Abstract
Background
FLT3-internal tandem duplication (ITD) mutations are found in approximately 30% of AML patients. FLT3 inhibitors have shown clinical activity in AML with FLT3-ITD but responses are usually short lived.
Methods
We reviewed 69 FLT3 mutated AML patients, treated with different FLT3 inhibitors to analyze emergence of new mutations.
Results
At baseline 87% of patients had an ITD mutation, 7% had a D835/I836 mutation and 6% had combined ITD and D835/I836 mutations. Responses occurred in 32% of patients, all with FLT3-ITD; none of the patients with D835/I836 or ITD+D835/I836 responded. Mutational assessment at the time FLT3 inhibitor discontinuation showed that 68% of patients were unchanged, 10% had become undetectable, and 22% of patients progressed from a single ITD to have combined ITD+D835/I836 mutations. In those patients with unchanged FLT3 mutation at progression the median survival was 5 months, while in those with undetectable and with combined ITD-D835/I836 mutations the median survival was 7 months respectively.
Conclusion
This data confirm in vitro observations that a secondary TKD mutation may arise after the use of FLT3 inhibitors in patients with single FLT3-ITD mutated AML, a phenomenon that is associated with resistance and a poor prognosis.
Keywords: FLT3 inhibitors, FLT3-ITD, FLT3-TKD, secondary FLT3 mutations, AML
Introduction
Acute myeloid leukemia (AML), the most common acute leukemia in the United States,1 is a heterogeneous disease that carries a variable prognosis based on pretreatment characteristics such as age, performance status, comorbid conditions, prior cancer treatments, antecedent hematologic disorders, and cytogenetic and molecular abnormalities.2-5 The FLT3 gene is located on chromosome 13q12 and encodes the FLT3 tyrosine kinase receptor. FLT3 has 993 amino acids in length, contains five extracellular immunoglobulin-like domains, a transmembrane domain, a juxtamembrane domain and two intracellular tyrosine kinase domains linked by a kinase-insert domain. 6-9 Under normal circumstances, cytoplasmic FLT3 undergoes glycosylation, which promotes localization of the receptor to the membrane. Binding to FLT3-ligand promotes receptor conformational changes and receptor homodimerization which in turn promotes phosphorylation of the tyrosine kinase domains and activation of downstream effectors such as the phosphatidylinositol 3-kinase (PI3K/AKT), mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and signal transducer and activator of transcription 5 (STAT5) pathways.8 Activating mutations of FLT3 have been identified in patients with acute myeloid leukemia (AML) including internal-tandem duplications (ITDs) of the juxtamembrane region (head to tail duplication of 3-400 base pairs in exons 14 or 15), tyrosine kinase domain 1, and mutations involving the D835/I836 residues and others of the tyrosine kinase domain (TKD).8,10-12 They occur in approximately 30% and 7% of AML patients respectively, and lead to constitutive activation of the tyrosine kinase domain.10,11,13,14
Patients with AML with FLT3-ITD mutation typically present with a normal karyotype, elevated white blood cell count, and higher percentages of blasts in peripheral blood and bone marrow. The presence of FLT3 mutations has been associated with a poor outcome, with a greater probability of relapse and shorter overall survival.15-19 Several FLT3 inhibitors have been developed in an attempt to overcome this aggressive outcome of FLT3-ITD AML.20 Clinical responses have been observed with agents such as sorafenib,21 quizartinib,22 midostaurin23 and others. Responses are frequently characterized by a rapid reduction in peripheral blood and/or bone marrow blasts, but they are usually transient with most patients eventually progressing. Recently, it has been reported that point mutations may confer in vitro resistance to FLT3 inhibitors.24,25
The frequency with which these mutations occur in the clinic among patients treated with FLT3 inhibitors and their clinical significance has not been fully described. We thus analyzed our experience among patients with AML with FLT3 mutations treated with various FLT3 inhibitors to define the frequency and clinical significance of this phenomenon.
Materials and Methods
Patients
We analyzed the records of 69 consecutive patients with AML with FLT3 mutations treated at our institution in clinical trials with different FLT3 inhibitors used as single agent from October 2002 to August 2011 and in whom we obtained mutational assessment before and after treatment. Patients were enrolled in studies 2009-0560 and 2006-0850 (AC-220, quizartinib), 2004-0702 (sorafenib), 2003-0719 and ID02-274 (lestaurtinib, CEP-701), and 2006-0275 (KW-2449). Studies were approved by the institutional review board and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before study entry. Patients were also included in a retrospective chart review approved by the IRB.
