; Van den Eede N.; Neels H.; Covaci A. Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania, 2010. Environ. Int. 2012, 49, 1–8. [DOI] [PubMed] [Google Scholar]
- Van den Eede N.; Dirtu A. C.; Ali N.; Neels H.; Covaci A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta 2012, 89, 292–300. [DOI] [PubMed] [Google Scholar]
- Ali N.; Harrad S.; Goosey E.; Neels H.; Covaci A. “Novel” brominated flame retardants in Belgian and U.K. indoor dust: Implications for human exposure. Chemosphere 2011, 83101360–1365. [DOI] [PubMed] [Google Scholar]
- Ali N.; Van den Eede N.; Dirtu A.; Neels H.; Covaci A. Assessment of human exposure to indoor organic contaminants via dust ingestion in Pakistan. Indoor Air. 2012, 223200–211. [DOI] [PubMed] [Google Scholar]
- Ali N.; Dirtu A. C.; Van den Eede N.; Goosey E.; Harrad S.; Neels H.; ’t Mannetje A.; Coakley J.; Douwes J.; Covaci A. Occurrence of alternative flame retardants in indoor dust from New Zealand: Indoor sources and human exposure assessment. Chemosphere 2012, 88111276–1282. [DOI] [PubMed] [Google Scholar]
- Bergman A.; Rydén A.; Law R. J.; Boer J.; Covaci A.; Alaee M.; Birnbaum L.; Petreas M.; Rose M.; Sakai S.; den Eede N. V.; van der Veen I. Environ. Int. 2012, 49, 57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C. A.; Herzke D.; Vorkamp K. Brominated flame retardants in the Arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408152885–2918. [DOI] [PubMed] [Google Scholar]
- Hoh E.; Zhu L. Y.; Hites R. A. Novel flame retardants, 1,2-bis(2,4,6-tribromophenoxy)ethane and 2,3,4,5,6-pentabromoethylbenzene, in United States’ environmental samples. Environ. Sci. Technol. 2005, 3982472–2477. [DOI] [PubMed] [Google Scholar]
- Yang R.; Wei H.; Guo J.; Li A. Emerging brominated flame retardants in the sediment of the Great Lakes. Environ. Sci. Technol. 2012, 4663119–3126. [DOI] [PubMed] [Google Scholar]
- Gauthier L. T.; Potter D.; Hebert C. E.; Letcher R. J. Temporal trends and spatial distribution of non-polybrominated diphenyl ether flame retardants in the eggs of colonial populations of Great Lakes Herring Gulls. Environ. Sci. Technol. 2009, 432312–317. [DOI] [PubMed] [Google Scholar]
- Kierkegaard A.; Bjorklund J.; Friden U. Identification of the flame retardant decabromodiphenyl ethane in the environment. Environ. Sci. Technol. 2004, 38123247–3253. [DOI] [PubMed] [Google Scholar]
- Salamova A.; Hites R. A. Discontinued and alternative brominated flame retardants in the atmosphere and precipitation from the Great Lakes Basin. Environ. Sci. Technol. 2011, 45208698–8706. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Luo X. J.; Yuan J. G.; Wang J.; Wang Y. T.; Chen S. J.; Mai B. X.; Yang Z. Y. Levels and sources of brominated flame retardants in human hair from urban, e-waste, and rural areas in South China. Environ. Pollut. 2011, 159123706–3713. [DOI] [PubMed] [Google Scholar]
- Guerra P.; Alaee M.; Jimenez B.; Pacepavicius G.; Marvin C.; MacInnis G.; Eljarrat E.; Barcelo D.; Champoux L.; Fernie K. Emerging and historical brominated flame retardants in peregrine falcon (Falco peregrinus) eggs from Canada and Spain. Environ. Int. 2012, 40, 179–186. [DOI] [PubMed] [Google Scholar]
- Kalachova K.; Hradkova P.; Lankova D.; Hajslova J.; Pulkrabova J. Occurrence of brominated flame retardants in household and car dust from the Czech Republic. Sci. Total Environ. 2012, 441, 182–193. [DOI] [PubMed] [Google Scholar]
- Kim U. J.; Oh J. E. Tetrabromobisphenol A and hexabromocyclododecane flame retardants in infant-mother paired serum samples, and their relationships with thyroid hormones and environmental factors. Environ. Pollut. 2014, 1841193–200. [DOI] [PubMed] [Google Scholar]
