Abstract
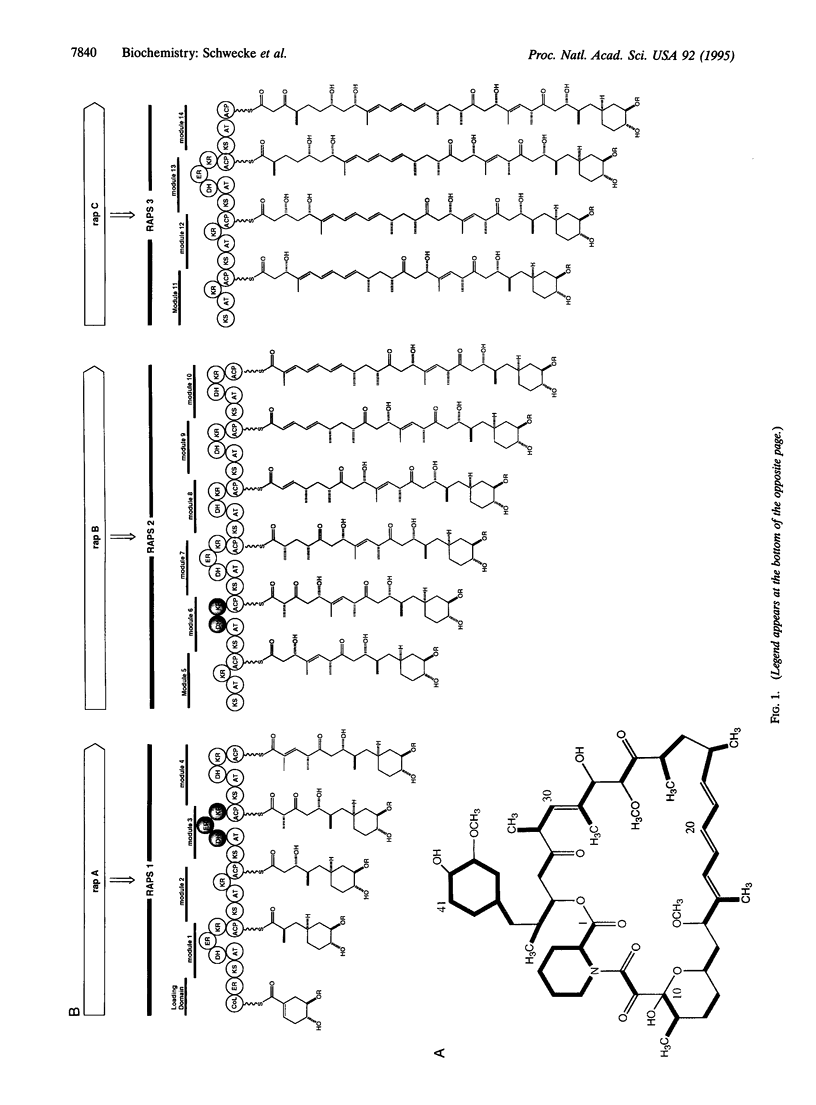
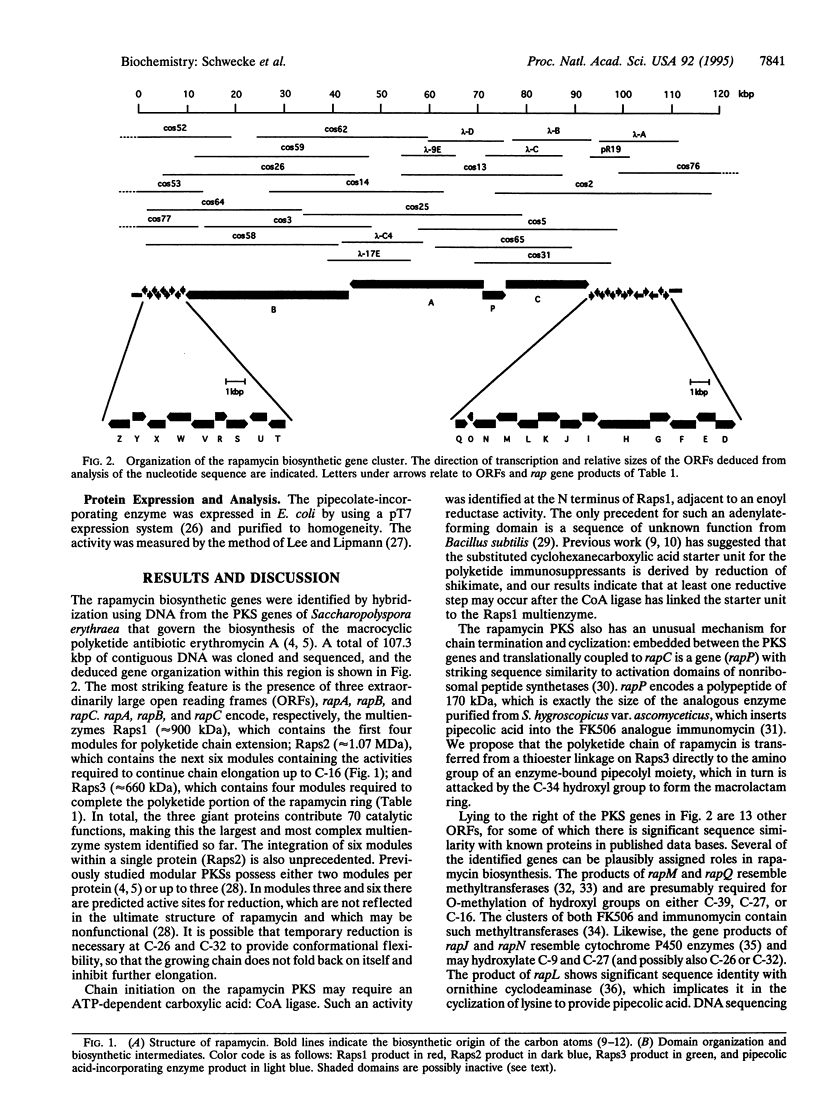
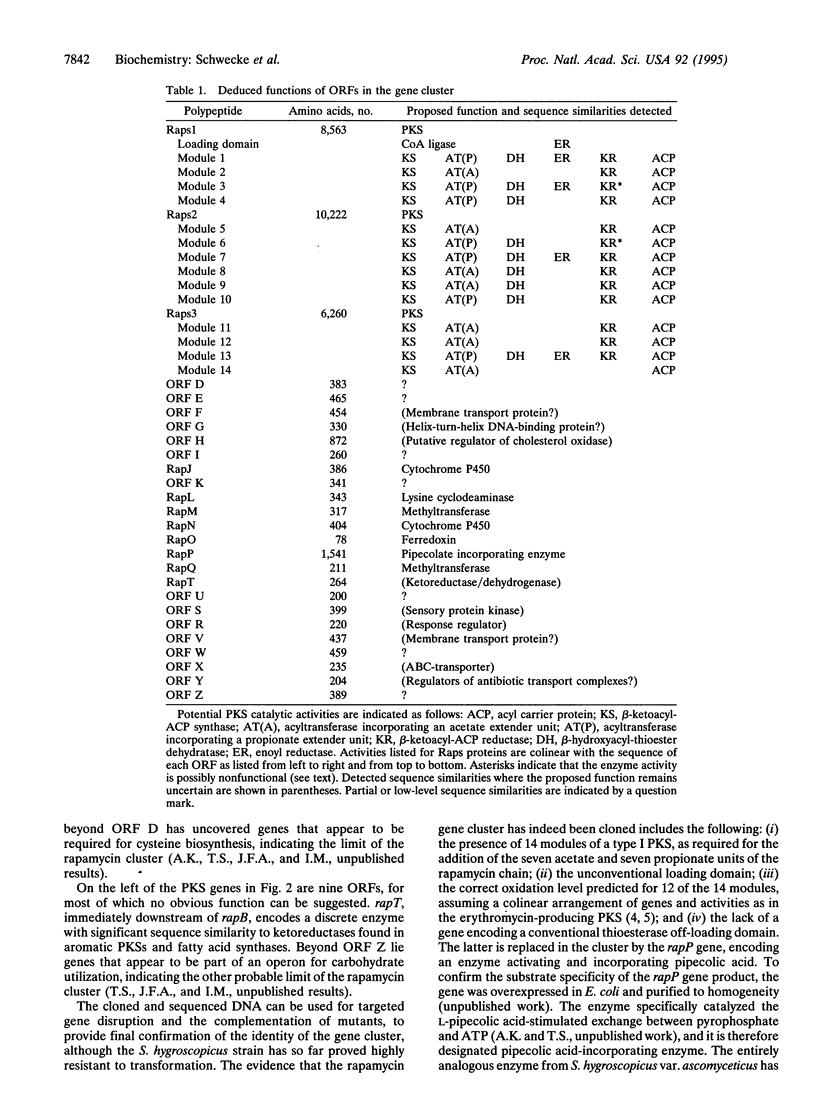
The macrocyclic polyketides rapamycin and FK506 are potent immunosuppressants that prevent T-cell proliferation through specific binding to intracellular protein receptors (immunophilins). The cloning and specific alteration of the biosynthetic genes for these polyketides might allow the biosynthesis of clinically valuable analogues. We report here that three clustered polyketide synthase genes responsible for rapamycin biosynthesis in Streptomyces hygroscopicus together encode 14 homologous sets of enzyme activities (modules), each catalyzing a specific round of chain elongation. An adjacent gene encodes a pipecolate-incorporating enzyme, which completes the macrocycle. The total of 70 constituent active sites makes this the most complex multienzyme system identified so far. The DNA region sequenced (107.3 kbp) contains 24 additional open reading frames, some of which code for proteins governing other key steps in rapamycin biosynthesis.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Caramori T., Scoffone F., Scotti C., Galizzi A. Sequence around the 159 degree region of the Bacillus subtilis genome: the pksX locus spans 33.6 kb. Microbiology. 1995 Feb;141(Pt 2):299–309. doi: 10.1099/13500872-141-2-299. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aparicio J. F., Caffrey P., Marsden A. F., Staunton J., Leadlay P. F. Limited proteolysis and active-site studies of the first multienzyme component of the erythromycin-producing polyketide synthase. J Biol Chem. 1994 Mar 18;269(11):8524–8528. [PubMed] [Google Scholar]
