Abstract
Objective
Describe the surgical technique, complications and long term outcomes of total pancreatectomy and islet auto transplantation (TP-IAT) in a large series of pediatric patients.
Summary Background Data
Surgical management of childhood pancreatitis is not clear; partial resection or drainage procedures often provide transient pain relief, but long term recurrence is common due to the diffuse involvement of the pancreas. Total pancreatectomy (TP) removes the source of the pain, while islet auto transplantation (IAT) potentially can prevent or minimize TP-related diabetes.
Methods
Retrospective review of 75 children undergoing TP-IAT for chronic pancreatitis who had failed medical, endoscopic or surgical treatment between 1989–2012.
Results
Pancreatitis pain and the severity of pain statistically improved in 90% of patients after TP-IAT (p =<0.001). The relief from narcotics was sustained. Of the 75 patients undergoing TP-IAT, 31 (41.3%) achieved insulin independence. Younger age (p=0.032), lack of prior Puestow (p=0.018), lower body surface area (p=0.048), IEQ per Kg Body Weight (p=0.001) and total IEQ (100,000) (0.004) were associated with insulin independence. By multivariate analysis, 3 factors were associated with insulin independence after TP-IAT:(1) male gender, (2) lower body surface area and the (3) higher total IEQ per kilogram body weight. Total IEQ (100,000) was the single factor most strongly associated with insulin independence (OR = 2.62; p value < 0.001).
Conclusions
TP-IAT provides sustained pain relief and improved quality of life. The β cell function is dependent on islet yield. TP-IAT is an effective therapy for children with painful pancreatitis that fail medical and or endoscopic management
Introduction
Chronic pancreatitis (CP) in children is most often due to hereditary causes with specific identifiable genetic mutations in many.1 Children with CP usually present with abdominal pain, initially with elevation of serum amylase and lipase, and with imaging of acute and/ or chronic pancreatitis. The disease is usually progressive, with recurrent hospitalizations, increasing pain and narcotic dependence, loss of school days, and impaired quality of life. In these cases, progression to exocrine and endocrine insufficiency is common; in addition, lifetime risk of pancreatic adenocarcinoma is elevated.2,3 Initial treatment is directed at relieving pain and restoring quality of life; treatments include narcotic analgesics, pancreatic enzymes to reduce pancreatic stimulation and treat pancreatic exocrine insufficiency, nerve block procedures such as celiac plexus blocks, and endoscopic decompression by pancreatic sphincterotomy, stone extraction, stricture dilation and stent placement.4–6 Those who fail these medical and endoscopic interventions may be candidates for surgical intervention.
The role of surgical management for pediatric patients with chronic pancreatitis is not clear; A number of surgical techniques have been used in attempt to ameliorate pain and restore quality of life, including partial resection, or drainage procedures such as lateral pancreaticojejunostomy (such as Puestow), or variants (such as Frey, Beger procedures).7,8 Patients often have transient pain relief, but due to the diffuse nature and involvement of the entire pancreas, pain eventually recurs in up to 50% of patients; 9–14 exocrine and endocrine insufficiency often develops over time.15 Total pancreatectomy (TP) removes the source of the pain, and potentially eliminates risk of pancreatic cancer. However, when used in isolation, TP results in brittle diabetes, and as a result is rarely done as a first procedure in children with CP.
In 1977, a novel approach was developed for treatment of CP in adults: total pancreatectomy, and isolation and auto transplantation of the patient’s islets (TP – islet auto transplantation TP-IAT).16 The goal of the islet auto transplantation was to prevent or minimize TP- related diabetes. Since then there have been several reports of TP-IAT in the adult population;17–24 however, there have been limited reports in children.25,26 We report techniques, complications and long term outcomes of TP-IAT in a large series of pediatric patients.
Methods
Between 1989 and 2012, 484 TP-IATs were performed at the University of Minnesota and University of Minnesota Amplatz Children’s Hospital. Of these, 75 were done in children and formed the study population. Our criteria for selection of patients with CP for TP-IAT has evolved over the years and have been standardized for the last 5 years.17 Currently, to qualify for TP-IAT, the patient must have had abdominal pain of > 6 months duration with impaired quality of life e.g., inability to attend school, inability to participate in ordinary activities, repeated hospitalizations, or constant need for narcotics, each coupled with failure to respond to maximal medical treatment or endoscopic pancreatic duct drainage procedures. In addition, there must be objective findings of CP, including at least one of the following: (1) pancreas calcifications on CT scan, or abnormal ERCP, or ≥ 6/9 criteria on endoscopic ultrasound( EUS); or (2) any two of following three: (1) ductal or parenchymal abnormalities on secretin stimulated magnetic resonance cholangiopancreatography (MRCP), EUS of pancreas with 6/9 criteria positive, or abnormal pancreatic function tests with peak bicarbonate < 80 mmol/L).; or (2) Histopathologic confirmed diagnosis of chronic pancreatitis from previous operations; or (3) Hereditary pancreatitis (PRSS1 gene mutation, (SPINK1 gene mutation, CFTR gene mutations), with a compatible clinical history ; or (4) History of recurrent acute pancreatitis with > 3 episodes of pain associated with imaging diagnostic of acute pancreatitis and/or elevated serum amylase or lipase 3 times normal.17
The current study was approved by the University of Minnesota Institutional Review Board. Informed consent and assent were obtained from parents and patients for all patients participating in quality of life assessments.
Surgical Technique
The main variation in the surgical technique for children receiving an Islet auto transplantation is preserving the blood supply to the pancreas until the dissection is completed for resection, thus minimizing the warm ischemia time, and maximizing islet cell preservation. In addition, important surgical steps in the pediatric patient include special attention to avoid any inadvertent injury or spasm of the small vessels to pancreas, pylorus preservation, and use of a Roux-en-Y loop for duodenojejunostomy to minimize post operative gastrointestinal complications such as bile reflux gastritis. For the procedure, we use a midline incision, as it is associated with less pain compared to a bilateral sub-costal incision. After opening the abdominal cavity, and performing any necessary adhesion lysis, a Kocher maneuver is performed to completely mobilize the duodenum and the pancreatic head until the left renal vein and the superior mesenteric artery are well visualized. The peritoneum on the anterior surface of the portal triad is opened; the gastroduodenal artery is dissected and looped. Papaverine (1%) is sprayed on the artery to prevent spasm. The short gastric vessels are divided; the spleen is mobilized by dividing the spleno-renal and spleno-colic ligaments. Using the spleen as a handle, the tail and body of the pancreas are mobilized all the way medially to the level of the superior mesenteric vein. The fibro-fatty tissue around the splenic artery and splenic vein both anteriorly in front of and behind are dissected, splenic artery is identified and looped on the superior border of the pancreas. The splenic vein is looped distal to the entry of the inferior mesenteric vein. The duodenum is transected 3 cm distal to the pylorus using a GIA stapler (1 Proximate, reloadable linear cutter stapler 55 mm, Ethicon Endo-surgery, USA). The right gastric and gastro-epiploic blood vessels are preserved and the stomach is reflected upwards and laterally to expose the head and body of the pancreas. The distal duodenum is transected at the ligament of Trietz. Superior mesenteric vein is identified and carefully dissected. The pancreatic neck is elevated off the portal vein. The bile duct is identified and transected at the superior border of the pancreas. Careful examination is done to look for any accessory right hepatic artery arising from the superior mesenteric artery. If one exists, it is carefully preserved. The uncinate process of the pancreas is mobilized off the portal vein by dividing all the small tributaries to the portal vein. At this stage the pancreas is connected only by its vascular structures and the islet cell isolation team is alerted.