- Rawn D. F.; Ryan J. J.; Sadler A. R.; Sun W. F.; Weber D.; Laffey P.; Haines D.; Macey K.; Van Oostdam J. Brominated flame retardant concentrations in sera from the Canadian Health Measures Survey (CHMS) from 2007 to 2009. Environ. Int. 2014, 63226–34. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Jiao Y.; Hu Y.; Sun Z.; Zhou X.; Feng J.; Li J.; Wu Y. Levels of tetrabromobisphenol A, hexabromocyclododecanes and polybrominated diphenyl ethers in human milk from the general population in Beijing, China. Sci. Total Environ. 2013, 452–453510–18. [DOI] [PubMed] [Google Scholar]
- Carignan C. C.; Abdallah M. A.; Wu N.; Heiger-Bernays W.; McClean M. D.; Harrad S.; Webster T. F. Predictors of tetrabromobisphenol-A (TBBP-A) and hexabromocyclododecanes (HBCD) in milk from Boston mothers. Environ. Sci. Technol. 2012, 462112146–12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. N.; Siddique S.; Lavoie L.; Takser L.; Abdelouahab N.; Zhu J. Hexachloronorbornene-based flame retardants in humans: Levels in maternal serum and milk. Environ. Int. 2014, 66, 11–17. [DOI] [PubMed] [Google Scholar]
- He S.; Li M.; Jin J.; Wang Y.; Bu Y.; Xu M.; Yang X.; Liu A. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of Laizhou Bay, China. Environ. Toxicol. Chem. 2013, 3261242–1247. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. TSCA Work Plan and Action Plan Risk Assessments and Data Collection Activities; http://www.epa.gov/oppt/existingchemicals/pubs/2013wpractivities.html. 2013.
- Government of Canada. Certain Organic Flame Retardants Substance Grouping; http://www.chemicalsubstanceschimiques.gc.ca/group/flame_retardant-ignifuges/index-eng.php. 2013.
- Bearr J. S.; Stapleton H. M.; Mitchelmore C. L. Accumulation and DNA damage in Fathead Minnows (Pimephales Promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster (R) 550 and Firemaster (R) Bz-54. Environ. Toxicol. Chem. 2010, 293722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Ma X.; Lin Z.; Na G.; Yao Z. Congener specific distributions of polybrominated diphenyl ethers (PBDEs) in sediment and mussel (Mytilus edulis) of the Bo Sea, China. Chemosphere 2009, 747896–901. [DOI] [PubMed] [Google Scholar]
- Liang Y.; Tse M.; Young L.; Wong M. Distribution patterns of polycyclic aromatic hydrocarbons (PAHs) in the sediments and fish at Mai Po Marshes Nature Reserve, Hong Kong. Water Res. 2007, 4161303–1311. [DOI] [PubMed] [Google Scholar]
- Sjoedin A.; Wong L. Y.; Jones R. S.; Park A.; Zhang Y.; Hodge C.; Dipietro E.; McClure C.; Turner W.; Needham L. L.; Patterson D. G. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polyhrominated biphenyl (PBB) in the united states population: 2003–2004. Environ. Sci. Technol. 2008, 4241377–1384. [DOI] [PubMed] [Google Scholar]
- Frederiksen M.; Thomsen C.; Froshaug M.; Vorkamp K.; Thomsen M.; Becher G.; Knudsen L. E. Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int. J. Hyg. Environ. Health 2010, 2134233–242. [DOI] [PubMed] [Google Scholar]
- Sjoedin A.; Wong L. Y.; Jones R. S.; Park A.; Zhang Y.; Hodge C.; Dipietro E.; McClure C.; Turner W.; Needham L. L.; Patterson D. G. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polyhrominated biphenyl (PBB) in the united states population: 2003–2004. Environ. Sci. Technol. 2008, 4241377–1384. [DOI] [PubMed] [Google Scholar]
- Shen H.; Maine K. M.; Andersson A. M.; Damgaard I. N.; Virtanen H. E.; Skakkebaek N. E.; Toppari J.; Schramm K. W. Concentrations of persistent organochlorine compounds in human milk and placenta are higher in Denmark than in Finland. Human Reprod. 2008, 231201–210. [DOI] [PubMed] [Google Scholar]