Patient Monitoring and Response Criteria
Patients were followed with complete blood counts at least weekly during the first 4 weeks of therapy, then every other week during the next 4-8 weeks, and then every 1-3 months based on response or clinical status. AML response criteria followed the recommendations of the International Working Group.26,27 Briefly, complete remission (CR) was defined by the presence of <5% blasts in the BM with >1 ×109/L neutrophils and >100 ×109/L platelets in the peripheral blood. Morphologic complete remission with incomplete platelet recovery (CRp), was defined in patients with CR but persistent platelet count <100 ×109/L. Morphologic complete remission with incomplete blood count recovery (CRi), was defined in patients with persistent neutrophil count <1 ×109/L, or without platelet recovery. Patients showing a significant decrease (>50%) bone marrow blast reduction (BMBR), without peripheral blood counts recovery are described separately. A relapse was defined by >5% blasts in a bone marrow aspirate or by the presence of extramedullary disease. Induction death was defined as death that occurred within 6 weeks from start of therapy.
Molecular Analysis for FLT3 Mutations
Genomic DNA extracted from fresh BM aspiration specimens using the Autopure extractor (QIAGEN/Gentra, Valencia, CA) was used for mutation analysis. FLT3 (ITD and D835) mutations were screened using polymerase chain reaction (PCR) followed by capillary electrophoresis on an ABI Prism 3100 or 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA), as previously described.28,29 To facilitate the detection of PCR products by capillary electrophoresis forward primers for ITD and D835 were labeled with a fluorescent dye, 6-carboxyfluorescein (FAM). The presence of any PCR fragment larger than the WT allele was considered positive for ITD. For FLT3 codon 835 point mutation analysis, PCR products were digested with EcoRV before capillary electrophoresis. The WT allele cut by this enzyme resulted in 2 fragments of 64 and 48 base pairs.29 In contrast, mutations at D835 alter the EcoRV recognition site and result in 1 112 base-pair fragment. The sensitivity of these assays is approximately 2%, that is, 1 mutated cell in 50 total cells, as established by dilution studies.
Statistical Methods
Patient characteristics are summarized using median (range) for continuous variables and frequency (percentage) for categorical variables. Categorical and continuous variables were compared by using the Fisher exact test and the Wilcoxon test, respectively.30 Event-free survival (EFS) was calculated from the beginning of treatment until an event, defined as relapse, resistance to induction therapy, or death. Disease free survival (DFS) was calculated from the moment of CR until relapse or death in CR. Overall survival (OS) was calculated from the start of treatment until death. Patients who remained alive at the last follow-up were censored. The probabilities of OS and EFS were estimated using the Kaplan-Meier method,31 and were compared among subgroups of patients using the log-rank test.32 All statistical analyses were conducted using SAS 9.0.
Results
Patient Characteristics (Table 1)
Table 1. Baseline Patient Characteristics.
| Patient Characteristics | Median [Range], or No. (%) |
|---|---|
|
| |
| Females | 35 (51) |
|
| |
| Median Age | 54 [18-87] |
|
| |
| Median No. of Prior Treatments | 2 [1-6] |
|
| |
| Cytogenetic | Diploid: 24 (35) |
| Complex: 12 (17) | |
| Miscellaneous: 11 (16) | |
| +8, -7, 11q: 5 (7) | |
|
| |
| ITD | 60 (87) |
|
| |
| D835/I836 | 5 (7) |
|
| |
| ITD and D8535/I836 | 4 (6) |
|
| |
| NPM1 Mutation | 15 (22) |
|
| |
| RAS Mutation | 9 (13) |
|
| |
| CEBPA Mutation | 3 (4) |
Before the start of therapy with a FLT3 inhibitor, 60 (87%) of the 69 patients had a FLT3 ITD mutation, 4 (6%) had a D835/I836 kinase domain mutation, and 5 (7%) had combined ITD and D835/I836 mutations. The median age for the 69 patients was 54 years (range, 18-87 years), and the median number of prior leukemia treatments was 2 (range 1-6), including 16 (23%) patients with prior stem cell transplantation (SCT). Karyotype was diploid in 24 (35%) patients, complex in 12 (17%), 5 (7%) patients had either -7, 11q, or +8, miscellaneous in 11 (16%), and an additional 17 (25%) patients had either insufficient metaphases or cytogenetics were not done at the time FLT3 inhibitor therapy was started. Concomitant NPM1, RAS, and CEBPA mutations were observed in 22%, 13%, and 4% of the patients, respectively.
