Abstract
Objective
The purpose of this study was to estimate the incidence, timing of onset, risk factors, and mortality rate of pregnancy-associated pulmonary embolism (PAPE).
Methods
We analyzed PAPE cases that occurred between January 2005 and December 2012 at Cheil General Hospital & Women's Healthcare Center. Those cases that were not confirmed by computed tomography scan or were confirmed as amniotic fuid embolisms were excluded. We analyzed various risk factors such as previous surgery, mode of delivery, maternal age, and obesity in PAPE.
Results
There were 57,092 deliveries over 8 years. Of them, 13 cases (0.023%) were diagnosed with PAPE. All cases occurred in the postpartum period after cesarean delivery. There were no cases of PAPE after vaginal deliveries. Of the total cases, 10 cases (76.9%) were diagnosed in the early postpartum period within 48 hours. Eight cases (61.5%) had a history of previous surgery. There were 3 cases (23.1%) of multiple pregnancy and 3 cases (23.1%) of preterm delivery. No cases had a history of venous thromboembolism. Among 13 cases, 10 cases improved with only anticoagulation, 2 cases received surgical thrombectomy, and one case was maternal death.
Conclusion
Our results indicated that the incidence of PAPE was very low (0.023%) and occurred mainly in the postpartum period after cesarean section. However, its maternal mortality rate was significantly high (7.7%). Therefore, we suggest that immediate diagnosis and prompt treatment should be prioritized for improvement of PAPE patients' survival rate.
Keywords: Peripartum period, Pregnancy, Pulmonary, Thromboembolism
Introduction
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism, is a complication in 0.5 to 3.0 of every 1,000 pregnancies [1]. Pregnancy-associated pulmonary embolism (PAPE) is still one of the leading causes of maternal morbidity and death in the industrialized part of the world [2].
There are several factors that increase the risk of VTE. Virchow described a triad of initiating factors for venous thrombosis, namely hypercoagulability, venous stasis, vascular damage and all of which occur during pregnancy [3]. Each component of Virchow's triad is present at some point during pregnancy. Pregnancy itself induces a hypercoagulable state in pregnant women in order to prevent hemorrhage [4]. These factors lead to an increased incidence of VTE. The incidence of VTE during pregnancy and the postpartum period has been estimated to be 5.5 to 6 times higher than in the general female population of childbearing age [5,6].
VTE can cause significant maternal mortality and morbidity. It accounts for 1.1 deaths per 100,000 deliveries [7,8], or 10% of all maternal deaths [9]. Despite recent medical technology development, VTE remains an unresolved problem in obstetrics. Of the two types of VTE, PAPE occurs suddenly and is not easy to diagnose promptly because the clinical presentation of PAPE is extremely variable in pregnancy and the signs and symptoms of a suspected PAPE such as dyspnea can frequently overlap with that of a normal pregnancy. Moreover, mortality by PAPE occurs within 30 minutes of the sentinel event, making timely intervention difficult [10]. Mortality from PAPE in pregnancy is related to challenges in targeting the right population for prevention, suspecting the diagnosis and adequately evaluating appropriate patients, and initiating timely and the best possible treatment when indicated [11]. Thus, it is very important for obstetricians to recognize the risks of PAPE during pregnancy [12]. When women with risk factors are appropriately managed and provided thrombophylaxis, the mortality from PAPE can be reduced.
Although PAPE is directly related to maternal health, there are little clinical data about this disease in South Korea. The purpose of this study was to estimate the incidence, onset timing, risk factors, and mortality rate of PAPE over eight years in one center.
Materials and methods
We retrospectively analyzed 13 cases of PAPE confirmed by computed tomography (CT) scan at Cheil General Hospital & Women's Healthcare Center, Kwandong University between January 1, 2005 and December 31, 2012. This study included some cases of PAPE previously reported in the Korean Journal of Medicine, 2008 [13].
Over eight years, a total of 30 cases were examined by CT scan for suspicion of pregnancy-associated PAPE. All hospital charts were analyzed with a radiologist and a cardiologist. Of them, 13 cases with objective evidence of PAPE were eligible for inclusion. The other 15 cases were excluded because CT identified pulmonary effusion or edema and not PAPE. An additional 2 cases were also excluded because amniotic fluid embolism was suspected. In addition, patients that were examined by CT scan or treated with heparin for clinical suspicion of PAPE but were not objectively diagnosed were excluded from analysis.
