Abstract
While it is known that islet cell mass increases considerably after birth, general uncertainty surrounds the source of new beta cells in humans. Chronic pancreatitis (CP) presents a natural injury model for studying postnatal beta-cell regeneration in the human pancreas. In this report, we present histological evidence from human CP pancreases to support the theory that islet neogenesis can occur from ductal precursor cells after birth. Three young patients (ages 16, 12, and 28 years) underwent total pancreatectomy for the management of CP followed by islet isolation and autologous transplantation to prevent or minimize post-surgical diabetes. In all cases, the pancreases had extensive fibrosis, a rock-like consistency, and calcifications in the ducts. During islet isolations, we observed the unusual release of islets with many ductal fragments. In histopathological evaluation of these pancreases, solid cords of cells sometimes formed islet like structures intraductally or extending from ductal structures. Immunofluorescence staining for chromogranin, insulin, proinsulin, PDX1, glucagon and cytokeratins confirmed these structures to be composed of chromogranin-positive endocrine cells which included both β-cells and α-cells. Labeling for Ki67 to demonstrate mitotic activity showed frequent labeling of duct epithelial cells and of some periductal cells. Using insulin and wide-spectrum cytokeratin double-immunofluorescent labeling, we found insulin positive cells to be present within the ductal lumens, among the cytokeratin positive ductal epithelium, and extending from the ductal epithelium into surrounding connective tissues, providing evidence for a ductal origin of islet neogenesis.
Keywords: islet neogenesis, chronic pancreatitis, islet precursor cell, beta cell, islet progenitor cell
Introduction
Islet autotransplantation (IAT) has emerged as an effective treatment for reducing and eliminating the risk of surgical diabetes in patients undergoing total pancreatectomy (TP) for the relief of intractable pain associated with chronic pancreatitis (CP) [1]. Chronic pancreatitis is a progressive and destructive inflammatory process of multifactorial etiology that leads to irreversible obliteration of pancreatic tissue [2]. Parenchymal destruction, ductal changes and dilatation, and other injury induce genomic changes and increases cell turnover [3]. In addition, signaling mechanisms that play a role in the development of the embryonic pancreas are reactivated, and initiate repair and regeneration [4, 5]. Many animal studies have investigated the potential of pancreatic regeneration to restore functional beta-cell mass after injury [6–8]. In humans, CP is characterized by many of the same damaging effects used in early injury-response studies in rodents, and presents a natural injury model for the study of human islet regeneration and its application to novel diabetes therapies. Islet neogenesis, seen as budding of hormone-positive cells from the ductal epithelium [9], is postulated to be one mechanism for normal islet growth and regeneration after birth, and has suggested the presence of pancreatic stem cells [10]. However, whether islet neogenesis from ductal precursors truly occurs in the human pancreas is debated amongst investigators [11].
In our laboratory, during clinical islet isolations from the pancreases of patients with CP, we have sometimes observed unusual cell release patterns, specifically numerous ductal cells with islet and acinar cells. Interestingly, we observed these findings in severely fibrotic and inflamed pancreases from young patients. These observations prompted further histological and immunofluorescence investigation of the pancreases of three young patients with CP which ultimately showed strong evidence of ductal islet neogenesis. In this report, we describe the isolation and histological characteristics of these three cases.
Case Reports
Patients with CP underwent TP and subsequent IAT (TP-IAT) at the University of Minnesota Medical Center. The diagnosis of CP was based on the results of the patient’s medical history and imaging studies of the pancreas. For all patients, the indication for TP was intractable pain. Three cases, unique in age of CP onset and isolation characteristics, were selected for histological study. All patients (or parents) gave written informed consent to participation in a prospective follow up study cohort, including specific permission to evaluate pancreatic histopathology. Biopsy samples were obtained from the pancreases before the isolation procedure. The isolation procedure and histolopathologic evaluation of biopsy specimens were similar for all cases, and are described below.