Survival Outcomes
Patients received therapy for a median of 78 days (range 19-838). Forty-three (62%) patients did not achieve response to therapy, and 4 (6%) patients died within 42 days after the start of therapy. Four (6%) patients achieved CR, 9 (13%) patients achieved CRp, and 9 (13%) had a significant BMBR from a median of 66% (range 20-92) blasts to a median of 5% (range 2-26); of note 3 of them had documented BM blasts less than 5% (i.e., morphologic leukemia free state) but did not reach hematologic parameters for CR, CRp or CRi. All responders had FLT3 ITD alone. None of the patients with D835/I836, whether alone or concomitant with FLT3 ITD achieved a response. All patients eventually discontinued therapy. Among responders, nine patients received stem cell transplantation (CR=1, CRp=3 and BMBR=5), and 7 of them relapsed with a median time to relapse after transplant of 4 months. Of note only two patients, 1 in CR and 1 in CRp had a negative FLT3 ITD by PCR prior to transplant. Two patients who were transplanted in CRp achieved complete remission after SCT and are the only two survivors, without relapse at the time of this analysis (3.5 and 5 years after transplant, respectively). Ten (14%) patients in the non-response group received SCT, 6 immediately after FLT3 inhibitor (median bone marrow blast percentage at the time of SCT 38%) and 4 after another salvage combination chemotherapy (IA, FA-sorafenib, decitabine + gemtuzumab ozogamicin, and MEC, respectively). The median time to progression post-transplant for these 10 patients was 2.3 months (range, 0 to 24 months). Among patients not transplanted, 4 (1 in CR, 1 in CRp and 2 with BMBR) discontinued FLT3 inhibitor due to toxicity and eventually progressed. All other patients discontinued therapy because of progressive disease and all patients (except those two still alive) eventually progressed with same malignant clone at presentation.
All patients had assessment of their FLT3 mutation status at the time FLT3 inhibitor was discontinued. In 15 (22%) patients where a single FLT3 ITD mutation had been identified at baseline, both FLT3 ITD and D835/I836 mutations were identified at the end of therapy. In 7 (10%) patients (6 with FLT3 ITD at baseline and 1 with D835/I836) no FLT3 mutation was identified at the time treatment was discontinued. In all other 47 (68%) patients the mutations status was unchanged.
Patient characteristics of 15 patients developing a secondary FLT3 mutation are summarized in table 2, their median age was 43, and median number of prior treatments was 2, including 4 patients with prior SCT (one of them with 2 prior transplants). The median duration of treatment with FLT3 inhibitors was 126 days (range 77-570 days). Best response to therapy included NR in 7, significant BMBR in 3, CRp in 3 and CR in 2. In this group we include 2 patients that received a SCT after FLT3 inhibitor, one with no response but having low burden disease (patient number 9) and the other (patient number 12) in whom BM blast had decreased to 4 %. In these two cases, the D835/I836 mutation was detected at disease progression 2 and 3 months post-transplant, respectively.
Table 2. Patient Characteristics of patients that developed D835 Mutation upon clinical resistance to FLT3 inhibitors.
| Patient | Age | # Prior Treatments | Baseline | Best response to FLT3 inhibitor |
At Disease Progression | Treatment after FLT3 Inhibitor |
Response | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (x109/L) |