In total, we analyzed 13 cases with obstetric, internal medicine, and clinical test databases. Maternal characteristics of age, parity, height, weight, time during pregnancy or postpartum at which thromboembolism occurred, presenting clinical symptoms and signs, and managements at time of diagnosis were analyzed with hospital charts. Coexisting medical conditions or comorbidities were specified according to a prespecified list. Gestational age was defined as the weeks of gestation recorded in the hospital chart. Postpartum was defined as the first 6 weeks after delivery. We analyzed risk factors for thromboembolism, including maternal age over 35 years, parity of three or more, body mass index (BMI) over 30, multiple pregnancies, anemia, method of delivery, gestational age at delivery, gestational diabetes mellitus, and preeclampsia [14]. Immobilization was defined as bed rest more than 3 days according to previous studies [15,16].
Maternal demographics and clinical characteristics, including risk factors and clinical symptoms, were also analyzed.
Results
During the 8-year period, there were 57,092 births at Cheil General Hospital & Women's Healthcare Center, with 21,918 (38.4%) cesarean and 35,174 (61.6%) vaginal deliveries. Thirteen pregnancies (0.023%) were complicated by PAPE, for a total incidence of one per 4,392 births. The mean age of women diagnosed with PAPE was 33.3±3.3 years. The mean gravidity was 2.0±1.0. The average weight gain in patients with PAPE was 13.1±4.8 kg (range, 6-22 kg).
Table 1 presents the incidence of PAPE according to various risk factors of pregnancy-related thrombosis. Six cases of PAPE (46.2%) occurred in advanced maternal age. There was a higher prevalence of PAPE in nulliparous women than primiparous or multiparous women (8 versus 5 cases). A pregnancy