Abstract
The lung is a complex structure that is interdigitated with immune cells. Understanding the 4-dimensional process of normal and defective lung function and immunity has been a centuries-old problem. Challenges intrinsic to the lung have limited adequate microscopic evaluation of its cellular dynamics in real time, until recently. Because of emerging technologies, we now recognize alveolar-to-airway transport of inhaled antigen. We understand the nature of neutrophil entry during lung injury and are learning more about cellular interactions during inflammatory states. Insights are also accumulating in lung development and the metatastatic niche of the lung. Here we assess the developing technology of lung imaging, its merits for studies of pathophysiology and areas where further advances are needed.
Introduction
The lung is a site of constant immune surveillance at the interface between self and the environment. A better understanding of how cells of the immune system assemble and dissemble within specific regions of the lung would allow for a more sophisticated understanding of how homeostasis is maintained, how pathogenesis is incurred and propagated throughout the lung, and, eventually, how we may be able to intervene at the bed-side. However, because of the complexities of the lung architecture as well as technical limitations inherent in observing this dynamic organ ‘in action’, it has historically been difficult to study the behaviors of collectives of immune cells within the lung.
Extraordinary advances in live imaging have recently enabled observation of the immune dynamics of the lung without requiring the isolation of the organ from the remainder of the system. Here, we discuss the challenges of imaging the lung at sub-cellular resolution and review current solutions that are meeting this challenge. Further, we discuss what dynamic imaging studies have revealed about the allergic response, injury, pulmonary development, and cancer within the lung. Finally, we provide a perspective of how this exciting technology may enable the advancement of treatments for these various lung pathologies.
History of Imaging of the Lung
Understanding the spatiotemporal aspects of lung biology requires 4 dimensional imaging. Attempts at imaging the living lung began nearly four hundred years ago, in the quest to understand pulmonary blood flow. In letters written in the mid seventeenth century, Marcello Malpighi, an Italian physician scientist, described the advantages of using microscopy to study circulation within the lungs. “By the arrangement of the instruments and light, you will observe the movement of the blood through the vessels in question. You will yourself be able to contrive it by different degrees of light, which escape description by the pen.” [1]
To understand blood flow in the lung, however, necessitates the study of circulation in a living animal with a beating heart and breathing lungs. Live lung imaging was thus advanced by the study of pulmonary circulation, specifically in the early twentieth century. Early work by Olkon and Joannides in 1930 described new methods using a binocular stereoscopic microscope in attempts to minimize motion artifact and improve depth of view to image dog, frog and alligator lungs. This group and, soon after, Wearn et al. advanced these studies by developing a pleural window in cats to study pulmonary circulation.[2,3](Fig. 1a) A seminal advance in intravital lung imaging occurred a few years later, however, with the advent of a thoracic window. By drawing air out from the pleural cavity by an exhaust tube, the lung adhered to a cover glass window that sat in the thoracic window. This method, with the advantage of greatly decreased movement of the lung, was first described in Science in 1939 by Terry. [4]
Figure 1.
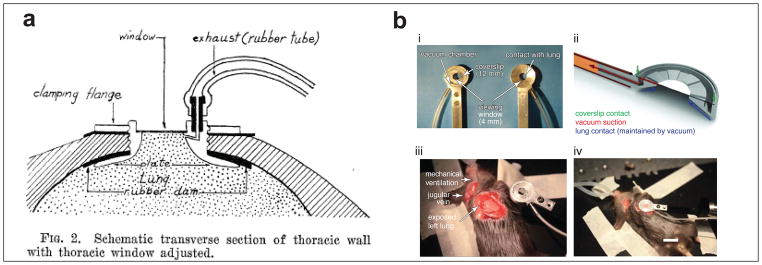
Evolution of the thoracic window. (a) The original thoracic window as described by Terry in 1939 for use in cats. (From Terry, R. J. (1939) A Thoracic Window for Observation of the Lung in a Living Animal. Science. 90, 43–44. Reprinted with permission from AAAS.) (b) The thoracic window as described by Looney et al in 2011. Shown in panel is (i) the thoracic window itself, (ii) a schematic side-view representation of the vacuum suction and contact to coverslip and lungs, (iii) the surgical preparation and location of thoracic window on a mouse in the right lateral decubitus position with a left thoracotomy and an exposed left lung, and finally (iv) the surgical preparation of a mechanically ventilated mouse with thoracic window in place. (Reprinted by permission from Macmillan Publishers Ltd: Nature Methods. Looney, M.R. et al. Stabilized imaging of immune surveillance in the mouse lung.8(1) copyright 2011)
To understand why such stabilization is important, consider that the lung moves on the order of centimeters (millimeters in rodents) per inspiration, but it is typically desirable to clearly view cells or subcellular features that are on the order of 10−3 centimeters in diameter, at most. Thus, tissue movement is much greater than the objects being visualized within that tissue; furthermore, this movement occurs on the order of 1–4 times per second (the respiratory rate of large animals to small rodents). Together, this creates the potential for considerable blurring particularly when images are not captured fast enough.