PBB (%) | BMB (%) | Ratio Allele Burden ITD |
Cytogenetic | WBC (x109/L) |
PBB (%) | BMB (%) | Ratio Allele Burden ITD/D835 |
Cytogenetic | ||||||
| 1 | 37 | 2 | 2.4 | 33 | 66 | ND | Misc | CRp | 20.5 | 81 | 56 | ND | ND | IA+ Sorafenib | NR |
| 2 | 87 | 1 | 7.1 | 89 | 80 | ND | Dip | BMBR | 23 | 81 | 82 | ND | Dip | Azacytadine + Sorafenib | NR |
| 3 | 69 | 2 | 5.4 | 20 | 86 | Size 371 and 39.7% | Dip | CR | 14 | 71 | 91 | 0.47/0.5 3 | Dip | Phase 1 Protocol | NR |
| 4 | 25 | 4 | 1.7 | 33 | 60 | ND | Com | NR | 10.1 | 97 | 76 | ND | Com | Phase 1 Protocol | NR |
| 5 | 79 | 1 | 21 | 51 | 52 | 0.007 and 0.167 | Dip | NR | 5.1 | 25 | 63 | 0.029 and 0.318/0. 210 | Dip | Azacytadine + Sorafenib | NR |
| 6 | 38 | 5 | 9.8 | 85 | 95 | ND | Dip | NR | 17.9 | 63 | 70 | ND | Dip | Sorafenib | NR |
| 7 | 43 | 1 | 60 | 95 | 92 | 0.384 | -7 | CRp | 28 | 86 | 47 | 0.388/0. 265 | Dip | Plerixafor + Sorafenib | NR |
| 8 | 23 | 4 | 74 | 92 | 86 | ND | ND | NR | 67 | 96 | 88 | ND | Com | Phase 1 Protocol | NR |
| 9* | 21 | 2 | 3 | 13 | 55 | ND | −7q | NR | 1.8 | 0 | 23 | 0.231/0. 384 | −7q | SCT | Relapsed within 2 months |
| 10 | 67 | 2 | 60 | 58 | 66 | 0.26 | Dip | BMBR | 13.9 | 83 | 55 | 0.023/0. 33 | Dip | Plerixafor + Sorafenib. FA | NR |
| 11 | 38 | 4 | 7.8 | 90 | 90 | ND | Com | NR | 43 | 91 | 80 | ND | Com | Decitabine + GO | NR |
| 12* | 33 | 3 | 0.2 | 0 | 25 | 0.065 | Ins | BMBR | 23 | 67 | 74 | 0.124/0. 134 | Misc | SCT | Relapsed within 3 months |
| 13 | 52 | 2 | 19.2 | 89 | 93 | ND | Misc | CRp | 105 | 55 | 80 | ND | Misc | Phase 1 Protocol | NR |
| 14 | 75 | 2 | 65.2 | 1 | 40 | ND | Dip | CR | 37.6 | 6 | 32 | 0.856/0.393 | Dip | Plerixafor + Sorafenib | NR |
| 15 | 64 | 2 | 1.6 | 6 | 20 | ND | Dip | NR | 2.6 | 80 | 78 | Ratio 0.01/0.159 | ND | ND | NR |
WBC: White Blood Cells, PBB: Peripheral Blood Blasts, BMB: Bone Marrow Blasts, CR: Complete Remission, CRp: Complete Remission with incomplete platelet recovery, NR: No Response, BMBR: Bone Marrow Blast Reduction, ND: Not Done, Dip: Diploid, Misc: Miscellaneous, Insu: Insufficient, Com: Complex, I: Idarubicin, F: Fludarabine, A: Ara C, GO: gemtuzumab Ozogamicin, SCT: Stem Cell Transplant.
These 2 patients had documented combined ITD/D835 mutation at the time of disease progression after transplantation.
Second line FLT3 inhibitor treatment was attempted in 7 out of 15 patients with both FLT3 ITD and D835/I836 mutations. None of the patients responded to further therapy. In patients in whom FLT3 mutation was unchanged or became negative, the median white blood cell count (WBC), peripheral blood blasts (PBB) and bone marrow blasts (BMB) at baseline were 5.5 ×109/L, 27%, 68.5%, and at the time of disease progression 2.6 ×109/L, 7.8%, 70%, respectively. In those who developed both mutations they were 7.8, 51%, 66%, and 20.5, 80% and 74% respectively.
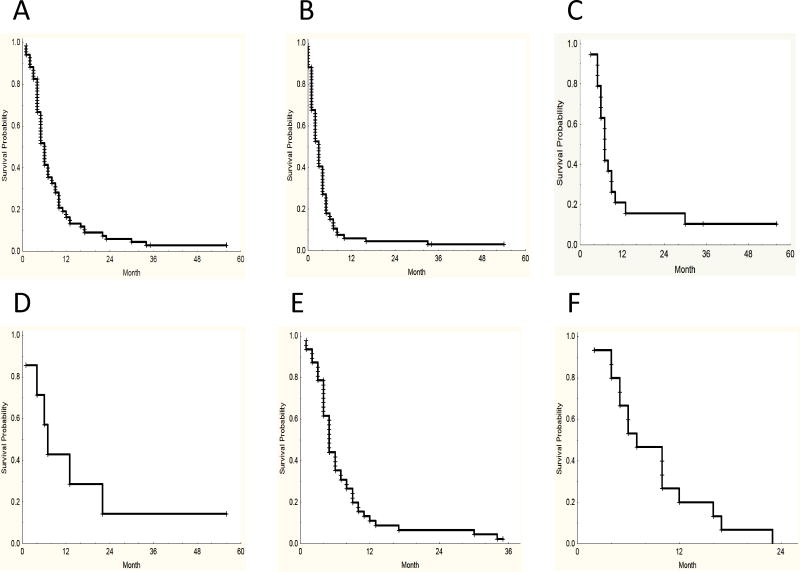
At the time of this analysis 67 (97%) patients had died. The median survival in the entire group from the time the FLT3 inhibitor was started was 6 months. In the subgroup analysis, those patients in whom the FLT3 mutation became negative the median survival was 7 months, compared to 5 months for those with in no change in mutation status and 7 months in those with ITD-D835/I836 mutations. The median survival for transplanted patients was 7 months and the median survival from time of treatment discontinuation in the entire group was 3 months (Figure 1).