Isolation Procedure
All islet isolations were performed at the University of Minnesota Molecular and Cellular Therapeutics GMP facility in compliance with federal regulations. Tissue dissociation was performed by injecting cold dissociation enzymes, collagenase and neutral protease (SERVA Electrophoresis GmbH, Heidelberg, Germany), through the main pancreatic duct (MPD) using a pressure-controlled pump system. The pancreas was then digested using a modification of Ricordi’s semi-automated method described elsewhere [12]. The switch from the digestion phase to the dilution phase occurred when most of the islets were free from exocrine tissue. When calcification deposits were observed, the particles were allowed to sediment to the bottom of the collection conicals where they would be quickly aspirated out with a pipette while the islets were agitated and suspended in the media. This was repeated and finished with a final washing of the sediment in a 50 mL conical with 40 mL of media to rescue any lost islets originally aspirated with the calcium deposits. The islet yield and purity were assessed by counting duplicate aliquots stained with diphenylthiocarbazone (Sigma, St. Louis, MO). The liberated islets were then suspended in Connaught Medical Research Laboratories-1066 medium (Mediatech, Inc, Manassas, VA) supplemented with 25 mM HEPES, antibiotics and 2.5% human serum albumin (American Red Cross, Washington, DC). The islets were collected in a transplant bag for delivery to the operating room [13].
Histopathologic Evaluation
Pancreatic biopsy samples were fixed in 10% formalin, embedded in paraffin by routine methods, and stained with hematoxylin and eosin, Alcian blue, and Masson’s trichrome stains.
Immunohistochemistry
For insulin, glucagon, and chromograinin staining, 4 μm sections were cut, deparaffinized, and rehydrated, followed by incubation with 3% hydrogen peroxide to quench endogenous peroxidase activity. Sections were then incubated with the primary antibody (guinea pig anti-swine insulin, cat.#A0564, Dako, Carpinteria, CA; rabbit anti-human glucagon, cat.#NB120-1846 Novus Biologicals, Littleton, CO; mouse monoclonal antibody to Ki67 MiB-1, cat.#ab6526, Abcam, Cambridge, England). Antigen detection was done with anti-rabbit polymer or anti-mouse polymer (EnVision, Dako) and color visualization was done using DAB (Dako).
Immunofluorescence
Double immunofluorescence staining for insulin/cytokeratins or insulin/PDX1 was done on formalin fixed, paraffin-embedded sections cut at 4 μm thickness. For insulin labeling, sections were first deparaffinized, rehydrated, and incubated with guinea pig anti-swine insulin (Dako) for 60 minutes. Next, sections were incubated in the dark with FITC-conjugated, goat anti-guinea pig immunoglobulin for 60 minutes. Sections were next incubated with antibodies to either wide spectrum cytokeratin (Rabbit anti-cytokeratin, cat. #Z0622, Dako), or PDX1 (cat.# BAF2419, R&D systems), followed by incubation in the dark with Streptavidin-AlexaFluor-594 for 60 minutes. Finally, nuclear staining was done using TOPRO-3 (Molecular Probes, Eugene, OR). Labeled sections were examined with a Biorad laser confocal microscope.
Case 1
A 16 year-old female underwent TP for the management of idiopathic CP. The patient’s medical history before TP was remarkable for onset of disease at 5 years of age, history of a failed Puestow procedure, numerous hospitalizations, and progression to daily narcotic dependence (methadone and fentanyl patch). The resected pancreas was small, with a trimmed pancreas weight of 59 grams. The organ was noted to be extensively fibrotic with a rock-like consistency, and had low surface fat and visible calcifications with intraductal stones. On arrival to the laboratory, the pancreas was trimmed and split into two sections, head (35g) and body/tail (24g). Biopsy samples were taken from the cannulated region of the pancreas and fixed in 10% formalin.
Tissue dissociation was performed by injecting cold dissociation enzymes through the MPD using a pressure-controlled pump system. While the head was cannulated with a 16 gauge catheter, the MPD was completely obstructed by intraductal stones in the body/tail section and required interstitial enzyme perfusion (parenchymal injection). Due to the firmness of the organ, however, interstitial distention was not effective in delivering the desired volume of enzyme, and thus the body/tail was sectioned into 22 small pieces to increase exposure to the enzyme in the digestion chamber.
Isolation results are summarized in Table 1. A majority (60%) of the islets were embedded within acinar tissue. Undigested tissue weighing 35.5 grams (60%) remained in the digest chamber. Only 4,130 total islet equivalents (IEQ) (70 IEQ/g pancreas) were isolated, a number insufficient to meet release criteria for transplant and so IAT was not performed. As expected, the patient required full insulin supplementation, with a basal insulin analog and rapid-acting insulin analog postoperatively. She did report complete resolution of pain and was off narcotic medications at 3 months after pancreatectomy.