The vascular structures are divided in the following order, gastro-duodenal artery, splenic artery and splenic vein. The pancreas is then immediately placed in cold sterile preservative solution and transported to the islet isolation laboratory. While the processing of the pancreas is continued in the laboratory, gastrointestinal reconstruction in initiated as follows: the proximal jejunum is mobilized and brought into the infra hepatic region on the right side. A choledochojejunostomy in end-to-side (end of bile duct to the side of jejunum) is constructed using multiple interrupted absorbable sutures. Twenty centimeters downstream, the jejunum is divided using a GIA a stapler. A 40 cm Roux-en-Y limb is fashioned. A duodenal jejunostomy is constructed in a end-to-side fashion in two layers using multiple absorbable interrupted sutures. A Gastrojejunostomy tube is placed in the stomach using the Stamm technique and the tip of the jejunal tube placed in the distal jejunum. The entire procedure lasted 9.8 ± 1.1 hours, including the islet preparation time of 4.8 ± 0.1 hours.
Islet Isolation
Our islet isolation technique is describe elsewhere.17, 27,28,29 For the pediatric pancreata, we currently use a new enzyme mixture that consists of a higher proportion of intact C1:C2 Clostridium histolyticum (Ch) collagenase and Ch neutral protease. The new enzyme mixture improved islet yields in pediatric pancreata without compromising their functional capacity in vivo.27,28 Although the pancreas size was smaller in children we have consistently obtained high islet yield per gram pancreas in children compared to adults.27,28 Currently, if the post-digest tissue volume of tissue is < 0.25 cc/Kg the islet cells are considered purified.
Islet cell infusion
Prior to starting islet infusion, the patient is given 70 units per kilogram of heparin and the heparin is allowed to circulate for at least 3 minutes. The islet product also contains 30 units per kilogram of heparin. The patient is also started on dextran 0.5 cc per kilogram to a maximum of 10 cc per hour continuous infusion. Dextran specifically inhibits the extrinsic pathway of coagulation. The splenic vein stump or the middle colic pain is cannulated and attached to pressure tubing with an in-line manometer, which is typically zeroed prior to starting the infusion. The islet preparation is infused by gravity into the portal vein system. Baseline portal pressure is first recorded and the pressure is measured every 3 minutes. If the pressure increases to greater than 25 cm of saline, infusion is paused for 15 minutes and the pressure measured. Most of the time, there is auto regulation and the portal pressure decreases. It is important to closely monitor pressures changes at 3 minute intervals in children. If the pressure is less than 25cm of saline, the infusion is restarted. If the pressure is more than 25 cm saline (after waiting for 15 minutes to autoregulate), or if a total tissue volume of 0.25/kg, the portal infusion is stopped and the rest of the islet preparation is implanted in the peritoneal cavity as a thin-film. The total time spent on infusing islets ranged from 60–110 min.
Post-operative management
All patients are started on an insulin drip to maintain blood sugar control between 80 and 120 mg/dL, in the immediate post operative to relieve β-cell functional stress during islet engraftment.30–33 The portal vein is evaluated by Doppler ultrasound on post-operative days 1 and 7. Dextran 40 is continued for 48 hours, after which aspirin 2 mg/kg is started. The heparin drip is continued at 10 units per kilogram for seven days. If the Doppler ultrasound revealed any portal vein clots, anti-coagulation with warfarin is continued for three months.
Delayed gastric emptying is common in the early post-operative period, requiring gastric decompression via the gastric port of the gastrojejunal tube (placed in the operating room) to decompress the stomach and simultaneously give jejunal feeds. As soon as the small bowel ileus resolves, enteral feeding is started through the jejunal port of the tube. A polymeric formula appropriate for the age of the child is used with pancreatic enzymes supplied in the tube feeding. Oral diet is started as the delayed gastric emptying resolves and evident by low gastrostomy tube outputs. Platelet counts were checked every other day and if the count was more than >106/mm3 hydroxyurea 500 mg po twice a day is initiated.
Post-operative follow-up
During the first three months post TP-IAT nearly all patients receive exogenous insulin to relieve beta cell functional stress during the engraftment (neovascularization) stage.30–33 Thereafter, insulin is weaned as long as blood glucose levels remain in a near normal target range (fasting < 125 mg/dl post prandial < 180 mg/dl and glycosolated hemoglobin ≤ 6.5 %). If these parameters were not achieved the patients were maintained on insulin.
Diet is advanced and tube feeds are stopped when the patient was able to take adequate oral calories and protein. Patients are seen after surgery in outpatient clinics at three months, six months and one year and thereafter annually. Routine laboratory studies are obtained at these intervals, including fasting glucose and C-peptide, stimulated glucose and C-peptide, and hemoglobin A1C level for assessment of metabolic control and islet graft function
Management of exocrine pancreatic insufficiency
When weaned to oral feeds, all patients are educated on the use of pancreatic enzymes, with a recommended initial dose of 1000 lipase units/kg/meal (half for snacks) and a goal dose of 1500 lipase units/kg/meal. All patients are started on a fat soluble vitamin supplement (source CF or AquaADEKS). Fat soluble vitamin levels and B12 levels are monitored at 3 months, 6 months, and 1 year after surgery, then yearly. All patients meet with our dietician at each visit, and receive counseling regarding a low oxalate diet to prevent kidney stones. Weight and height are measured and monitored at each visit to assess adequacy of pancreatic enzyme replacement.
Splenectomy Management
All patients have splenectomy as part of the TP-IAT. All children are fully immunized against Haemophilus influenzae type b, Meningococcus and Pneumococcus. Vaccination is completed at least 2 weeks before splenectomy. All children are maintained on prophylactic antibiotics for 1 year, and all patients and their parents receive counseling regarding the risks and management of splenectomy.
Pain Management
All patients were on narcotics before the operation. Patients are post operatively started on intravenous narcotics, and as they resume gastrointestinal function, oral narcotics are started. Patients are weaned off narcotics in the outpatient clinics.
Data Review
For the 75 patients having TP-IAT, medical records were reviewed and clinical data prospectively stored in a long-term auto islet database analyzed. We studied surgical complications, narcotic use before and after the procedure, short and long-term success of the islet transplantation, and postoperative quality of life. For the analysis of factors predicting insulin independence, we included 58 patients, who had sufficient long-term follow-up (>1 year) to attain insulin independence and excluded the remaining 17 patients from analysis. Narcotic use was determined from the medical records and from self-reported survey data. Patients were classified as either being on narcotics (no matter the dose) or off narcotics, meaning they took no narcotics daily or intermittently. Patients were asked if they had any pain during follow-up and if any component of the pain was similar to what they considered pancreatic pain similar to the time before TP-IAT.