The advent of the thoracic window stabilized the lung sufficiently to permit study of basic physiological parameters within the vasculature of the lung including changes within the microcirculatory flow, vascular pressures and alveolar pressures, both at steady state and in response to mild trauma and edema.[5–8] Intravascular dyes such as fluorescent dextran conjugates and fluorescent microspheres used to label vessels furthered the understanding fluid flow in pulmonary microcirculation. [7–11]
Through this initial focus on the flow of blood through the circulation, microscopy began to be used to observe the behavior of leukocytes within the vasculature several decades ago. Bright-field microscopy was initially used to visualize neutrophil rolling (margination) along the endothelium, followed by activation and adhesion (sequestration) within the pulmonary capillary bed prior to endothelial transmigration.[6,8–11] Newer imaging modalities, including confocal scanning microscopes and two photon microscopy,[12–14], were soon applied to pulmonary research.[15,16] (Box 1) With the recent optimization of preparation techniques, fluorophores and microscopes, live-imaging is now being applied to the spatiotemporal cellular dynamics of lung immunity, and modern smaller variants of thoracic windows are part of the enabling tools for these approaches. [17–19] (Figure 1b)
Box 1. An Introduction to Microscopy.
| Brightfield | This classic form of microscopy uses illumination with a conventional light source aimed towards a lens, through the specimen, through an objective, and to the eye of a second magnifying lens (the ocular). |
| Conventional Epifluorescence | A short wavelength beam of light is reflected through an objective and introduces a uniform illumination to the entire specimen before passing through a chromatic reflector to the ocular. |
| Confocal | A single point of excitation light, usually from scanning lasers, is used to illuminate a single point at one time; then the reflected light from the specimen passes through a detecting pinhole, allowing for focused illumination. |
| Two Photon | Multi-photon excitation uses the simultaneous absorption of two photons directed at a specimen from high energy pulsed lasers, using long wavelengths and penetrating deeper into tissue with excitation only at a focus plane. |
A final note on the advent of live-imaging of the lung: Intravital live imaging has also recently intersected with slice-model imaging,[19] a method which produces organ fragments that show many characteristics of the intact (albeit not respirating) organ. Procedures to slice the lung for the purpose of observing agonist-induced smooth muscle contraction are by now well-established [20–22]; lungs treated appropriately maintain high cellular viability for greater than a week, including robust smooth muscle responsiveness and ciliary beating similar to behaviors observed in intravital preparations.[22] This provides a convenient and higher throughput method to study dynamics in the lung -- so long as one recognizes its various limitations. In particular, lack of lymphatic and blood flow, lack of enervation, lack of mechanical expansion/contraction and the potential for immune cells to redistribute in the lung. Our group was able to compare data obtained with intravital imaging with data obtained using the slice model to show that many parameters of dendritic cell behavior were accurately recapitulated in the slice model.[19] While this method has its limitations, filling and cutting of the lung provides particular optical advantages for imaging, as discussed in the next section. As a general rule, correlation of observations made in slice-model imaging with those seen in intravital imaging or with those observed by taking images at fixed time points offers a complementary methodology with which to understand cellular dynamics during various immune insults.