Table 1.
Patient Characteristics & Islet Isolation Results
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Sex (M/F), Age (yrs), BMI (kg/m2) | Female, 16, 26.4 | Male, 12, 18.3 | Female, 28, 20.8 |
| Cause of Pancreatitis | Unknown | Familial | Sphincter of Oddi dysfunction |
| Pancreas Weight | 59 | 107 | 39 |
| Condition of Pancreas | Rock-like, low fat, medium blood content | Rock-like, medium fat, high blood content | Rock-like, medium fat, medium blood content |
| Condition of Ducts | Intraductal stones | Dilated duct | Dilated duct |
| Digestion Time (min) | 64 | 32 | 37 |
| Dilution Time (min) | 5 | 24 | 20 |
| Undigested Pancreas (%) | 40 | 61 | 90 |
| Postdigest IEQ (103) | 4.13 | 111.98 | 10.28 |
| Postdigest IEQ/g Pancreas | 70 | 1047 | 265 |
| Postdigest IEQ/Islet Number | 0.3 | 0.7 | 0.5 |
| Embedded Islet (%) | 60 | 50 | 75 |
| Islet Viability (FDA/PI; %) | ND | 83.4 | 94.2 |
| Final Product Purity (%) | ND | 30 | 10 |
| Final Tissue Volume (mL) | 1.5 | 3.0 | 2.0 |
| Post-operative insulin use | Dependent | Independent | Dependent |
| HbA1c (after surgery, %) | 9.3 | 5.3 – 6.0 | 7.5 – 8.5 |
| C-peptide (after surgery, ng/mL) | n/a | 0.8 – 4.9 | <0.1 – 0.3 |
BMI, body mass index; IEQ, islet equivalent; Percent recovery, postpurification IEQ/postdigest IEQ × 100; FDA, fluorescein diacetate; PI, propidium iodide
Case 2
A 12 year-old male with autosomal dominant hereditary CP with symptom onset at age 6 years underwent TP-IAT for management of intractable pain and narcotic dependence. The excised pancreas weighed 107 grams. Similar to case #1, the pancreas was very hard with complete fibrotic infiltration. Several intraductal stones up to 3mm in diameter were found in the head section. Two large metal catheters were used to cannulate the two sections of the pancreas due to the presence of a dilated main pancreatic duct (Figure 2). During enzyme perfusion (described in case 1), it was visually hard to monitor the extent of intraductal distention due to the rigidity of the pancreas tissue; however, perfusion of the enzyme and the distention was deemed good. The pancreas sections were cut into 30 small pieces to increase enzyme exposure during the digestion phase of isolation.
Figure 2.
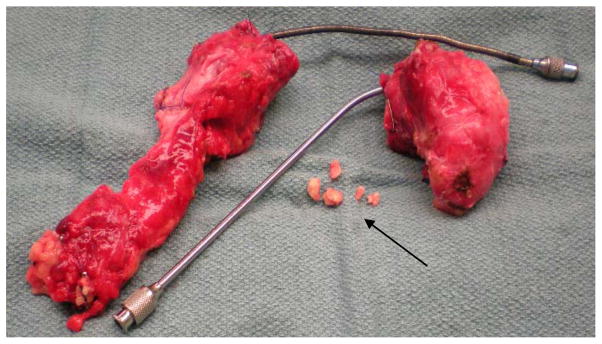
Chronic pancreatitis pancreas resected from male pediatric donor at age 12. Noted were severe fibrosis (white tissue color), rock-like texture, heavy blood infiltration, and medium level of parenchymal fat. Depicted is the pancreas split into head and body/tail sections with large metal catheters used during surgical cannulation to accommodate the dilated main pancreatic duct. Also, several calcification deposits (arrow) up to 3mm in diameter were found intraductally in the head region.