Quality of life Assesments (Qol)
Beginning in 2007, quality of life surveys were administered to patients undergoing TP-IAT (n= 30).25 Patients (families) were asked to complete a comprehensive survey before surgery and at 3, 6, 12 months and annually after surgery. The RAND Medical Outcomes Study 36-item Short Form (SF-36) health survey was used as a measure of generic health related quality of life. Additional survey items included questions about school attendance, pain symptoms, narcotic use and insulin requirements.25 Parents were asked to report whether chronic pancreatitis kept their child from attending school and for the number of limited activity days during the past 28 days.
Statistical Analysis
Continuous variables were summarized as means and standard errors of the mean. Categorical variables were summarized as counts and percents. All analyses were performed using SAS/STAT™ 9.2 software. The prevalence of pain was assessed for (1) pancreatitis pain and (2) the severity of pain. Assessments were made pre-transplant and post-TPIAT at one-month, 6-months, 1-year and annually thereafter. Trajectory of changes were evaluated using either a generalized linear mixed models for categorical outcomes or general linear mixed models for continuous variables. Global tests for a difference in prevalence and pain severity over time were tested using a type III test for fixed effect of time. The underlying variance structure for both approaches used compound symmetry. Changes from pre-transplant were evaluated by examining change scores at each point-in-time after TPIAT.
For the analysis, patients were classified as follows: (1) Insulin-independent, (2) Partial graft function (C-peptide positive defined as > 0.6ng/dl or C-peptide unknown, the ability to maintain near normal glucose and glycohemoglobin levels on once daily long acting insulin only or with only occasional supplementation (less than daily) with short acting insulin), or (3) Insulin-dependent (C-peptide < 0.6 ng/ml; or if unknown on both long and short acting insulin [basal-bolus regimen] to maintain glycemic control.17 Individual patient classifications as to islet function could change over time. For example, a patient insulin-independent could revert to partial graft function or insulin-dependence; or an insulin-dependent could attain partial function or if partial function could change to either of the other categories. The rate of insulin independence was evaluated using Kaplan-Meier, time-to-event analysis. For this analysis, the number of months to insulin independence was depicted as a cumulative incidence function. The likelihood of becoming insulin independent was evaluated using multivariate logistic regression. Bivariate analyses were used to screen possible risk factors for inclusion in a multivariate adjusted model for insulin independence. We excluded the later 17 patients in our series from the analysis and these patients by definition lacked the follow up or length of time after the islet transplant time necessary for patients to attain insulin independence. Recipient characteristics separately statistically associated with insulin independence were included in a full model and evaluated as independent risk factors. Multivariate logistic regression was used to construct the final adjusted model. In constructing the model, forward step-wise and backward step-wise regression was used to arrive at a parsimonious list of factors for inclusion in the final model. A final, reduced multivariate model was based on risk factors that were statistically associated with insulin independence at 5% level of significance.
Patients and Demographics
A total of 75 TP-IATs were done in 75 pediatric patients. Of these, 11 had a previous procedure and underwent completion pancreatectomy and 64 had one-stage TP (Table 1). Baseline demographic characteristics at the time of TP-IAT are presented in Table 1. By etiology of CP, the idiopathic/familial/genetic constituted the majority.
Table 1.
Characteristics for Pediatric Total Pancreatectomy Islet Auto Transplants (TP-IAT) between July 7, 1989 and November 15, 2012.
| Mean ± SEM | N | % | |
|---|---|---|---|
| Number of Primary Pediatric TPIAT | 75 | ||
| Age | 13.8 ± 0.4 | ||
| Age Group | |||
| 5 to 12 years of age | 27 | 36.0% | |
| 13 to 19 years of age | 48 | 64.0% | |
| Gender | |||
| Male | 33 | 44.0% | |
| Female | 42 | 56.0% | |
| Etiology | |||
| Cystic Fibrosis | 4 | 5.3% | |
| Idiopathic | 21 | 28.0% | |
| Hereditary | 37 | 49.3% | |
| Other | 13 | 17.3% | |
| Body Mass Index | 22.9 ± 0.8 | ||
| Abdominal Pain (years) | 7.5 ± 0.5 | ||
| Chronic Pancreatitis (years) | 5.7 ± 0.6 | ||
| Narcotic Use (years) | 1.9 ± 0.4 | ||
Previous treatment given to the patients
Of the 75 patients, all patients had failed medical treatment, endoscopic therapy or were not amenable to endoscopic treatment due to anatomical considerations or failed surgical treatment or progressive hereditary disease. The previous surgical procedures performed are listed in Table 2.
Table 2.
Previous Interventions, Concurrent Interventions and Procedures by Age Group.
| 5 to 12 years of age | 13 to 19 years of age | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | N | % | Mean ± SEM | N | % | Mean ± SEM | N | % | |
| Direct Pancreas Surgery | 6 | 22.2% | 13 | 27.1% | 19 | 25.3% | |||
| Puestow | 4 | 14.8% | 9 | 18.8% | 13 | 17.3% | |||
| Whipple | 1 | 3.7% | 0 | 0.0% | 1 | 1.3% | |||
| Beger-Frey | 0 | 0.0% | 3 | 6.3% | 3 | 4.0% | |||
| Distal Pancreatectomy | 4 | 14.8% | 3 | 6.3% | 7 | 9.3% | |||
| Non-Direct Pancreas Surgery | 11 | 40.7% | 13 | 27.1% | 24 | 32.0% | |||
| Sphincterotomy | 11 | 40.7% | 12 | 25.0% | 23 | 30.7% | |||
| Sphincteroplasty | 0 | 0.0% | 1 | 2.1% | 1 | 1.3% | |||
| ERCP | |||||||||
| Any | 19 | 70.4% | 37 | 77.1% | 56 | 74.7% | |||
| Number of ERCPs | 2.6 ± 0.6 | range = 0, 15 | 1.9 ± 0.3 | range = 0, 10 | 2.1 ± 0.3 | range = 0, 15 | |||
| Endoscopic Duct Drainage | 16 | 59.3% | 28 | 58.3% | 44 | 58.7% | |||
| Stent | |||||||||
| Any | 13 | 48.1% | 25 | 52.1% | 38 | 50.7% | |||
| Number of Stents | 1.7 ± 0.6 | range = 0, 6 | 0.9 ± 0.2 | range = 0, 15 | 1.2 ± 0.2 | range = 0, 15 | |||
| Celiac Blocks | |||||||||
| Any | 3 | 11.1% | 9 | 18.8% | 12 | 16.0% | |||
| Number of Celiac Blocks | 0.2 ± 0.1 | range = 0, 2 | 0.8 ± 0.1 | range = 0, 4 | 0.3 ± 0.1 | range = 0, 4 | |||
Surgical and all cause of mortality
There was one in-hospital mortality (1.3%). The cause of death was sepsis resulting from enteric perforation due to a feeding tube placed in the operating room. In addition, there were two late deaths unrelated to TP-IAT. One patient had an infection of a paraspinal rod placed for scoliosis 4 years prior to TP-IAT, she died of ARDS associated with para spinal infection 7 years after TP-IAT. At the time of death, she was pain free and insulin independent. The second patient died 8 years after TP-IAT, a sudden death due to sepsis, at an outside emergency room, an autopsy was not performed. In both the cases, we were unable to obtain the culture reports from the treating facility to ascertain the causative infection. Since these two deaths, we have instituted post splenectomy prophylaxis in all our patients. The median follow up was 3.86 Years (Range - 0.6 years to 23.49 years; Interquartile Range = 6.09 years)
Surgical complications
Surgical complications occurred in 15 (20%) of patients (Table 3). The Complication rate was significantly lower in younger (<12 years) children (p=0.041).