parity over 3 was not in PAPE cases. Three cases of PAPE (23.1%) found in multiple pregnancies and in assisted reproduction technique, respectively. There were 2 cases (23.1%) with a prepregnancy BMI of 25 to 29 and no one had a prepregnancy BMI over 30. In analysis of BMI at delivery, women with BMI of 25 to 29 showed higher prevalence than women with BMI greater than 30 (7 versus 5 cases). All cases found in the postpartum periods after cesarean delivery. The emergency cesarean section rate was 53.8% (7 cases). There were 8 cases with a previous surgical history (61.5%). Four of 8 cases had undergone previous cesarean section, 2 had undergone laparotomy, and an additional 2 cases had laparoscopic surgery. PAPE cases with preeclampsia, transfusion, and immobilization were 2 (15.4%), 1 (7.7%), and1 (7.7%), respectively. Women with risk factors such as gestational diabetes mellitus, smoking, heart disease were not in PAPE cases.
Table 1.
Associated risk factors of pulmonary embolism in 13 cases
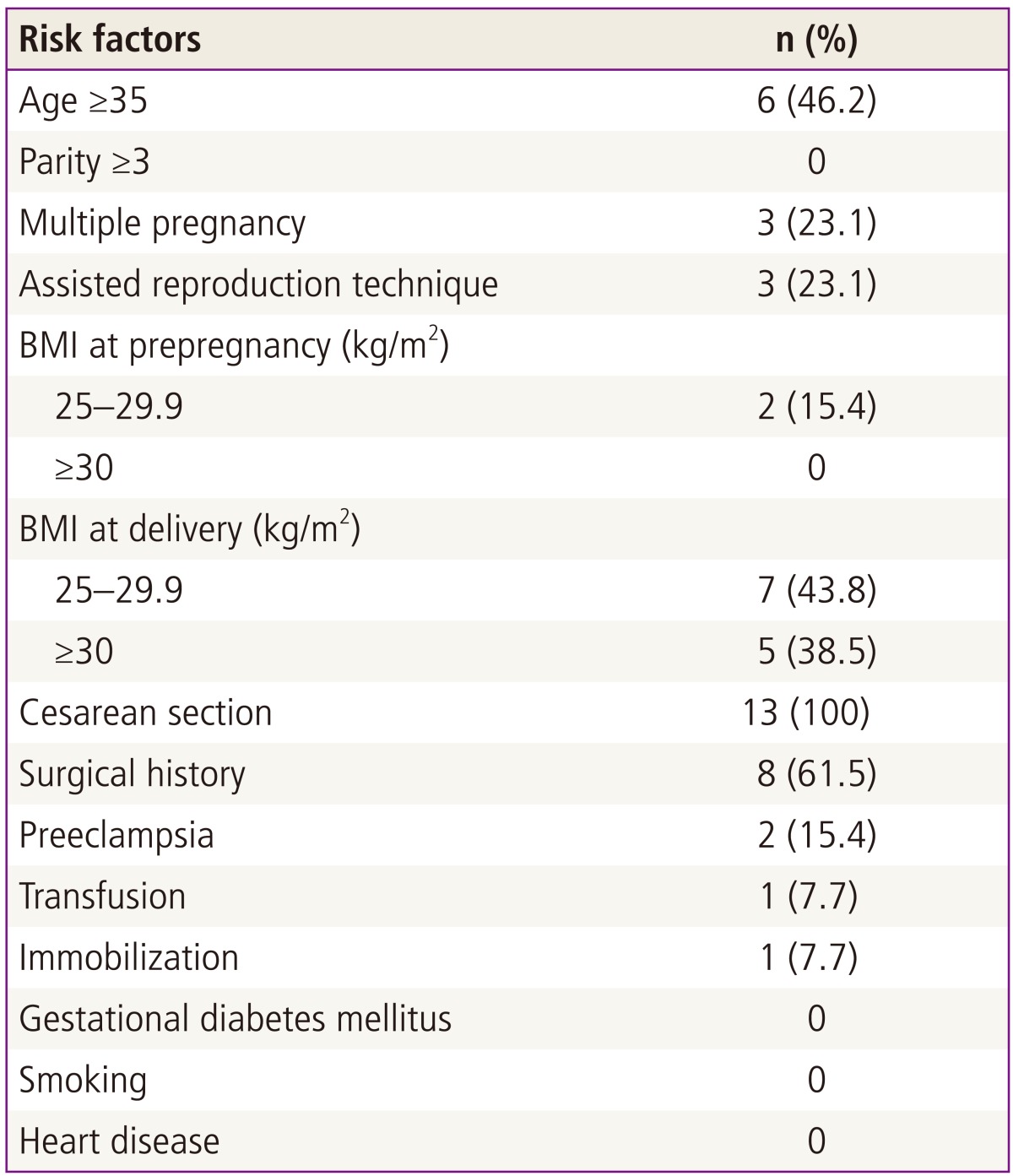
BMI, body mass index.
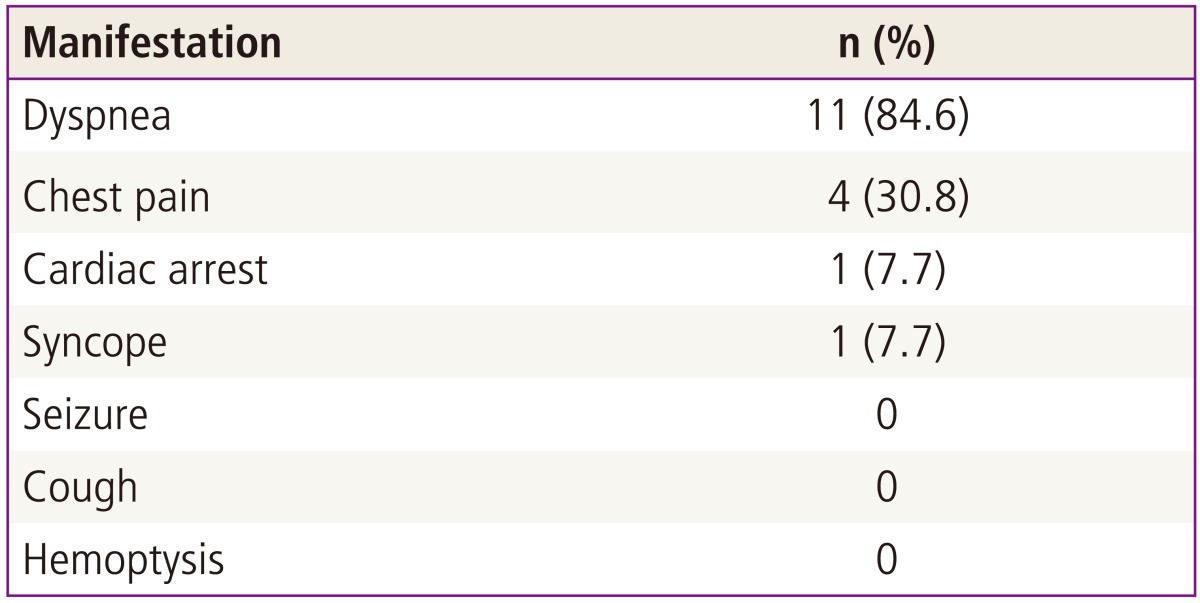
Table 2 shows the clinical manifestations of the study cases. The most common symptom of PAPE patients was dyspnea (11 cases, 84.6%) or chest pain (4 cases, 30.8%). Cardiac arrest and syncope were detected in one case, respectively. Manifestations including seizure, cough, and hemoptysis were not detected in PAPE cases. The results of chest X-ray at the time of manifestation were as below: normal in 10 of 13 cases (76.9%), pleural effusion in 1 (7.7%), pulmonary edema in 1 (7.7%), and cardiomegaly in 1 (7.7%). The electrocardiogram was abnormal in 4 of 13 patients (30.8%); two patients had tachycardia (15.4%), one patient had an ST-T abnormality (7.7%), and 1 patient had right axis deviation (7.7%).