- Baeder W. L., Sredy J., Sehgal S. N., Chang J. Y., Adams L. M. Rapamycin prevents the onset of insulin-dependent diabetes mellitus (IDDM) in NOD mice. Clin Exp Immunol. 1992 Aug;89(2):174–178. doi: 10.1111/j.1365-2249.1992.tb06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevitt D. J., Cortes J., Haydock S. F., Leadlay P. F. 6-Deoxyerythronolide-B synthase 2 from Saccharopolyspora erythraea. Cloning of the structural gene, sequence analysis and inferred domain structure of the multifunctional enzyme. Eur J Biochem. 1992 Feb 15;204(1):39–49. doi: 10.1111/j.1432-1033.1992.tb16603.x. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., Collier D. S., Lim S., Pollard S. G., Samaan A., White D. J., Thiru S. Rapamycin for immunosuppression in organ allografting. Lancet. 1989 Jul 22;2(8656):227–227. doi: 10.1016/s0140-6736(89)90417-0. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Cortes J., Haydock S. F., Roberts G. A., Bevitt D. J., Leadlay P. F. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature. 1990 Nov 8;348(6297):176–178. doi: 10.1038/348176a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadio S., Staver M. J., McAlpine J. B., Swanson S. J., Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991 May 3;252(5006):675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- Douros J., Suffness M. New antitumor substances of natural origin. Cancer Treat Rev. 1981 Mar;8(1):63–87. doi: 10.1016/s0305-7372(81)80006-0. [DOI] [PubMed] [Google Scholar]