Table 3.
Surgical Complications by Age Group.
| 5 to 12 years of age | 13 to 19 years of age | Total | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Post operative complications* | 2 | 7.4% | 13 | 27.1% | 15 | 20.0% |
| Abdominal Hemorrhage | 0 | 0.0% | 4 | 8.3% | 4 | 5.3% |
| Biliary Leak | 1 | 3.7% | 0 | 0.0% | 1 | 1.3% |
| Duodenal Leak | 0 | 0.0% | 1 | 2.1% | 1 | 1.3% |
| Anastomosis Leak Enteric | 0 | 0.0% | 1 | 2.1% | 1 | 1.3% |
| Abdominal Abscess | 1 | 3.7% | 2 | 4.2% | 3 | 4.0% |
| Wound Infection | 0 | 0.0% | 1 | 2.1% | 1 | 1.3% |
| Gastrointestinal Obstruction | 0 | 0.0% | 4 | 8.3% | 4 | 5.3% |
p value = 0.041
Intra abdominal bleeding occurred in 4 patients all of whom had high post islet cell infusion portal pressures (>25 mmHg). Post splenectomy thrombocytosis (>106/mm3) occurred in 30 (40%) patients, and was self-limiting or easily treated with hydroxyurea. No patient developed portal vein thrombosis. However, at the time of TP-IAT, 1 patient was noted to have a stenosis of the portal vein; he later developed variceal bleeding and was treated by surgical shunt at another medical center. There were no vascular injuries to the hepatic artery, superior mesenteric artery or celiac axis.
Pain and narcotic use
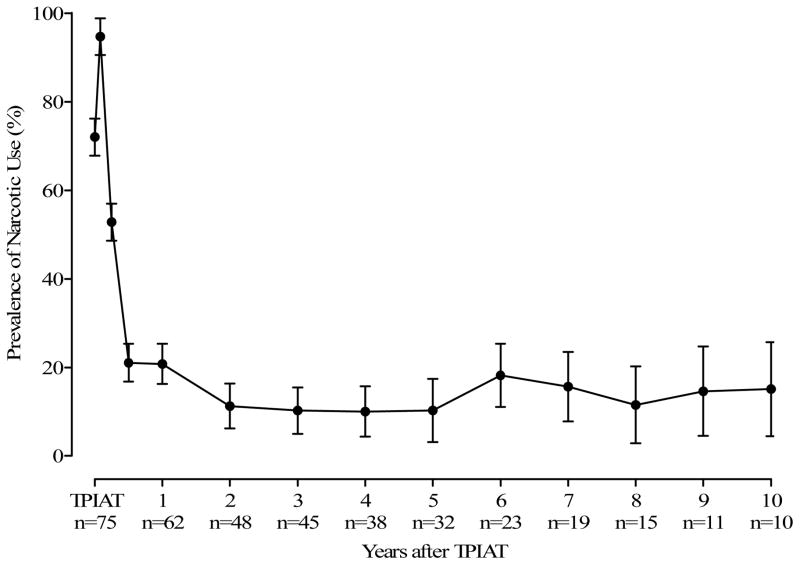
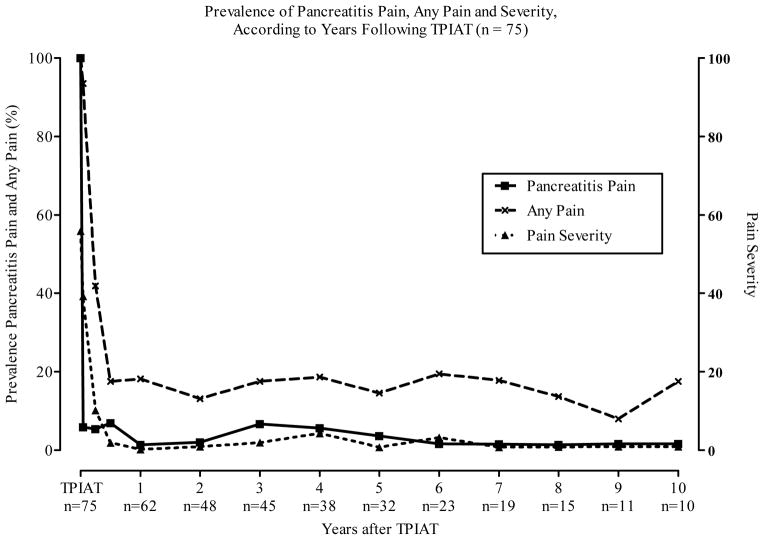
All patients were on narcotics prior to TP-IAT (Figure 1). Figure 2 gives the prevalence of pain at each point-in-time after TPIAT. Pancreatitis pain and the severity of pain statistically improved over time (p = < 0.001) following TPIAT. Nearly all improvement in pancreatitis pain occurred in the first 3 months after TPIAT (Figure 2). Figure 1 shows that except for the first month after TPIAT, narcotic use was statistically reduced after TP-IAT. Much of this decline occurs in the first year after surgery and remains relatively constant at between 10 % and 20 % from the first year after TPIAT forward. The relief from narcotics is sustained (Figure 1).
Figure 1.
Prevalence of Narcotic Use by Years Following TP-IAT (n = 75).
Figure 2.
Prevalence of Pancreatitis Pain and the Severity of Pain by Years Following TP-IAT.