Table 2.
Presenting manifestations in patients with pregnancy-associated pulmonary embolism
Ten cases of PAPE occurred within 2 days of delivery (76.9%) (Table 3). Preterm birth was detected in 3 cases (23.1%). All patients with PAPE had anticoagulation therapy with heparin and warfarin, and 4 patients (30.8%) were transferred to a tertiary hospital due to rapid deterioration in condition. Two of them underwent surgical thrombectomy (case 5,13), and there was one maternal death (case 10). The patient was found unconscious in her sickroom 2 days after an elective cesarean section. She was transferred to a tertiary hospital after immediate cardiopulmonary resuscitation and heparin treatment. CT scan showed almost totally blocked bilateral PAPE. One case underwent hysterectomy due to massive bleeding during cesarean section (case 11). There were no cases with hereditary thrombophilia or past history of thromboembolism. Case 1 was complicated by preterm labor and hospitalization for a week with acute fatty liver. She was found to have classic type myotonic dystrophy, and her baby was diagnosed with congenital myotonic dystrophy.
Table 3.
Clinical characteristics of pulmonary embolism
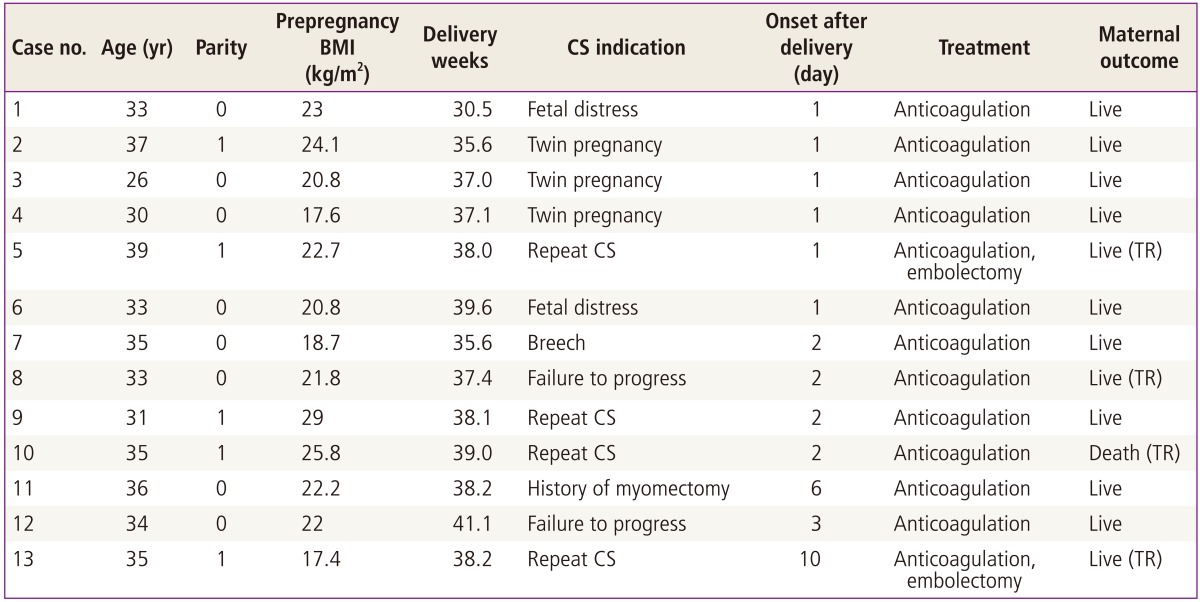
BMI, body mass index; CS, cesarean section; TR, transfer to tertiary center.