Challenges in Imaging of the Lung
The lung presents unique challenges for imaging. First and quite simply are issues related to gaining access to the organ itself; the proper functioning of the lung requires an intact thoracic cavity to generate the vacuum that inflates the lungs during inhalation. Thus, the very act of exposing the lungs and violating this cavity has the potential to prevent autonomous breathing. While some thoracic windows may tightly seal the thoracic cavity such that normal lung inflation can be achieved, more typically these devices necessitate the use of a ventilator, particularly for longer-term experiments. (Figure 1b)
A second challenge in cellular imaging of the lung is the significant motion of the organ (millimeters to centimeters) compared to the cells themselves. To ameliorate this problem, a thoracic window minimizes the shifts perpendicular to the front face of the microscope lens and, to a lesser extent, minimizes the movement in the ‘z’ axis (along the imaging axis). Movement in z, as during respiration, can be corrected by either carefully timing imaging to take place in between breathing cycles or to average multiple rapid exposures, effectively selecting for data from the more prominent resting state. Alveolar expansion/contractions still occur under the modest vacuum [17] and it is presumed that any limitations in movement at the site of the imaging window per se are compensated for by normal expansion/contraction more distal to the window.
In rodents, respiration and associated lung movements occur on a 2–4Hz frequency, whereas larger mammals breathe at a rate of 0.2–1 Hz. In all cases, the fast rate of motion means that, in order to collect ‘in between breaths’, a sensitive and high-speed camera, capable of collecting frames considerably faster than the respiratory rate, is a considerable asset. In our own work, video rate (30Hz) has provided the speeds necessary to capture the stable phases of breathing. In a similar vein, the motion of cells through the vasculature can exceed 500 microns per second. [17,23] Collecting images of large fields (>500um on a side) at 30Hz or greater resolves motion blur that would otherwise prevent the tracking of such fast moving objects.
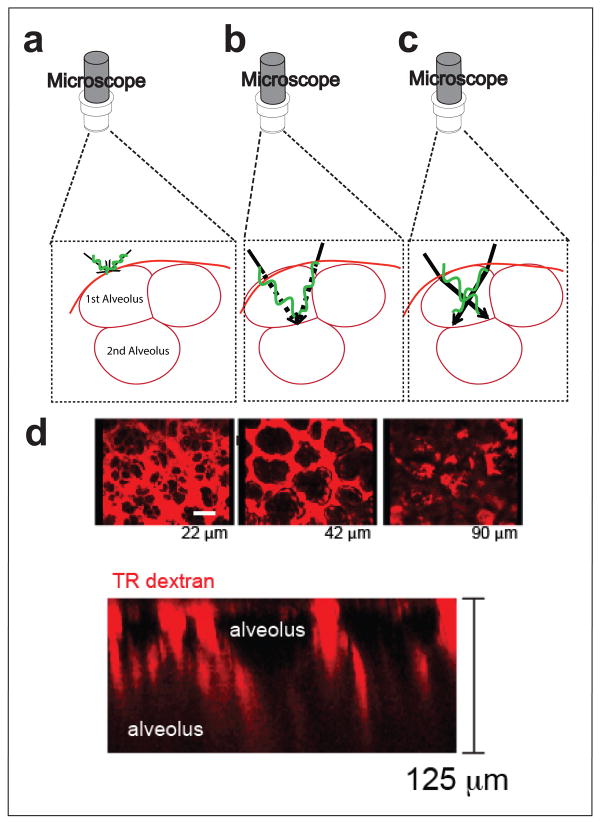
The most significant challenge of live imaging in the lung is the fact that the tissue is air-filled. This is problematic because tissue and air have quite different refractive indices for light and so passage of light from one to another causes light to be bent. Lungs consist of many of these air-water interfaces and the problem of light rays bending gets worse for each additional layer through which one tries to image. Intuitively, the effect of a refractive media mismatch can be understood by considering rays of light emerging from the side of a fish-tank; the rays bend as they pass from water (one refractive index) into air (a different index); this results in an apparent translation of the position of the fish when viewed from outside the tank (i.e. from air). In the lung, as light passes through tissue (which is effectively water), into air, it is similarly bent. In imaging, the highest resolution of objects is achieved when the light rays coming through all possible routes in the optical path constructively interfere only at the focal point (Figure 2a). However, when one passes through just one air-water interface, the rays no longer constructively converge at the desired focal point (Figure 2b,c), many excitation photons are lost and efficient excitation of fluorophores is lost. This results in rapidly degraded excitation and thus poor signal and resolution beyond the first intact alveolus. (Figure 2d)
Figure 2. Challenges of imaging air-filled lungs.