Insulin Use and Islet cell function
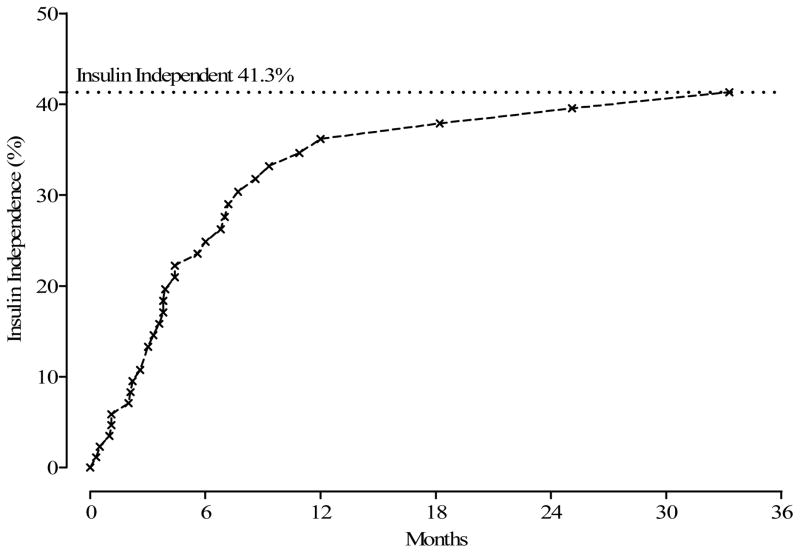
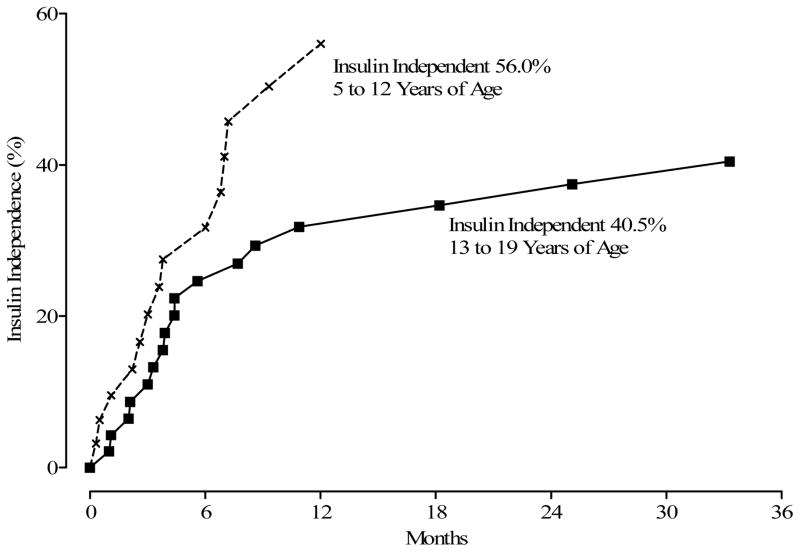
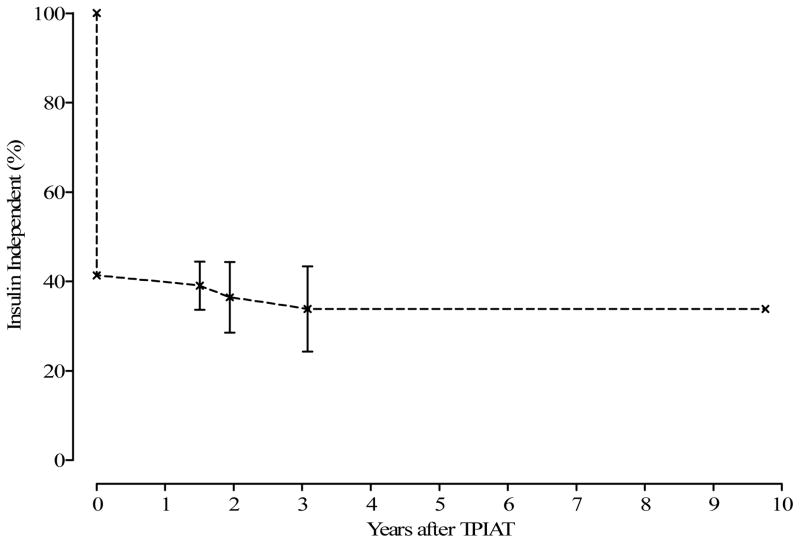
Of the 75 having TP-IAT, 31 (41.3%) achieved insulin independence (Figure 3). Time to insulin independence is displayed in Figure 3. Twenty-eight of the 31 patients achieved insulin independence within 12 months after TPIAT. Younger children (< 12 years) were more likely to achieve insulin independence than older children (p = 0.05) Figure 4.
Figure 3.
Months from TP-IAT to Insulin Independence.
Figure 4.
Months from TP-IAT to Insulin Independence by Age Group.
Insulin independence has been observed for as long as 10years after TP-IAT (Figure 5). Complete follow-up data on islet cell function at intervals 6 months, 1-year, 2-years and 3-years was available for 49 patients. The proportion of patients with insulin independence, partial graft function, and insulin dependence over the first 3 years post-operatively is shown in Table 4. Mean ± SEM hemoglobin A1c level for the entire cohort regardless of graft function was 6.88 ± 2.4% at 1 year, 5.8 ± 0.55% at 3 years and 5.7 ± 0.52% at 5 years. All insulin independent patients are confirmed or presumed to be C-peptide positive. Mean ± SEM stimulated C-peptide in insulin independent/ partial graft function patients was 2.21 ± 1.43 ng/mL, 3.38 ± 1.89ng/mL, 2.85 ± 0.07ng/mL at –1-year, 3-years and 5-years respectively.
Figure 5.
Months Insulin-Free Survival after First Insulin Independence (n = 75).
Table 4.
Islet Function Status According to Number of Islet Equivalents per Kilogram Transplanted.
| 6-Month | 12-Months | 24-Months | 36-Months | C-peptide-positive (> 0.6 ng/ml), % | HbA1C with mean < 7.0% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| < 2,500 IEQ / KG | 15 | 15 | 15 | 10 | 84 | 81 | ||||
| Insulin Independent | 2 | 13% | 2 | 13% | 2 | 13% | 1 | 10% | ||
| Partial Function | 8 | 53% | 6 | 40% | 6 | 40% | 3 | 30% | ||
| Insulin Dependent | 5 | 33% | 7 | 47% | 7 | 47% | 6 | 60% | ||
| 2,500 – 5,000 IEQ / KG | 17 | 17 | 17 | 14 | 100 | 96 | ||||
| Insulin Independent | 5 | 29% | 9 | 53% | 9 | 53% | 7 | 50% | ||
| Partial Function | 12 | 71% | 8 | 47% | 5 | 29% | 4 | 29% | ||
| Insulin Dependent | 0 | 0% | 0 | 0% | 3 | 18% | 3 | 21% | ||
| > 5,000 IEQ / KG | 17 | 17 | 17 | 12 | 100 | 100 | ||||
| Insulin Independent | 9 | 53% | 13 | 76% | 13 | 76% | 11 | 92% | ||
| Partial Function | 7 | 41% | 3 | 18% | 3 | 18% | 1 | 8% | ||
| Insulin Dependent | 1 | 6% | 1 | 6% | 1 | 6% | 0 | 0% | ||
Of the 31 patients who attained insulin independence, 3 reverted to insulin dependence (partial graft function. (Figure 5). The clinical details were as follows:
Case 1 is a 9 year old girl with an unusual underlying diagnosis of Goldston syndrome (renal cysts + Dandy Walker malformation and in her case pancreatic cysts with pancreatitis), with developmental delay and feeding and motility issues. She received an islet mass of 148,907 IEQ, in her case 6,053 IEQ/kg, and was initially insulin independent. However, about 1.5 years post-TPIAT she was admitted for motility evaluation and enteral feed initiation at an outside hospital, where she had a precipitating event of Escherichia coli bacteremia which was associated with sudden and severe hyperglycemia. She had a documented HbA1c level of 5.8% and measured glucoses 70–116 mg/dL only 4 days prior to the onset of bacteremia, confirming that this patient was normoglycemia off insulin prior to infection. With Escherichia coli infection, initial glucose on day of infection onset was first documented at 328 mg/dL and then remained 216–428 mg/dL until hospital discharge. Hyperglycemia was inadequately treated and not communicated to our institution until after patient discharge, 10 days after event, at which time glucoses remained frequently >200 mg/dL during tube feeding, and we initiated basal insulin coverage. Patient remains with partial graft function at last follow up. We believe patient has suffered partial loss of islet mass due to sustained hyperglycemia in this case.