- Haydock S. F., Dowson J. A., Dhillon N., Roberts G. A., Cortes J., Leadlay P. F. Cloning and sequence analysis of genes involved in erythromycin biosynthesis in Saccharopolyspora erythraea: sequence similarities between EryG and a family of S-adenosylmethionine-dependent methyltransferases. Mol Gen Genet. 1991 Nov;230(1-2):120–128. doi: 10.1007/BF00290659. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Horii M., Ishizaki T., Paik S. Y., Manome T., Murooka Y. An operon containing the genes for cholesterol oxidase and a cytochrome P-450-like protein from a Streptomyces sp. J Bacteriol. 1990 Jul;172(7):3644–3653. doi: 10.1128/jb.172.7.3644-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Donadio S. Polyketide synthesis: prospects for hybrid antibiotics. Annu Rev Microbiol. 1993;47:875–912. doi: 10.1146/annurev.mi.47.100193.004303. [DOI] [PubMed] [Google Scholar]
- Lee S. G., Lipmann F. Tyrocidine synthetase system. Methods Enzymol. 1975;43:585–602. doi: 10.1016/0076-6879(75)43121-4. [DOI] [PubMed] [Google Scholar]
- MacNeil D. J., Occi J. L., Gewain K. M., MacNeil T., Gibbons P. H., Ruby C. L., Danis S. J. Complex organization of the Streptomyces avermitilis genes encoding the avermectin polyketide synthase. Gene. 1992 Jun 15;115(1-2):119–125. doi: 10.1016/0378-1119(92)90549-5. [DOI] [PubMed] [Google Scholar]
- Marahiel M. A. Multidomain enzymes involved in peptide synthesis. FEBS Lett. 1992 Jul 27;307(1):40–43. doi: 10.1016/0014-5793(92)80898-q. [DOI] [PubMed] [Google Scholar]
- Marsden A. F., Caffrey P., Aparicio J. F., Loughran M. S., Staunton J., Leadlay P. F. Stereospecific acyl transfers on the erythromycin-producing polyketide synthase. Science. 1994 Jan 21;263(5145):378–380. doi: 10.1126/science.8278811. [DOI] [PubMed] [Google Scholar]