- Mannetje A.; Coakley J.; Bridgen P.; Brooks C.; Harrad S.; Smith A. H.; Pearce N.; Douwes J. Current concentrations, temporal trends and determinants of persistent organic pollutants in breast milk of New Zealand women. Sci. Total Environ. 2013, 458–460, 399–407. [DOI] [PubMed] [Google Scholar]
- Siddique S.; Xian Q.; Abdelouahab N.; Takser L.; Phillips S. P.; Feng Y. L.; Wang B.; Zhu J. Levels of dechlorane plus and polybrominated diphenylethers in human milk in two Canadian cities. Environ. Int. 2012, 39150–55. [DOI] [PubMed] [Google Scholar]
- Ben Y. J.; Li X. H.; Yang Y. L.; Li L.; Di J. P.; Wang W. Y.; Zhou R. F.; Xiao K.; Zheng M. Y.; Tian Y.; Xu X. B. Dechlorane Plus and its dechlorinated analogs from an e-waste recycling center in maternal serum and breast milk of women in Wenling, China. Environ. Pollut. 2013, 173, 176–181. [DOI] [PubMed] [Google Scholar]
- Karlsson M.; Julander A.; van Bavel B.; Hardell L. Levels of brominated flame retardants in blood in relation to levels in household air and dust. Environ. Int. 2007, 33162–69. [DOI] [PubMed] [Google Scholar]
- Norrgran J.; Jones B.; Lindquist N. G.; Bergman A. Decabromobiphenyl, polybrominated diphenyl ethers, and brominated phenolic compounds in serum of cats diagnosed with the endocrine disease feline hyperthyroidism. Arch. Environ. Contam. Toxicol. 2012, 631161–168. [DOI] [PubMed] [Google Scholar]
- Frederiksen M.; Vorkamp K.; Thomsen M.; Knudsen L. E. Human internal and external exposure to PBDEs—A review of levels and sources. Int. J. Hyg. Environ. Health 2009, 2122109–134. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority Website; http://www.efsa.europa.eu/en/efsajournal/doc/2908.pdf. 2012.
- Zhou S. N.; Reiner E. J.; Marvin C.; Kolic T.; Riddell N.; Helm P.; Dorman F.; Misselwitz M.; Brindle I. D. Liquid chromatography-atmospheric pressure photoionization tandem mass spectrometry for analysis of 36 halogenated flame retardants in fish. J. Chromatogr. A 2010, 12175633–641. [DOI] [PubMed] [Google Scholar]
- Watkins D. J.; McClean M. D.; Fraser A. J.; Weinberg J.; Stapleton H. M.; Sjoedin A.; Webster T. F. Exposure to PBDEs in the office environment: Evaluating the relationships between dust, handwipes, and serum. Environ. Health Perspect. 2011, 11991247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N.; Herrmann T.; Paepke O.; Tickner J.; Hale R.; Harvey E.; La Guardia M.; McClean M. D.; Webster T. F. Human exposure to PBDEs: Associations of PBDE body burdens with food consumption and house dust concentrations. Environ. Sci. Technol. 2007, 4151584–1589. [DOI] [PubMed] [Google Scholar]
- Coakley J. D.; Harrad S. J.; Goosey E.; Ali N.; Dirtu A. C.; Van den Eede N.; Covaci A.; Douwes J.; Mannetje A. Concentrations of polybrominated diphenyl ethers in matched samples of indoor dust and breast milk in New Zealand. Environ. Int. 2013, 59, 255–261. [DOI] [PubMed] [Google Scholar]
- EPA. WSKow v 1.41, United States Environmental Protection Agency: Washington, DC, 2009, 2013. [Google Scholar]
- EPA. Estimation Programs Interface Suite for Microsoft® Windows, v 4.10. United States Environmental Protection Agency, Washington, DC, USA. 2012, 2013. [Google Scholar]
- Advanced Chemistry Development (ACD/Labs) Software v 11.02, Toronto, Canada. 2013.
- SPARC Online Calculator. 2013.
- Agency for Toxic Substances and Disease Registry. U.S.A., 2004. 2013. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Agency for Toxic Substances and Disease Registry. U.S.A., 2004. 2013. [PubMed]