Case 2 is a 13 year old male with history of a prior Puestow procedure and the first pediatric case in the series. He received 3,100 IEQ/kg, was off insulin therapy with HbA1c low to mid-6% range, until HbA1c elevated to 7% at nearly 2 years post-operatively and insulin was initiated. This case had a lower than average islet mass, and using our current protocols, might have been maintained on basal insulin therapy due to borderline HbA1c.
Case 3 is an 11 year old male with severe PRSSI disease who received only 1,926 IEQ/kg at time of transplant. Despite low islet mass, he weaned off insulin entirely and maintained normal HbA1c (multiple documented levels <6%) until 3 years after TPIAT. At that time, his HbA1c elevated to 6.7% with modest hyperglycemia detected by home meter, and insulin was resumed. At that time, islet mass had decreased to about 1,300 IEQ/kg.
Factors Predicting Insulin Independence
For the 58 patients with adequate long-term follow-up to allow for insulin independence, (> 12 months), younger age (p=0.032), No prior Puestow (p=0.018), lower body surface area (p=0.048), higher IEQ per Kg body weight (p=0.001) and total IEQ transplanted (100,000) (0.004) were associated with a higher probability of insulin independence (Table 5). Using multivariate logistic regression, 3 factors were associated with a higher probability of insulin independence after TP-IAT (Table 6): (1) male gender, (2) lower body surface area and the (3) total IEQ transplanted. Total IEQ given in units of (100,000) is by far and away the single factor most strongly associated with insulin independence (OR = 2.62; p value < 0.001). The odds of insulin independence in boys are nearly 17 times greater than girls. Increased body surface area is associated with a decreased likelihood of insulin independence, suggesting that body surface area is a proxy for patient age, which was associated with independence in the unadjusted analysis. Overall, the statistics for final multivariate adjusted logistic regression model support good discrimination and calibration.
Table 5.
Unadjusted Odds Ratio for Association between Risk Factors and Insulin Independence (n=58).
| Odd Ratio | LCL | UCL | p value | |
|---|---|---|---|---|
| Age | 0.85 | 0.73 | 0.99 | 0.032 |
| Female | 0.50 | 0.17 | 1.42 | 0.190 |
| Etiology | ||||
| Cystic Fibrosis | 3.23 | 0.32 | 33.05 | 0.323 |
| Hereditary | 1.00 | 0.35 | 2.84 | 1.000 |
| Idiopathic | 2.22 | 0.72 | 6.85 | 0.166 |
| Other | 0.17 | 0.03 | 0.85 | 0.031 |
| Years with Pancreatitis | 0.91 | 0.81 | 1.02 | 0.097 |
| Years with Pain | 0.87 | 0.76 | 1.01 | 0.059 |
| Years on Narcotics | 0.87 | 0.61 | 1.24 | 0.432 |
| Prior Surgery and Procedures | ||||
| Puestow | 0.14 | 0.03 | 0.72 | 0.018 |
| Stent | 1.15 | 0.41 | 3.22 | 0.793 |
| Celiac Block | 0.50 | 0.13 | 1.95 | 0.320 |
| Sphincterotomy | 1.65 | 0.53 | 5.20 | 0.389 |
| ERCP | 0.82 | 0.24 | 2.83 | 0.753 |
| Endoscopic Duct Drainage | 1.54 | 0.54 | 4.45 | 0.422 |
| Pancreas Fibrosis Severity | 0.85 | 0.69 | 1.05 | 0.132 |
| Body Mass Index | 0.93 | 0.86 | 1.02 | 0.123 |
| Body Surface Area | 0.25 | 0.06 | 0.99 | 0.048 |
| IEQ per KG Body Weight | 1.05 | 1.02 | 1.08 | 0.001 |
| Change in Portal Pressure | 1.19 | 0.82 | 1.73 | 0.364 |
| Total IEQ (100,000) | 1.81 | 1.21 | 2.72 | 0.004 |
Table 6.
Multivariate Adjusted Logistic Regression Model for Insulin Independence (n=58).
| Risk Factor | Odds Ratio | LCL | UCL | p value |
|---|---|---|---|---|
| Male gender | 4.17 | 1.00 | 16.67 | 0.050 |
| Total IEQ (100,000 Units) | 2.62 | 1.48 | 4.64 | 0.001 |
| Body Surface Area | 0.95 | 0.91 | 0.98 | 0.005 |
C Statistic = 0.832
- Chi-Square = 6.50
- DF = 8
- p value = 0.591
Quality of life data
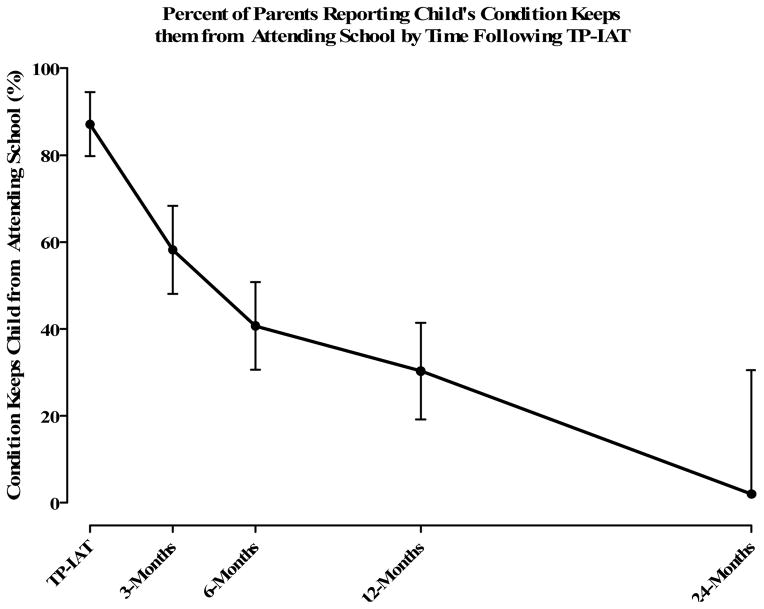
Ninety two quality of life surveys were completed by 30 recipients. Completion rate was 97% at baseline and 60–81% at any time point after surgery. Responses to the SF-36 Health Survey among those 12 years of age and older show a statistical improvement in physical and mental health following TP-IAT using mixed model methods. The Physical Component Summary Scale (PCS) score improved with time (p value = 0.007) changing from pre-transplant by nearly 2 standard deviations (17.2 points). The Mental Component Summary Scale (MCS) MCS was similarly improved (p value = 0.024). The mental component of the health status score improved by over 2 standard deviations (22.2 points). Significant improvements were seen in all 8 subscales, with the most dramatic improvements in raw score for role-physical (14 ±7 at baseline vs. 83 ±10 at 1 year, p<0.001) and bodily pain (25 ±5 at baseline vs. 70 ±7 at 1 year, p<0.001). Figure 6 shows the percent of parents reporting their condition kept them from attending school. Based on a generalized linear mixed model, the percent of parents reporting lost days at school statistically declined (p value < 0.001). Pre-TPIAT 87% of the parents reported lost school days compared with a negligible value at 2 years. The number of limited activities showed a similar statistical decline over the same period (p value < 0.001). The number of limited activity days at 24 months was about 20% of what was reported pre-TP-IAT (Figure 6).