Figure 1. Survival Curves.
A: Overall Survival from beginning of treatment (Median 6 months), B: Overall Survival from FLT3 Inhibitor treatment discontinuation (Median 3 months), C: Survival Patients Transplanted (Median 7 Months), D: Survival Patients with Undetectable Mutation After Treatment (Median 7 months), E: Survival Patients with Unchanged Mutation Status (Median 5 months), F: Survival Patients Developing Both Mutations (Median 7 months).
Discussion
It has been established that FLT3 ITD mutated, normal karyotype AML carries a poor prognosis. Although several multivariate analyses have failed to establish a direct impact in the ability to achieve a complete remission, they have proved a strong correlation with an increased risk of relapsed and poor overall survival.10,11,13-17,19,33 Therefore it is generally accepted that these patients should undergo hematopoietic cell transplantation in first remission if feasible. However the outcome of these patients is also inferior after allogeneic stem cell transplantation compared to patients comparable patients with no FLT3 mutation. In one recent report of patients transplanted in first remission, the 2-year relapse rate was 30% vs. 16% for those with no FLT3 ITD, and the leukemia-free survival probability lower (58% vs. 71%).34 Thus there is a need to investigate new treatment options for these patients. Among them, FLT3 inhibitors have emerged in recent years as promising additions to the treatment armamentarium that may improve the outcome of these patients.20
Several inhibitors of FLT3 kinase activity have entered the clinical development arena. Sorafenib has shown inhibition of the phosphorylation of tyrosine residues in ITD mutant in vitro using primary human AML cells and in an animal model.35 A phase I study conducted in patients with MDS and relapsed or refractory AML, showed CR or CRp in 10% of patients; significant reduction in bone marrow and or peripheral blood blasts was seen in an additional 34% patients (all responses in patients with FLT3-ITD mutation).21 Activity of midostaurin (PKC412) was reported in a phase IIB trial with 95 patients with AML or MDS, with either wild-type (n=60) or mutated (n=35) FLT3; one patient achieved PR and reduction in peripheral blood or bone marrow blasts of at least 50% was observed in 71% of patients with FLT3-mutant and 42% of patients with FLT3 wild-type.23 Quizartinib (AC220) is a second-generation FLT3 inhibitor with greater kinase selectivity.36 In a phase 1 study, 76 patients with relapsed refractory AML, irrespective of FLT3-ITD mutation status, received quizartinib at escalating doses. Of 17 patients with FLT3-ITD mutation 53% responded, and among 37 patients without the mutation 14%responded. There were 22 patients with FLT3-ITD indeterminate or not tested status, and among them, 41% responded. The median duration of response was 13 weeks; median survival was 14 weeks.37 Earlier inhibitors such as lestaurtinib inhibited multiple kinases and produced mostly partial responses.38 Despite this promising clinical activity, responses are usually transient and insufficient to induce a durable response.39,40
The current cohort of patients, treated with a variety of inhibitors, followed the same pattern as reported in these studies; several patients achieved responses, many of them able to be bridged to transplant, but in all instances (except occasionally when transplanted) the responses were eventually lost. Many of these patients were treated during a time when the allelic burden was not routinely measured, but it has been suggested that patients with relapsed disease carry a greater allelic burden than untreated patients and their disease depends more on the aberrant FLT3 signaling, a phenomena that could explain the greater cytotoxicity observed with more specific FLT3 inhibitors. 41 Interestingly, in a subset of patients the mutated clone was no longer detectable upon progression. This subset of patients carried a similar median survival when compared with the group developing the second mutation, but disease burden and proliferation seem to be increased in the later. Undoubtedly this is a small cohort and a retrospective analysis, making these observations only preliminary, but if these observations can be confirmed in larger, prospective series, this might identify a subset of patients whose biology is changed by the introduction of FLT3 inhibitors, perhaps favoring the emergence of clones that are more proliferative and no longer inhibited by FLT3 inhibitors that have lesser (e.g., quizartinib) or no (e.g., sorafenib) activity against unmutated FLT3. It is also entirely possible that the FLT3 mutated clone is present, only below the level of detection of our assay. Still, this would suggest that another clone has become more prominent. Ongoing studies are testing these hypotheses.