The islet yield was 111,980 total IEQ (1,047 IEQ/g pancreas), with 41.5 g (39%) of pancreas remaining undigested. Approximately 50% of the islets were embedded within acinar tissue with an overall preparation purity of 30%. The final islet preparation was transplanted. The patient discontinued insulin and narcotics approximately 6 weeks after surgery. He has remained pain-free and insulin independent, with euglycemia as evidenced by normal self-monitored blood sugars and hemoglobin A1c levels (5.3–6.0%, most recently 5.7% at 24 months post-IAT).
Case 3
A 28 year old female with acute relapsing pancreatitis diagnosed at age 18 years and progressing to idiopathic chronic pancreatitis underwent TP-IAT for management of pain and 4 year history of daily narcotic use. She had C-peptide positive diabetes mellitus, with a preoperative hemoglobin A1c level of 6.9% and stimulated C-peptide level of 2.7 ng/mL, and thus underwent IAT to preserve beta cell mass. Prior to surgery, she was on metformin and once daily glargine for management of her diabetes. The resected pancreas was small, 38.3 grams in weight, and similar to prior cases, was very hard and rock-like in firmness with severe fibrosis. Unique to this case, the pancreas was atrophic in appearance. Medium levels of blood and fat were observed in the pancreatic parenchyma and calcifications were present throughout the tissue. A large metal catheter was used to cannulate the dilated duct in both sections of the pancreas and a partial distention was observed during enzyme perfusion. The pancreas was cut into 25 pieces to enhance the enzyme exposure during digestion in the Ricordi chamber.
Although the pancreas was nearly completely digested and 4 g (10% of the 39 g pancreas) remained, only 10,280 total IEQ or 265 IEQ/g pancreas were isolated. A majority of these islets were embedded (75%) and the final preparation was impure (10% purity). Nevertheless, the patient underwent the transplant. Postoperatively, the patient continued to require intermittent narcotic medications, and remained on insulin therapy (continuous subcutaneous insulin infusion by pump) with hemoglobin A1c levels of 7.5–8.5% and minimal C-peptide production (0.1–0.3 ng/mL fasting and stimulated).