(a) The unobstructed view of a single alveolus in which rays from the microscope converge at a single point. (b) With a second alveolus and thus a second air-water interface, ideally rays would still converge at a single point. (c) In reality, with a second alveolus, rays interfere with each other and converge at distinct points. (d) Intravital imaging through a thoracic window in a mouse injected i.v. with Texas Red Dextran showing loss of resolution and signal at progressively deeper layers (22 um, 42 um, 90 um, and (bottom) showing a z-projection).
While tissue is oriented randomly and we occasionally succeed in imaging features past this limit, it is clear that considerable advances in adaptive optics [24] will be necessary to overcome and image into airway regions in normal, air-filled lungs. One other possible solution is the ventilation of lungs with inert liquids whose index of refraction might be selected to better match that of the tissue, although caveats to this approach are multiple. Another solution is simply to rely on slices, derived from agarose-filled lungs, to reach these deep, largely inaccessible areas of the lung.[19,20,22]
Lessons Learned from Imaging of the Lung
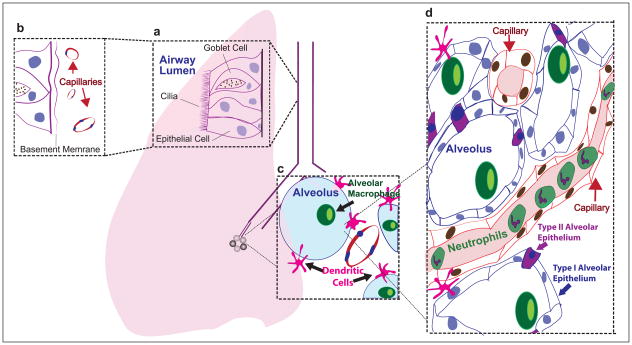
As a primer, Figure 3 shows an overview of the lung and its structures as we currently appreciate them. How diseases affect processes in each part of the lung can now be addressed through direct imaging.
Figure 3. The immune system of the lung.
(a) Epithelial cells line the airways to defend the host from antigen. Cilia mechanically move antigen out of the airway. Goblet cells produce mucous to help eliminate antigen. (b) The epithelium forms both a physical barrier to antigen entry into the lung and an immune barrier by its ability to incite an immune response to antigen. (c) The alveolus is a site of immune surveillance by alveolar macrophages within the alveolus, and by dendritic cells within the interstitum which extend processes into the alveolus. (d) Capillaries course in close proximity to alveoli composed of thin type I and type II alveolar epithelial cells and contain leukocytes, particularly neutrophils (in addition to erythrocytes, not illustrated) which marginate in the lungs searching for breach of immunity.
Antigen Uptake and processing in the Normal and Inflamed Lung
Most antigen that is inhaled into the lung is either expelled in the mucus or digested by alveolar macrophages (AMs) in a manner that minimizes an inflammatory response. At times, however, antigen does (or should) trigger a rapid innate response. Typically, this requires it to be carried to the lymph node and processed into the Class I and/or Class II MHC pathways for presentation to T cells.
Until recently, antigen uptake in the lung was inferred from static imaging and tissue digestion/flow cytometry-based studies.[23,25,26] Based on studies from trachea[27,28] and nasal mucosa[29] it was assumed that dendritic cells (DCs) sent processes between the epithelial cells of large airways to sample inhaled material. [25,28,30,31] Recent work with imaging using static and slice imaging have shown that airways are rarely, or never, sites of such transepithelial dendritic cell projections.[19]
The alveoli present a different picture, however. Slice and intravital imaging have shown that within the alveolus, the major phagocytes, AMs, are relatively immotile and become phagocytic following encounter with antigen. Through their large numbers, they effectively digest most of the material that evades the effects of the ‘mucosal elevator’ and reach the alveoli. In contrast, DCs project dendrites into the alveoli with dramatic movements that allow them to provide an alternative path to uptake by AMs.[19] Live-imaging and pulse-chase methods show that after DCs take up antigen in the alveoli, they may traffic to airways where they release antigen into the interstitium. (Figure 4a) In this way, these DCs offer an alternative to AMs in the alveoli, differentiating innocuous insults from dangerous ones: though AMs can inactivate limiting amounts of particulate and pathogens that reach the alveoli, a profuse amount in the terminal alveoli will result in transport of this material upward by DCs to the branching airways, and perhaps ultimately to a lymph node. This upward flow on DC culminates in local (e.g. airway proximal) or systemic activation of T cells.