Resistance to FLT3 tyrosine kinase inhibition occurs eventually in virtually all patients and has been attributed to compensatory activation of downstream effectors such as STAT5 or MAPK despite complete inhibition of FLT3 tyrosine phosphorylation.42-44 Other mechanisms of resistance include up-regulation of FLT3 ligand and FLT3 receptor or up-regulation of anti-apoptotic genes and down-regulation of pro-apoptotic signals, or compensatory activation of secondary signaling pathways such as Ras or PI3K.45-49 Recently a novel FLT3-ITD mutation located in the non-juxtamembrane domains of FLT3 (FLT3_ITD627E) mediated constitutive phosphorylation of FLT3 and of STAT5, and induced transformation of hematopoietic 32D cells leading to a lethal myeloproliferative disease in a syngeneic mouse model.12 Upon further characterization authors also suggested that (FLT3_ITD627E) induces dramatic up regulation of the anti-apoptotic myeloid cell leukemia 1 protein (MCL-1), an effect that appeared to be due to an enhanced and sustained binding of the adaptor protein GRB-2 to the FLT3_ITD627E receptor, which was independent from the inhibitor midostaurin-induced inhibition of the receptor kinase.44 Sensitivity to FLT3 inhibitors seems to be variable in different mutants, 35,50,51 and in vitro exposure to different FLT3 tyrosine kinase inhibitors has been associated with development of secondary FLT3 resistant mutations, which interestingly were not overlapping like in the case of Bcr-Abl kinase inhibitors (imatinib, dasatinib, nilotinib).52 More recently, Smith et al.24 reported the emergence of resistance mutations in cells from 8 patients treated with AC220 (quizartinib). D835Y (an activation loop mutation) and F691L (a gatekeeper mutation) were each detected in 3 instances, with 2 others showing D835V.
Our results confirm the emergence of secondary TKD mutations, in our series specifically D835/I836, as a common mechanism of resistance after treatment with FLT3 inhibitors in patients with FLT3-ITD AML occurring in approximately 20% of patients. To our knowledge, this is the first report to analyze the development of these mutations while on therapy with FLT3 inhibitors. De novo FLT3-TKD mutations are also associated with normal karyotype AML and higher blast percentage, but while several groups have noted a strong association with worse disease free and overall survival,14,15,53 others noted no significant prognostic impact.11,54,55 Recently Bacher et al.56 characterized the mutational status of 3082 patients with newly diagnosed AML; unlike FLT3-ITD, FLT3-TKD did not influence prognosis, however authors found an unfavorable prognosis in patients harboring double mutations, including those with both FLT3 ITD and TKD. In our series, none of the patients expressing TKD mutations, whether alone or in combination with ITD responded to FLT3 inhibitors. The emergence of resistant clones with this or other mutations that are resistant to agents being used to treat the patient would likely alter the outcome of patients. New agents, such as crenolanib57 and PLX339758 are being developed that may overcome these resistant mutations.
Our series represents a retrospective analysis of patients treated with a variety of inhibitors. It is possible that the rate and type of mutations will vary clinically depending on the inhibitor being used as has been described in the laboratory.52 Importantly, we did not look for other mutations that have been described as mechanisms of resistance of FLT3 inhibitors, e.g. N676K as reported by Heidel et al.25 Thus, it is likely that the incidence of resistant mutations is higher than reported here.
We conclude that emergence of resistant mutations is a common mechanism of resistance to FLT3 inhibitors used clinically, with such mutations emerging in at least 20% of patients. This shows that in these cases, survival of AML blasts depends to a great extent on FLT3 signaling. Patients treated with these agents need to be assess for emergence of these mutations, and new agents that may overcome or, ideally, prevent the emergence of such mutations need to be developed.
Key Points.
Secondary FLT3-TKD mutations can arise after treatment with FLT3 inhibitors in patients with FLT3-ITD mutated AML.
D835/I836 mutations were observed in at least 20% of patients after treatment, and are associated with resistance and poor prognosis.
Acknowledgments
This research is supported in part by the MD Anderson Cancer Center Support Grant CA016672 and AML P01 Grant CA055164 19 from the National Institutes of Health.