Histopathology of Pancreas
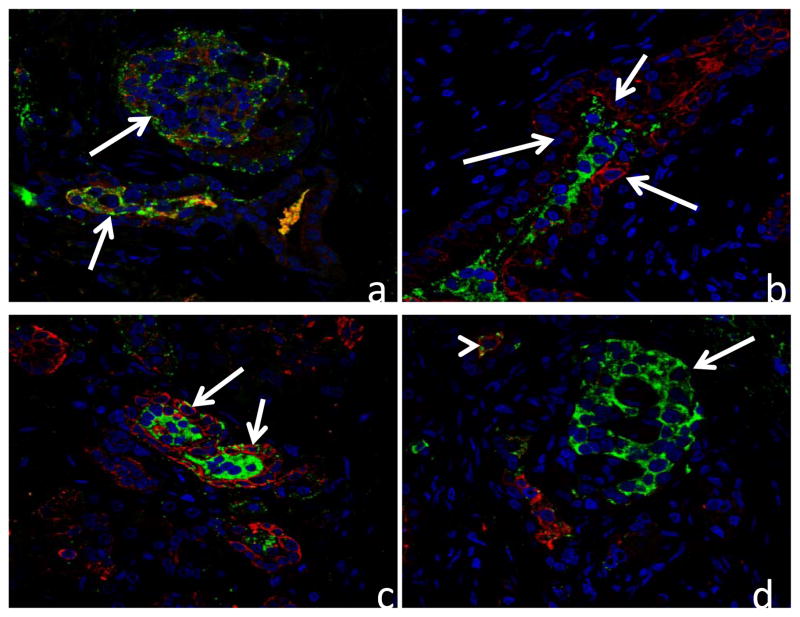
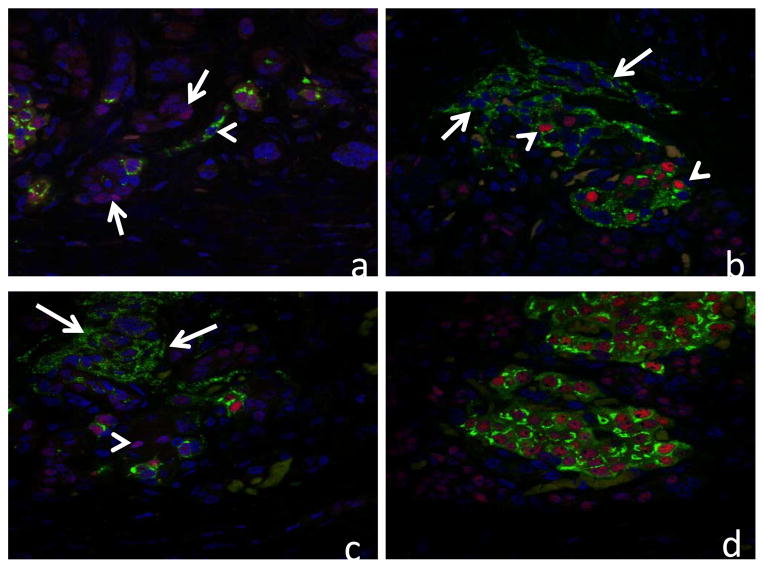
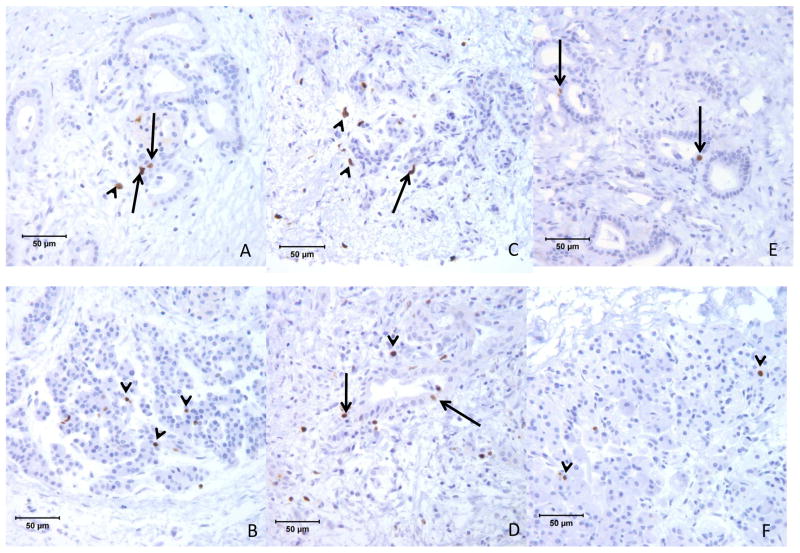
In all 3 cases the pancreas showed marked diffuse loss of exocrine acini with extensive fibrosis, and generally mild accumulations of lymphocytes and plasma cells scattered within areas of fibrosis. Remnant tissue in each pancreas consisted of small and medium ducts which were often found in association with solid cords of polyhedral cells forming islet-like structures characteristic of nesidioblastosis (Figure 1a & 1b). Cells in these clusters were diffusely positive for chromogranin-A, and the majority were insulin positive (β-cells) (Figure 3). Glucagon positive cells (α-cells) were often observed near the periphery of the islet-like structures (not shown). Confocal microscopy evaluations of insulin/wide-spectrum cytokeratin (wsCK) double immunofluorescent stains of pancreas from each case demonstrated islets located in close proximity to ducts as well as islets which were apparently within the lumens of ductal structures (Figure 3). In the latter instance, clusters of insulin-positive β-cells were present in the lumen and were surrounded by wsCK positive duct epithelial cells (Figure 3). Furthermore, many cells in the intraductal islets and among the duct epithelial cells were expressing both insulin and wsCK (Figure 3) suggesting a transition from wsCK positive/insulin negative cells to double positive cells. Further confocal microscopic analysis of the pancreas of each patient with proinsulin/PDX1 double immunofluorescent labeled sections demonstrated many typical β-cells which were positive for both proinsulin and PDX1 as well as cells in the islet-like structures that were proinsulin positive but lacked nuclear PDX1 labeling (Figure 4). In addition, in regions external to the islet-like structures there were many cells which were proinsulin negative but which showed nuclear PDX1 labeling. Immunohistochemical labeling for the marker for mitotic activity, Ki67, showed many labeled ductal epithelial cells as well as some labeling of periductal cells, consistent with mitotic activity associated with islet neogenesis (Figure 5).
Figure 1.