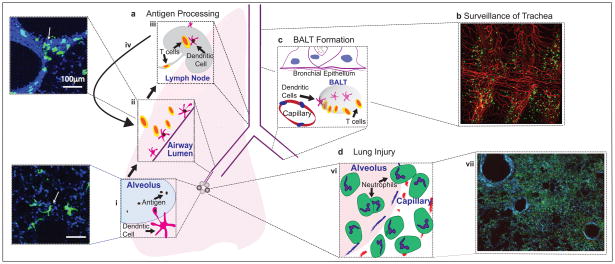
Figure 4. Lessons learned from lung imaging.
(A) Alveolar dendritic cells send processes into alveoli where they take up antigen, seen both in schematic and in a 2-photon image of a lung slice in a CD11c-eYFP mouse, with dendritic cells in green (with arrow) (i) and then traffic up along airways where they interact with T cells in allergic airways, seen both in schematic and in a 2-photon image of a lung slice ina CD11c-eYFP mouse, with dendritic cells in green (with arrow) (ii) and then traffic to mediastinal lymph nodes to further prime and activate T cells (iii) which then return to the airways (iv). B) CD11c+ cells (primarily DCs) survey the large airways, including the trachea, in steady state, and surround CD31+ vasculature in a tracheal slice of a CD11c-yfp (green) mouse with surface staining of CD31-PE (red), imaged using a 2-photon microscope. (C) Bronchus Associated Lymphoid Tissue (BALT) may be formed between bronchial epithelium and arteries in response to inflammation where T cells interact with DCs. (D) Lung injury results in neutrophil sequestration, extravasation and clustering in alveoli (vi) which results in massive infiltration in both parenchyma and the alveoli, as seen in a 2-photon image of a lung slice of transgenic mice 48 hrs after intratracheal LPS, with neutrophils in green (cfms-gfp), T cells in red (CD2-rfp) and actin in blue (actin-cfp). (vii)
Imaging has thus informed that significant particulate antigen uptake does not take place in airways but that alveolar uptake is robust and results in materials ingested in the alveolus being moved to the airway. Interestingly, while DC uptake and presentation account for only a fraction of uptake of particulate from alveolar at steady state, uptake and transport are enhanced during allergic responses.[19] This is driven, in part, not by a change in the ability of DCs to gain access to the alveoli but by the dramatic rise in the numbers of monocyte-derived DCs in the alveoli during allergen challenge.
The possible ability of larger airways to pass small molecule antigens across their barriers, as an alternative mechanism to particulate uptake in the alveoli, has yet to be addressed. Such a possibility is raised by recent findings that goblet cells of the gut can serve as backward conduits for the passage of relatively small molecular weight material into the lamina propria for uptake and presentation.[32] Further, imaging of the trachea and large conducting airways reveals a large number of antigen presenting cells such as CD11c+ cells (mainly DCs) a few cell layers beneath the luminal surface, underlining that for these to get antigen, some form of transepithelial transport may be required.[28] (Figure 4b) Imaging of the sampling of a variety of molecular weights across larger airways would corroborate prior views of large airway antigen presentation,[27,30] demonstrating perhaps the complexity and redundancy of antigen presentation in the lung.
Asthma and the Allergic Response in the Lung
Asthma is a complex chronic airway disease with various etiologies, including but not limited to allergy. Allergic mouse systems have thus served as general models of airway inflammation and hypersensitivity for decades. Pathophysiologically, asthma is defined by reversible airflow obstruction, hyper-responsiveness of the smooth muscles in the lung and airway inflammation, irrespective of the underlying cause. Allergic eosinophilic asthma is largely driven by allergen-specific Th2 lymphocytes, whereas neutrophilic asthma is possibly driven by Th17 cells.[33]
Imaging of lung slices in an allergic OVA/alum model has demonstrated that DCs (likely of monocyte origin), which are necessary partners for T cells,[25,31] accumulate near the airways as with viral infection.[19] (Figure 4a) Allergen-reactive T cells did not engage in stable interactions with these DC but rather ‘swarmed’ around them. Notably, a similar swarming behavior was observed for activated T cells even when those T cells bore receptors that could not recognize the allergen. This suggests that a chemotactic environment exists under allergy that efficiently traps both antigen-loaded DCs as well as any activated T cell. While this may be designed for ensuring that circulating primed T cells can defend an infected lung, in the context of asthma, it may equally ensure reactivation of allergen-specific T cell clones.