Table 4 shows risk factors that possibly affected the disease occurrence in each case. These risk factors were classified as baseline, pregnancy-associated and delivery-associated. The frequency of various risk factors range from two to seven according to each case.
Table 4.
Possible risk factors for pulmonary embolism in 13 cases

ART, assisted reproductive technique; CS, cesarean section.
Discussion
Although incidence of VTE is low, it is the leading cause of maternal deaths and is fatal to women who have delivered or are pregnant [2]. Therefore, diagnosis and early management of VTE are important.
Two types of VTE are PAPE and DVT; PAPE occurs when clots break off from the vein wall and travel through the heart to the pulmonary arteries [14], whereas DVT occurs by the formation of a blood clot in the deep leg vein. Especially, PAPE has an increased probability of acute attack compared with DVT. Therefore, PAPE has been referred to as a more dangerous disease than DVT.
This study presents the incidence, onset timing, associated risk factors, and mortality of women diagnosed with PAPE. We identified 13 cases of PAPE over 8 years at our hospital, which is a rate of 0.23 per 1,000 deliveries. Our study shows the timing of onset of all PAPE cases was in the postpartum period. Similar to other studies [17], this study shows that the immediate postpartum period is the greatest risk period for PAPE.
There are many well-known risk factors associated with PAPE. These include surgical history, preeclampsia, high parity (≥3), elderly (age over 35), previous PAPE history, anemia, and others [18,19]. Of known several risk factors, our results showed that prevalence of PAPE was high in advanced maternal age (46.2%), a previous surgical history (61.5%), and cesarean delivery (100%). Especially, the highest prevalence of PAPE found in cesarean delivery. Therefore, we suggest that patients after cesarean delivery should be more carefully monitored than patients who have a vaginal delivery. It is assumed that the operation may affect the occurrence of PAPE because surgery results in many hematological changes.
Previous studies have demonstrated that the risk of PAPE is increase with obesity (BMI ≥30) [18], but our study did not support this conclusion. Many patients with PAPE had more than one known risk factor, thus patients with many risk factors need to be carefully monitored. Our results showed that the frequency of various risk factors range from two to seven according to each case (Table 4). Of the 13 cases in this study, there were two cases of patients who were in the active labor stage (cervical dilatation over 4 cm). However, it was not entirely clear whether being in the acive labor stage was a risk factor for PAPE or not.
PAPE is very difficult to diagnose in pregnancy and the postpartum period because clinical presentations of PAPE are atypical, subtle, and obscured by original pregnancy symptoms. In addition, PAPE may have absent or minimal symptoms. Atypical symptoms can interfere with the prompt diagnosis of PAPE, and so the true incidence is likely to be underestimated. Moreover, PAPE is fatal in nearly 15% of affected people, with two-thirds of the deaths occurring within 30 minutes of the embolic event [20]. In our study, patients with PAPE commonly showed dyspnea, and this result is consistent with previous studies [21]. The following symptoms may be overlooked. After cesarean delivery, transient dyspnea may happen due to anesthesia or other reasons; as such, the medical teams may have difficulty distinguishing this temporary symptom from a manifestation of PAPE. For this reason, delayed diagnosis of PAPE cases occur frequently, and delayed diagnosis may lead to delayed thrombophylaxis, which can increase maternal mortality.
A few limitations of the study were identified. Our study was not a case control study, but instead, was a case series of PAPE. Therefore, the purpose of this study was not to identify the relative risk of women with risk factors for PAPE compared with women without any risk factors. Additionally, our goal was not to discover any new risk factors of PAPE. The goal of our study was to attempt to determine the high frequency risk factors in Korean women from among the risk factors identified. Further research has been planned regarding the relative risk between women with and without risk factors, as well as any new risk factors of PAPE. The last, there is a possibility that the incidence of PAPE in this study was underestimated, as the patient population was restricted to our hospital. PAPE may have absent or minimal symptoms. Atypical symptoms can interfere with the prompt diagnosis of PAPE, and so the true incidence is likely to be underestimated.