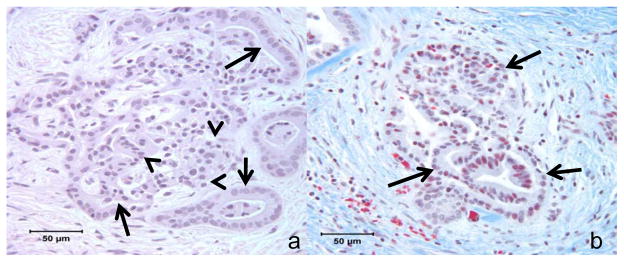
Figure 1a, b. Photomicrographs of case #1 showing a) interconnected duct-like structures (arrows) and endocrine like strucutres (arrow heads) within the pancreas (H&E stain); and b) ductuloinsular structures (arrows) surrounded by dense fibrosis (blue, Masson’s Trichrome stain).
Figure 3.
Confocal microscopy images of pancreas from 3 patients double immunolabeled for insulin (green) and wide-spectrum cytokeratins (red) and nuclear stained with To-Pro 3 (blue). a) Atypical islet-like structures in case #1 showing cells with both insulin and wide-spectrum cytokeratin immunoreactivity (arrows). b) Atypical islet-like structure in case #2 showing insulin positive cells surrounded by a duct-like structure (arrows) in which cells label for wide-spectrum cytokeratins. c) Atypical islet-like structure in case #3 showing insulin positive cells surrounded by a duct-like structure in which cells label for wide spectrum cytokeratins. Arrows demonstrate cells with both insulin and cytokeratin immunoreactivity. d) Normal islet (arrow) in case #3 in which β-cells do not show cytokeratin immunoreactivity. A single atypical cell (arrow head) labels for both insulin and cytokeratins.
Figure 4.
Confocal microscopy images of pancreas from 3 patients double immunolabeled for proinsulin (green) and PDX1 (red) and nuclear stained with To-Pro 3 (blue). a) Atypical islet structures from case #1 showing proinsulin-containing cell which does not exhibit nuclear PDX1 labeling (arrowhead) and several cells which are negative for proinsulin but show nuclear PDX1 labeling (arrows). b) Atypical islet structures from case #2 showing a mixture of typical β-cells with both proinsulin and nuclear PDX1 labeling (arrowheads) and atypical cells showing only proinsulin labeling (arrows). c) Atypical islet structures from case #3 showing cells with proinsulin labeling which lack nuclear PDX1 labeling (arrows) and some cells with nuclear PDX1 which lack proinsulin labeling (arrowhead). d) Normal islets from case #3 showing double labeling of β-cells for proinsulin and PDX1.
Figure 5.
Photomicrographs of pancreas from 3 patients immunohistochenically labeled for Ki67 MiB-1 demonstrating many labeled cells among the duct epithelium (arrows) and in periductal locations (arrow heads). A,B) patient 1, C,D) patient 2, and E,F) patient 3.
Discussion
While it is known that islet mass increases postnatally [14], the source of new islets remains less certain. Pancreatic biopsies from these 3 young patients who underwent TP-IAT for management of chronic pancreatitis provide unique evidence of islet neogenesis from pancreatic ductal progenitor cells. In these cases, islet-like structures staining positive for insulin and glucagon are observed arising from ductal cells and duct-like structures. Notably, these cases were unusual in the severity of CP at a young age, with extensive inflammation, fibrosis, and significant calcifications. Such severe injury may have stimulated islet neogenesis from ductal precursors [15, 16]. Based on these findings, we performed many histological screenings of other adult and pediatric CP pancreases and concluded that this type of phenomena is not common in most CP cases.
Evidence for islet neogenesis in response to injury from severe CP has been previously observed. In pediatric patients with CP undergoing TP-IAT, nesidioblastosis was observed more frequently in those patients with severe fibrosis [16]. In our 3 cases, the texture of these pancreases was described as exceptionally hard, consistent with extensive fibrosis. Phillips et al. [17] reported similar findings in adult patients with CP. Although islet neogenesis initiated from progenitor cells present within the ducts has been reported in animal models [15], the existence of such progenitor cells in the human pancreas has been debated. The present report sheds light on the occurrence of islet neogenesis in cases of pancreatic injury from CP, and in particular, provides evidence that pancreatic ductal precursors may be one important source of islet neogenesis.