Much of the pathophysiology in asthma and allergy takes place adjacent to airways and significant immune aggregates. In many cases, remodeling of the lung in asthma or chronic infection results in the development of bronchus associated lymphoid tissue (BALT),[34,35] often found in regions at airway junctions including the carina. Imaging has shown that viral-specific T cells also cluster within these airway branch-ponts, and this depends on the presence of DCs.[19,34] (Figure 4b)
While imaging of the allergic lung showed that attraction of T cells to the airway-adjacent regions was not allergen-specific, the use of calcium-sensitive dyes showed that allergen-specific cells are triggered during this interaction.[19] It remains to be determined as to whether local cytokine secretion by T cells during these or subsequent interactions at or near the airway lead to systemic inflammatory cytokine secretion by T cells. The cellular orchestration of the Th2 response also remains poorly understood, especially as to how eosinophils, mast cells and basophils physically interact with T cells to drive inflammation. Using two-photon microscopy to image lung slices following helminth infections, basophils were recently shown to be involved in T cells swarm, where they may be providing IL-4 to skew T cells to a Th2 phenotype in infected tissues.[36] This study, utilizing new transgenic strains of mice to label cells, serves as an example of how this advancing technology can inform cellular interactions in the lungs. Continuing forward, lung imaging can help further dissect out how cells crosstalk and interact in asthma and the allergic response in the lung.
Neutrophil Dynamics in the Response to Injury in the Lung
For several decades, the initial neutrophil response to injury was visualized in real time in extra-pulmonary sites including in the mesenteric venules of rabbits, [37] the cremaster muscle of mice,[38–41] and in the liver of mice.[42] This imaging, using bright-field, spinning disk confocal, and two-photon microscopy, allowed for in vivo descriptions of neutrophil migration to the site of inflammation and for the description of neutrophil margination, sequestration and transmigration. Neutrophil interactions with other cells including the endothelium and other innate immune cells were thus visualized.[42]
More recent applications of two photon microscopy to the lung have revealed an innate immune response that is specific to the pulmonary circulation and parenchyma: though margination of neutrophils occurs throughout the entirety of the vasculature, neutrophils marginate and sequester at surprisingly high numbers in the lung microcirculation, both at steady state and, to a much greater extent, in response to injury.[18] (Figure 3d) Several groups have used advanced microscopy in mice to visualize neutrophils trapped in both narrow and wide capillaries at steady state.[17–19] Following various models of lung injury, including intratracheal lipopolysaccharide,[15,17,18] intratracheal bacteria and lung transplant,[43] recruitment of neutrophils to these capillaries was dramatically increased, and these cells extravasated and clustered, all within minutes of inflammatory challenge. (Figure 4d)
Indeed, the abundant homeostatic neutrophil trapping in the lung allows for rapid neutrophil sequestration with transmigration and entry into interstitium of the lungs immediately following injury.[18] This process was previously only surmised to occur in the lung from in vitro experiments and from in vivo data in other organs. [42,44–47] Additionally, imaging of this process has suggested that the neutrophils may be following a leading monocyte which may drive the neutrophil response.[18]
The imaging of this process has revealed that the lungs are primed to respond to an insult rapidly due to their abundant number within the vasculature. This provides insight as to why the lung is vulnerable to injury originating from within the lung but also to injury from systemic, indirect insults; a triggering of the immune response will allow these neutrophils – seemingly waiting within in the lung -- to enter the lung and possibly degranulate. Further, the notion that the neutrophil response may be orchestrated not just by a lung intrinsic mediator but by a circulating monocyte may provide a new target for intervention in neutrophil-mediated lung diseases including acute respiratory distress syndrome (ARDS), for which no therapeutic intervention currently exists.[48] Continued efforts to image neutrophils dynamics within the lung may allow us to not only to understand where, when and with whom they work, but how the injurious reactions of neutrophils may be modulated.