Disclosures: JC received research support from Ambit, Arog, Novartis, Kyowa, and Astellas.
Footnotes
Author Contribution: YA, JC, designed the research, analyzed the data and wrote the manuscript. JC, HK, RL, FR, GB, GGM, MK, ZE, MA treated the patients. All authors reviewed the data and the manuscript and all authors agreed with the final version of the manuscript.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009 Sep 24;361(13):1235–1248. doi: 10.1056/NEJMoa0901409. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006 Mar 1;106(5):1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 4.Estey EH. General approach to, and perspectives on clinical research in, older patients with newly diagnosed acute myeloid leukemia. Semin Hematol. 2006 Apr;43(2):89–95. doi: 10.1053/j.seminhematol.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Grimwade D, Walker H, Harrison G, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001 Sep 1;98(5):1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 6.Rosnet O, Buhring HJ, deLapeyriere O, et al. Expression and signal transduction of the FLT3 tyrosine kinase receptor. Acta Haematol. 1996;95(3-4):218–223. doi: 10.1159/000203881. [DOI] [PubMed] [Google Scholar]
- 7.Agnes F, Shamoon B, Dina C, Rosnet O, Birnbaum D, Galibert F. Genomic structure of the downstream part of the human FLT3 gene: exon/intron structure conservation among genes encoding receptor tyrosine kinases (RTK) of subclass III. Gene. 1994 Aug 5;145(2):283–288. doi: 10.1016/0378-1119(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 8.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003 Sep;3(9):650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 9.Rosnet O, Birnbaum D. Hematopoietic receptors of class III receptor-type tyrosine kinases. Crit Rev Oncog. 1993;4(6):595–613. [PubMed] [Google Scholar]
- 10.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996 Dec;10(12):1911–1918. [PubMed] [Google Scholar]
- 11.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001 Apr 15;97(8):2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 12.Breitenbuecher F, Schnittger S, Grundler R, et al. Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood. 2009 Apr 23;113(17):4074–4077. doi: 10.1182/blood-2007-11-125476. [DOI] [PubMed] [Google Scholar]
- 13.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002 Sep 1;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 14.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008 Feb 1;111(3):1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002 Dec 15;100(13):4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 16.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002 Jul 1;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001 Sep 15;98(6):1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 18.Frohling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002 Dec 15;100(13):4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 19.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008 May 1;358(18):1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 20.Pemmaraju N, Kantarjian H, Ravandi F, Cortes J. FLT3 inhibitors in the treatment of acute myeloid leukemia: the start of an era? Cancer. 2011 Aug 1;117(15):3293–3304. doi: 10.1002/cncr.25908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borthakur G, Kantarjian H, Ravandi F, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011 Jan;96(1):62–68. doi: 10.3324/haematol.2010.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortes J, Foran J, Ghirdaladze D, et al. AC220, a Potent, Selective, Second Generation FLT3 Receptor Tyrosine Kinase (RTK) Inhibitor, in a First-in-Human (FIH) Phase 1 AML Study. Blood. 2010;114 Abstract #636. [Google Scholar]
- 23.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010 Oct 1;28(28):4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012 May 10;485(7397):260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006 Jan 1;107(1):293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003 Dec 15;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990 May;8(5):813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 28.Beran M, Luthra R, Kantarjian H, Estey E. FLT3 mutation and response to intensive chemotherapy in young adult and elderly patients with normal karyotype. Leuk Res. 2004 Jun;28(6):547–550. doi: 10.1016/j.leukres.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Murphy KM, Levis M, Hafez MJ, et al. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J Mol Diagn. 2003 May;5(2):96–102. doi: 10.1016/S1525-1578(10)60458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snedecor G, Cochran W. Statistical Methods. 7. Ames, Iowa: Iowa State University Press; 1980. [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 32.Mantel N. Evaluation of Survival Data and Two New rank order Statistics Arising in Its Considerations. Cancer Chemotherapy Reports. 1966;(50):163–170. [PubMed] [Google Scholar]