In conclusion, our study investigated the incidence, timing, risk factors, and mortality of pregnancy-associated PAPE. We confirmed that the incidence of PAPE in the postpartum period is very low but its maternal mortality was significantly high. Therefore, the prevention strategy of PAPE such as early ambulation and prophylactic heparin treatment needs to be considered. We also suggest that early detection and prompt intervention in cases of PAPE might be the only way to improve patients' survival rate.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Snow V, Qaseem A, Barry P, Hornbake ER, Rodnick JE, Tobolic T, et al. Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2007;146:204–210. doi: 10.7326/0003-4819-146-3-200702060-00149. [DOI] [PubMed] [Google Scholar]
- 2.Berg CJ, Atrash HK, Koonin LM, Tucker M. Pregnancy-related mortality in the United States, 1987-1990. Obstet Gynecol. 1996;88:161–167. doi: 10.1016/0029-7844(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 3.Laros RK, Jr, Alger LS. Thromboembolism and pregnancy. Clin Obstet Gynecol. 1979;22:871–878. doi: 10.1097/00003081-197912000-00008. [DOI] [PubMed] [Google Scholar]
- 4.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194:1311–1315. doi: 10.1016/j.ajog.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Coon WW. Epidemiology of venous thromboembolism. Ann Surg. 1977;186:149–164. doi: 10.1097/00000658-197708000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Swiet M. Thromboembolism. Clin Haematol. 1985;14:643–660. [PubMed] [Google Scholar]
- 7.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–516. doi: 10.1097/01.AOG.0000172428.78411.b0. [DOI] [PubMed] [Google Scholar]
- 8.James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564–1571. doi: 10.1161/CIRCULATIONAHA.105.576751. [DOI] [PubMed] [Google Scholar]
- 9.James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29:326–331. doi: 10.1161/ATVBAHA.109.184127. [DOI] [PubMed] [Google Scholar]
- 10.Committee on Practice Bulletins: Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 84 Prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol. 2007;110(2 Pt 1):429–440. doi: 10.1097/01.AOG.0000263919.23437.15. [DOI] [PubMed] [Google Scholar]
- 11.Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375:500–512. doi: 10.1016/S0140-6736(09)60996-X. [DOI] [PubMed] [Google Scholar]
- 12.Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood. 2005;106:401–407. doi: 10.1182/blood-2005-02-0626. [DOI] [PubMed] [Google Scholar]
- 13.Seo YY, Yang MS, Kim DY, Won HS, Lee SH, Hong YH, et al. Thromboembolism during pregnancy or postpartum. Korean J Med. 2008;75:658–664. [Google Scholar]
- 14.Goldhaber SZ, Morrison RB. Cardiology patient pages. Pulmonary embolism and deep vein thrombosis. Circulation. 2002;106:1436–1438. doi: 10.1161/01.cir.0000031167.64088.f6. [DOI] [PubMed] [Google Scholar]
- 15.Zakai NA, Wright J, Cushman M. Risk factors for venous thrombosis in medical inpatients: validation of a thrombosis risk score. J Thromb Haemost. 2004;2:2156–2161. doi: 10.1111/j.1538-7836.2004.00991.x. [DOI] [PubMed] [Google Scholar]
- 16.Kucher N, Kohler HP, Dornhofer T, Wallmann D, Lammle B. Accuracy of D-dimer/fibrinogen ratio to predict pulmonary embolism: a prospective diagnostic study. J Thromb Haemost. 2003;1:708–713. doi: 10.1046/j.1538-7836.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg JS, Brill-Edwards P, Burrows RF, Bona R, Prandoni P, Buller HR, et al. Venous thrombosis during pregnancy: leg and trimester of presentation. Thromb Haemost. 1992;67:519–520. [PubMed] [Google Scholar]
- 18.Friend JR, Kakkar VV. The diagnosis of deep vein thrombosis in the puerperium. J Obstet Gynaecol Br Commonw. 1970;77:820–823. doi: 10.1111/j.1471-0528.1970.tb04407.x. [DOI] [PubMed] [Google Scholar]
- 19.De Swiet M. Thromboembolic disease. In: James DK, Steer PJ, Weiner CP, Gonik B, editors. High risk pregnancy: management options. 2nd ed. London: Harcourt; 1999. pp. 901–909. [Google Scholar]
- 20.Manganelli D, Palla A, Donnamaria V, Giuntini C. Clinical features of pulmonary embolism. Doubts and certainties. Chest. 1995;107(1 Suppl):25S–32S. doi: 10.1378/chest.107.1_supplement.25s. [DOI] [PubMed] [Google Scholar]
- 21.Goldhaber SZ, Tapson VF DVT FREE Steering Committee. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol. 2004;93:259–262. doi: 10.1016/j.amjcard.2003.09.057. [DOI] [PubMed] [Google Scholar]