- McAlpine J. B., Swanson S. J., Jackson M., Whittern D. N. Revised NMR assignments for rapamycin. J Antibiot (Tokyo) 1991 Jun;44(6):688–690. doi: 10.7164/antibiotics.44.688. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Hsu M. J., Byrne K. M., Kaplan L. Biosynthesis of the immunosuppressant immunomycin: the enzymology of pipecolate incorporation. Biochemistry. 1991 Jun 11;30(23):5789–5796. doi: 10.1021/bi00237a023. [DOI] [PubMed] [Google Scholar]
- Paiva N. L., Demain A. L., Roberts M. F. Incorporation of acetate, propionate, and methionine into rapamycin by Streptomyces hygroscopicus. J Nat Prod. 1991 Jan-Feb;54(1):167–177. doi: 10.1021/np50073a015. [DOI] [PubMed] [Google Scholar]
- Pearson W. R. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N., Schindler U., Schröder J. Ornithine cyclodeaminase from Ti plasmid C58: DNA sequence, enzyme properties and regulation of activity by arginine. Eur J Biochem. 1988 Apr 5;173(1):123–130. doi: 10.1111/j.1432-1033.1988.tb13975.x. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992 Aug 7;70(3):365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Shafiee A., Motamedi H., Chen T. Enzymology of FK-506 biosynthesis. Purification and characterization of 31-O-desmethylFK-506 O:methyltransferase from Streptomyces sp. MA6858. Eur J Biochem. 1994 Oct 15;225(2):755–764. doi: 10.1111/j.1432-1033.1994.00755.x. [DOI] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vézina C., Kudelski A., Sehgal S. N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975 Oct;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]