Figure 6.
Percent of parents reporting child’s condition keeps them from attending school by time following TP-IAT.
Discussion
The primary goal of surgical treatment of childhood CP is to relieve pain and restore quality of life. Although many different operative procedures short of TP have been performed for CP, and pain is ameliorated initially, it recurs in more than 50% of patients.9–14 TP potentially eliminates the source of pain but results in brittle diabetes that can be difficult to treat, hence the addition of IAT. The first TP-IAT in a child was reported in 1996, done with the goal of eliminating pain and minimizing post surgical diabetes.26 Our current data in 75 children demonstrate that there is sustained pain relief, acceptable long-term glycemic control and improved quality of life after TP-IAT. Operative morbidity in our series was low compared to literature reports of TP for other indications.34 The surgical complication rate was 20%; most complications were relatively minor and none had long term sequelae. Younger children (<12 years) tended to have a lower complication rate, perhaps due to shorter duration of illness.
An important observation from our series is that the addition of islet auto transplantation to TP did not increase the morbidity associated with TP alone. Unlike the adult series, we have routinely measured the portal pressure at 3 minute intervals during islet cell infusion;12 because of concern about the possibility of development of portal vein thrombosis. We did not continue infusion if portal pressure reached 25cm of saline. We feel beyond this threshold there is an increased risk of bleeding and thrombosis.35 All 4 patients that had intra abdominal bleeding had pressures >25mm Hg. The maximum amount of islet cell infused intra portal in our series is 0.25cc/Kg. If infusion was stopped because of elevated portal pressure, the remainder of the islets were placed in the peritoneal cavity; Although we have used this site we do not have data to document the degree of engraftment at this site as opposed to the intra hepatic site of islet cell implantation.
Prior pancreatic surgery has significant impact on outcomes of TP-IAT. Previous surgery did not increase the surgical complication rate; however, prior Puestow operation resulted in a significantly lower islet yield and increased the risk of insulin dependence in long-term follow-up. Timing of the TP-IAT is likely a very important consideration as well. Younger children had a lower surgical complication rate and a higher chance of insulin independence. We believe that children with the PRSS1mutations and other forms of progressive hereditary pancreatitis are best served by TP-IAT performed earlier, due to impaired quality of life, progressive destruction of islet cells and increased lifetime risk of cancer. Of those with the PRSS1 genetic mutations, the association between chronic pancreatitis and cancer has been well established. Over time the risk approaches 40%.2 There is a theoretical risk of cancer developing with IAT, but to date, in our entire series of 75 patients, none have developed any malignancy
While we have no method to directly measure islet mass, and thus cannot definitively comment on whether transplanted islet mass grows with time in children, our data suggests some ability of the transplanted islet mass to compensate appropriately with growth of the child. This is most striking in our youngest children, who have the greatest growth potential. In our cohort, among the children age 5–9 years at transplant with >2 years of follow up for growth, there were 8 children with long-term insulin independence (2.5–8 years, n=7) or long-term minimal insulin use (2 units/day × 13 years, n=1), who maintained this insulin independence (or low insulin requirement in the latter case) despite significant growth. These 8 children were transplanted at a mean age of 8.0 ±1.7 years, most recently age 14.6 ±3.5 years. As expected, during this time of rapid growth, significant weight gain was observed, from 29 ±9 kg to 51 ±15 kg, and the relative islet mass for body size (calculated based on the number of islets transplanted) was reduced by 42%, from 6,325 ±3,816 IEQ/kg at transplant to 3,661 ±2,873 IEQ/kg at follow up. This series includes two 5 year olds, 6 and 8 years post-TP-IAT, both maintaining insulin independence despite change in calculated islet mass per kilogram body weight from 6,362 IEQ/kg to 3,406 IEQ/kg in the first case (a now 11 year old girl), and 10,647 IEQ/kg to 3,066 IEQ/kg in the second case (a now 14 year old teenage boy). Thus, we believe the data is optimistic in regards to the potential for compensatory growth in islet mass over time. While beta cell replication is mostly limited to infancy and early childhood, children in this young age group (<10 years) have been demonstrated to have increased capacity for beta cell replication compared to adolescents and adults on autopsy studies. 36 We have also demonstrated previously a capacity for neogenesis of islet like structures from ductal cells in the young patients with severe pancreatitis suggesting that ductal precursors transplanted in the “impure” fractions of the islet preparation could also theoretically provide a precursor for islet mass in these younger patients.37
Insulin Independence is clearly dependent on the number of islets transplanted. Although our numbers are too small to clearly determine a breakpoint, our data suggests that children that receive more than 2500 IEQ per kilogram are more likely to achieve insulin independence. Importantly, even the children who have partial function and need some insulin on a daily basis, have improved quality of life compared to their pre TPIAT status.
In summary, we present a large surgical series of islet auto transplantation in children. Total Pancreatectomy and auto islet cell transplant provide sustained pain relief, improved quality of life in the majority of children with chronic painful pancreatitis that fail medical and/or endoscopic treatment.
Acknowledgments
Dr. Arthur J Matas, MD, Professor of Surgery University of Minnesota for reviewing the manuscript;
Footnotes
Conflicts of Interest and Sources of Funding: Dr. Melena Bellin receives support from the National Institute of Diabetes, Digestive, and Kidney Diseases (1K23DK084315-01A1).