The proliferative capacity of adult pancreatic islet cells is limited [8]; however, the formation of new islets from ductal epithelium is achievable even in the adult pancreas, especially after injury to the organ. Historically, β-cell turnover in normal pancreases and an increase in β-cell mass in response to injury have been described by many investigators over the past century. Experimental models in rodents have shown strongly that β-cells can regenerate or expand after tissue injury [18]. When injury is induced, tissue remodeling occurs, characterized by acinoductal metaplasia of the exocrine tissue, an increase in mesenchyme, and hyperplasia of the endocrine cells as a result of islet neogenesis [19]. Understanding the mechanism of proliferation and how subsequent differentiation of putative precursor cells leads to islet neogenesis is important for the development of therapeutic interventions for type I diabetes [20].
This report highlights the opportunity to further study islet neogenesis using tissue biopsies from patients undergoing TP-IAT procedures for the treatment of CP. These preliminary findings in 3 patients support pancreatic ductal precursors as one source of islet neogenesis, but the mechanisms by which islets arise from ductal tissue remain unclear. Future studies which aim to isolate putative islet progenitor cells for in vitro culture, proliferation and differentiation may help in developing new sources of transplantable islet preparations and/or new drug therapies to induce beta-cell replication and regeneration in the native pancreas.
Acknowledgments
The authors would like to thank Josh Wilheim, Muhammad Abdulla, Bob Konz, Kate Mueller, Brian Perrault, Thomas Gilmore, Jeffrey Ansite, and Josh Parker for excellent technical support.
Funding source:
This work was supported in part by philanthropic gifts made to the Schulze Diabetes Institute and Schulze Family Foundation grant.
References
- 1.Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86:1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell RM, Byrne MF, Baillie J. Pancreatitis. Lancet. 2003;361:1447–1455. doi: 10.1016/s0140-6736(03)13139-x. [DOI] [PubMed] [Google Scholar]
- 3.Bhanot UK, Moller P. Mechanisms of parenchymal injury and signaling pathways in ectatic ducts of chronic pancreatitis: implications for pancreatic carcinogenesis. Lab Invest. 2009;89:489–497. doi: 10.1038/labinvest.2009.19. [DOI] [PubMed] [Google Scholar]
- 4.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59:2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology. 2000;141:1926–1929. doi: 10.1210/endo.141.6.7567. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg L. Induction of islet cell neogenesis in the adult pancreas: the partial duct obstruction model. Microsc Res Tech. 1998;43:337–346. doi: 10.1002/(SICI)1097-0029(19981115)43:4<337::AID-JEMT8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Bonner-Weir S, Inada A, Yatoh S, et al. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353–356. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- 10.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 59:2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solar M, Cardalda C, Houbracken I, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 13.Anazawa T, Balamurugan AN, Bellin M, et al. Human islet isolation for autologous transplantation: comparison of yield and function using SERVA/Nordmark versus Roche enzymes. Am J Transplant. 2009;9:2383–2391. doi: 10.1111/j.1600-6143.2009.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Manivel JC, Bellin MD, et al. Correlation of pancreatic histopathologic findings and islet yield in children with chronic pancreatitis undergoing total pancreatectomy and islet autotransplantation. Pancreas. 2010;39:57–63. doi: 10.1097/MPA.0b013e3181b8ff71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips JM, O’Reilly L, Bland C, Foulis AK, Cooke A. Patients with chronic pancreatitis have islet progenitor cells in their ducts, but reversal of overt diabetes in NOD mice by anti-CD3 shows no evidence for islet regeneration. Diabetes. 2007;56:634–640. doi: 10.2337/db06-0832. [DOI] [PubMed] [Google Scholar]
- 18.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 19.Bouwens L. Transdifferentiation versus stem cell hypothesis for the regeneration of islet beta-cells in the pancreas. Microsc Res Tech. 1998;43:332–336. doi: 10.1002/(SICI)1097-0029(19981115)43:4<332::AID-JEMT7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Weir GC, Bonner-Weir S. Beta-cell precursors--a work in progress. Nat Biotechnol. 2004;22:1095–1096. doi: 10.1038/nbt0904-1095. [DOI] [PubMed] [Google Scholar]