- 33.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99(12):4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 34.Brunet S, Labopin M, Esteve J, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012 Mar 1;30(7):735–741. doi: 10.1200/JCO.2011.36.9868. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008 Feb 6;100(3):184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 36.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML) Blood. 2009 Oct 1;114(14):2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cortes JE, Kantarjian H, Foran JM, et al. Phase I Study of Quizartinib Administered Daily to Patients With Relapsed or Refractory Acute Myeloid Leukemia Irrespective of FMS-Like Tyrosine Kinase 3-Internal Tandem Duplication Status. J Clin Oncol. 2013 Sep 3; doi: 10.1200/JCO.2013.48.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006 Nov 15;108(10):3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 39.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009 Jun 25;113(26):6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 40.Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010 Dec 9;116(24):5089–5102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- 41.Levis M. FLT3/ITD AML and the law of unintended consequences. Blood. 2011 Jun 30;117(26):6987–6990. doi: 10.1182/blood-2011-03-340273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006 Nov 15;108(10):3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knapper S, Mills KI, Gilkes AF, Austin SJ, Walsh V, Burnett AK. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006 Nov 15;108(10):3494–3503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]
- 44.Breitenbuecher F, Markova B, Kasper S, et al. A novel molecular mechanism of primary resistance to FLT3-kinase inhibitors in AML. Blood. 2009 Apr 23;113(17):4063–4073. doi: 10.1182/blood-2007-11-126664. [DOI] [PubMed] [Google Scholar]
- 45.Zhou J, Bi C, Janakakumara JV, et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009 Apr 23;113(17):4052–4062. doi: 10.1182/blood-2008-05-156422. [DOI] [PubMed] [Google Scholar]
- 46.Sato T, Yang X, Knapper S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011 Mar 24;117(12):3286–3293. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stolzel F, Steudel C, Oelschlagel U, et al. Mechanisms of resistance against PKC412 in resistant FLT3-ITD positive human acute myeloid leukemia cells. Ann Hematol. 2010 Jul;89(7):653–662. doi: 10.1007/s00277-009-0889-1. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009 Dec 3;114(24):5034–5043. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piloto O, Wright M, Brown P, Kim KT, Levis M, Small D. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007 Feb 15;109(4):1643–1652. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark JJ, Cools J, Curley DP, et al. Variable sensitivity of FLT3 activation loop mutations to the small molecule tyrosine kinase inhibitor MLN518. Blood. 2004 Nov 1;104(9):2867–2872. doi: 10.1182/blood-2003-12-4446. [DOI] [PubMed] [Google Scholar]
- 51.Bagrintseva K, Schwab R, Kohl TM, et al. Mutations in the tyrosine kinase domain of FLT3 define a new molecular mechanism of acquired drug resistance to PTK inhibitors in FLT3-ITD-transformed hematopoietic cells. Blood. 2004 Mar 15;103(6):2266–2275. doi: 10.1182/blood-2003-05-1653. [DOI] [PubMed] [Google Scholar]
- 52.von Bubnoff N, Engh RA, Aberg E, Sanger J, Peschel C, Duyster J. FMS-like tyrosine kinase 3-internal tandem duplication tyrosine kinase inhibitors display a nonoverlapping profile of resistance mutations in vitro. Cancer Res. 2009 Apr 1;69(7):3032–3041. doi: 10.1158/0008-5472.CAN-08-2923. [DOI] [PubMed] [Google Scholar]
- 53.Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. 2005 Aug;19(8):1345–1349. doi: 10.1038/sj.leu.2403838. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Wang XQ, Xu XP, Lin GW. Prevalence and prognostic significance of FLT3 gene mutations in patients with acute leukaemia: analysis of patients from the Shanghai Leukaemia Co-operative Group. J Int Med Res. 2010 Mar-Apr;38(2):432–442. doi: 10.1177/147323001003800206. [DOI] [PubMed] [Google Scholar]
- 55.Sheikhha MH, Awan A, Tobal K, Liu Yin JA. Prognostic significance of FLT3 ITD and D835 mutations in AML patients. Hematol J. 2003;4(1):41–46. doi: 10.1038/sj.thj.6200224. [DOI] [PubMed] [Google Scholar]
- 56.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters--an analysis of 3082 patients. Blood. 2008 Mar 1;111(5):2527–2537. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 57.Smith CC, Lasater E, Mccreery M, et al. Crenolanib (CP-868,596) Is a Potent and Selective Type I FLT3 Inhibitor That Retains Activity Against AC220 Resistance-Causing FLT3 Kinase Domain Mutants. ASH Annual Meeting Abstracts; November 16, 2012; 2012. p. 141. [Google Scholar]
- 58.Smith CC, Perl AE, Lasater E, et al. PLX3397 Is An Investigational Selective FLT3 Inhibitor That Retains Activity Against the Clinically-Relevant FLT3-ITD/F691L “Gatekeeper” Mutation in Vitro. ASH Annual Meeting Abstracts; November 18, 2011; 2011. p. 764. [Google Scholar]