References
- 1.Schmitt F, Henaff GL, Piloquet H, et al. Hereditary pancreatitis in children: surgical implications with special regard to genetic background. J Pediatr Surg. 2009;44:2078–2082. doi: 10.1016/j.jpedsurg.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clinical Gastroenterol Hepatol. 2004;2(2):252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P, Di Magno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–6. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 4.Steer ML, Waxman I, Freedman S. Chronic Pancreatitis. N Engl J Med. 1995;332:1482–1490. doi: 10.1056/NEJM199506013322206. [DOI] [PubMed] [Google Scholar]
- 5.Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss Med Wkly. 2006;136:166–174. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- 6.Choudari CP, Nickl NJ, Fogel E, et al. Hereditary Pancreatitis:clinical presentation, ERCP findings, and outcome of endoscopic therapy. Gastrointest Endosc. 2002;56:66–71. doi: 10.1067/mge.2002.125103. [DOI] [PubMed] [Google Scholar]
- 7.Clifton MS, Pelayo JC, Cortes RA, Grethel EJ, Wagner AJ, Lee H, Harrison MR, Farmer DL, Nobuhara KK. Surgical treatment of childhood recurrent pancreatitis. J Pediatr Surg. 2007;42:1203–1207. doi: 10.1016/j.jpedsurg.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Iqbal CW, Moir CR, Ishitani MB. Management of chronic pancreatitis in the pediatric patient:endoscopic retrograde cholangiopancreatography vs operative therapy. J Pediatr Surg. 2009;44:139–143. doi: 10.1016/j.jpedsurg.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Gachago C, Draganov P. Pain management in chronic pancreatitis. World J Gastroenterol. 2008;14:3137–3148. doi: 10.3748/wjg.14.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holmberg JT, Isaksson G, Ihse I. Long term results of pancreaticojejunostomy in chronic pancreatitis. Surg Gynecol Obstet. 1985;160:339–346. [PubMed] [Google Scholar]
- 11.Bradley EL., 3rd Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153:207–213. doi: 10.1016/0002-9610(87)90816-6. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg. 1994;129:374–379. doi: 10.1001/archsurg.1994.01420280044006. discussion 379–380. [DOI] [PubMed] [Google Scholar]
- 13.O’Neil SJ, Aranha GV. Lateral pancreaticojejunostomy for chronic pancreatitis. World J Surg. 2003;27:1196–1202. doi: 10.1007/s00268-003-7238-7. [DOI] [PubMed] [Google Scholar]
- 14.Cahen DL, Gouma DH, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676–684. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 15.Sasikala M, Talukdar R, Pavan kumar P, et al. β-Cell dysfunction in chronic pancreatitis. Dig Dis Sci. 2012 Jul;57(7):1764–72.2. doi: 10.1007/s10620-012-2086-7. [DOI] [PubMed] [Google Scholar]
- 16.Najarian JS, Sutherland DER, Matas AJ, Goetz FC. Human islet autotransplantation following pancreatectomy. Transplant Proc. 1979;ll:336–340. [PubMed] [Google Scholar]
- 17.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214:409–24. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad SA, Lowy AM, Wray CJ, et al. Factors associated with insulin and narcotic independence after islet autotransplantation in patients with severe chronic pancreatitis. J Am Coll Surg. 2005;201:680–687. doi: 10.1016/j.jamcollsurg.2005.06.268. [DOI] [PubMed] [Google Scholar]
- 19.Garcea G, Weaver J, Phillips J, et al. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: a series of 85 consectuive patients. Pancreas. 2009;38:7. doi: 10.1097/MPA.0b013e3181825c00. [DOI] [PubMed] [Google Scholar]
- 20.Clayton H, Davies J, Pollard C, et al. Pancreatectomy with islet autotransplantation for the treatment of severe chronic pancreatitis: the first 40 patients at the Leicester General Hospital. Transplantation. 2003;76:92–98. doi: 10.1097/01.TP.0000054618.03927.70. [DOI] [PubMed] [Google Scholar]
- 21.Dixon J, DeLegge M, Morgan K, Adams D. Impact of total pancreatectomy with islet cell transplant on chronic pancreatitis management at a disease-based center. Am Surg. 2008;74:735–738. doi: 10.1177/000313480807400812. [DOI] [PubMed] [Google Scholar]
- 22.Argo J, Contreras J, Wesley M, Christein J. Pancreatic resection with islet cell autotransplant for the treatment of severe chronic pancreatitis. Am Surg. 2008;74:530–536. [PubMed] [Google Scholar]
- 23.Sutton MJ, Schmulewitz N, Sussman JJ, et al. Total Pancreatectomy and islet cell auto transplantation as a means of treating patients with genetically linked pancreatitis. Surgery. 2010;148:676–86. doi: 10.1016/j.surg.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Webb M, Illouz S, Pollard C, et al. Islet auto transplantation following total pancreatectomy: a long-term assessment of graft function. Pancreas. 2008;37:282–287. doi: 10.1097/mpa.0b013e31816fd7b6. [DOI] [PubMed] [Google Scholar]
- 25.Bellin MD, Freeman ML, Schwarzenberg SJ, et al. Quality of life improves for pediatric patients after total pancreatectomy and islet autotransplant for chronic pancreatitis. Clinical Gastroenterology. 2011;9:793–9. doi: 10.1016/j.cgh.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahoff DC, Paplois BE, Najarian JS, et al. Islet Autotransplantation after total pancreatectomy in a child. J Pediatr Surg. 1996;31:132–5. doi: 10.1016/s0022-3468(96)90335-8. [DOI] [PubMed] [Google Scholar]
- 27.Balamurugan AN, Chang Y, Bertera S. Suitability of human juvenile pancreatic islets for clinical use. Diabetologia. 2006;49:1845. doi: 10.1007/s00125-006-0318-0. [DOI] [PubMed] [Google Scholar]
- 28.Balamurugan AN, Loganathan G, Bellin MD, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogenic human islet products. Transplantation. 2012;93:693–702. doi: 10.1097/TP.0b013e318247281b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anazawa T, Balamurugan AN, Bellin M, et al. Human islet isolation for autologous transplantation: comparison of yield and function using SERVA/Nordmark versus Roche enzymes. Am J Transplant. 2009;10:2383–91. doi: 10.1111/j.1600-6143.2009.02765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finzi G, Davalli A, Placidi C, Usellini L, La Rosa S, Folli F, Capella C. Morphological and ultrastructural features of human islet grafts performed in diabetic nude mice. Ultrastruct Pathol. 2005;29:525–33. doi: 10.1080/01913120500323563. [DOI] [PubMed] [Google Scholar]
- 31.Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 32.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate post transplantation period. Dynamic changes in structure and function. Diabetes. 1996;45:1161–7. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 33.Juang JH, Bonner-Weir S, Wu YJ, Weir GC. Beneficial influence of glycemic control upon the growth and function of transplanted islets. Diabetes. 1994;43:1334–9. doi: 10.2337/diab.43.11.1334. [DOI] [PubMed] [Google Scholar]
- 34.Barbier L, Jamal VV, Dokmak S, et al. Impact of total pancreatectomy:short and long term assessment. HPB; Oxford: 2013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilhelm JJ, Bellin MD, Dunn TB, et al. Proposed Thresholds for Pancreatic Tissue-Volume for Safe Intraportal Islet-Autotransplantation after Total-Pancreatectomy. Am J Transplant. 2013 Oct 21; doi: 10.1111/ajt.12482. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–94. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soltani SM, O’Brien TD, Loganathan G, et al. Severely fibrotic pancreases from young patients with chronic pancreatitis: evidence for a ductal origin of islet neogenesis. Acta Diabetol. 2013;50:807–14. doi: 10.1007/s00592-011-0306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]