Future Investigation in Lung Injury: More than just neutrophils
Observations of neutrophil dynamics within the lung are now being teased out using two-photon microscopy, but lung injury is not solely reliant on neutrophils; neutrophil biology in the lung intersects with other innate cells such as monocytes, AMs and platelets. Platelets, for example, are now being better appreciated as capable immune cells that participate in lung injury responses in a variety of experimental models.[49] A consequence of platelet activation is the formation of dynamic complexes with neutrophils and platelets that may lead to increased activation states. While these complexes have been measured in the peripheral blood of patients with sepsis, ARDS, and myocardial ischemia,[49–52] imaging may permit comparison of the sites of complex formation and localization with the increases in lung permeability to establish possible causality and, perhaps, guide new therapies.
Activated platelets are also responsible for neutrophil extracellular trap (NET) formation, which may be an important innate immune response leading to containment of serious bacterial or fungal infections. NETs may have an undesirable pathological role by inducing endothelial damage.[49] Real-time imaging of NET formation within the lung may demonstrate how and when NETS are formed, and, possibly, the destructive effects of NETs with subsequent vascular leak. This would provide evidence of their role in lung injury and, again, a possible source of intervention for a disease entity that currently has none.
Adaptive immune cells also play a role in lung injury. The presence of lymphocytes in the lung was recently found to be critical for the recovery of mice from LPS-induced lung injury.[53] Regulatory T cells (Tregs) were recently identified as necessary for the resolution of neutrophil-mediated lung injury, perhaps by modulating the immune response of the lung from a pro-inflammatory milieu to an anti-inflammatory one. Whether this function of Treg cells in lung repair following is mediated via interaction with innate cells or with adaptive cells, and where this modulation occurs, is still unclear but could be assessed by two-photon microscopy. Furthermore, the lung parenchyma contains a variety of cell types -- the epithelium itself and mesenchymal cells including fibroblasts -- that can potentially participate in epithelial repair;[54–56] migration of lung epithelial stem cells has been detected in the resolution phase of Influenza-mediated lung injury.[57] Visualizing the dynamics of these processes and the temporal contributions of immune cell subsets intravitally would provide insight into the relevant cellular interactions dictating the resolution of lung injury.
Beyond Immunity: Lung development and regeneration
Much of the current research into lung regeneration is based on the notion that regeneration and repair mimic lung morphogenesis and development.[58] Accordingly, the study of lung regeneration seeks insights from our understanding of embryonic morphogenesis, stem cell biology and fetal development.[59–61] Lung-branching morphogenesis has been imaged using confocal microscopy and time-lapsed sequences in lung explants, allowing visualization of epithelial branching in embryonic lungs. [62] Lung alveolarization and postnatal development have yet to be imaged in real time, although static three-dimensional renditions have shown that lung development continues into young adulthood. [63]
An important site for intravital imaging will be to study the role of immune cells at the site of repair. A few studies have used fixed lungs and immunohistochemistry, to suggest that intravascular dendritic cells, monocytes and neutrophils are necessary for lung regeneration post-injury.[64,65] However, the mechanisms by which these cells function in repair processes and lung growth remain undefined. The development of methods enabling real time imaging over days and even weeks of embryonic and post-natal lung development and morphogenesis, as well as lung repair and regeneration, would inform which cellular interactions and mechanisms underlie these processes. Subsequently, this could advance the field of bio-tissue engineering greatly by furthering the development of cellular or tissue implants for the regeneration of damaged lungs after devastating diseases such as necrotizing pneumonias or congenital disorders resulting in hypoplastic lungs, as well as artificial human lungs for transplant.
Real Time Imaging for the Lung as Metastatic Site
Intravital and live tissue imaging is now recognized as an important tool in cancer research. Recent years have seen a surge of studies employing these methodologies to study primary and metastatic tumors of liver, brain, bone, and breast, and have allowed, for example, detailed visualization and description of the extravasatory process undergone by tumor cells during the first steps in metastasis.[66] These approaches have, in addition, shown when and where interactions between tumor cells and immune cells take place during establishment of immune tolerance and have begun to show how immune macrophages ferry metastatic cells out of tumors [67,68]. These approaches have not, however, yet been applied to the lung.
The lung is the most common metastatic site for many cancers, and lung cancer is one of the most common cancer diagnoses globally.[69–71] However, why this is has remained essentially unexplored in vivo.
Though no true intravital studies have yet been performed exploring the lung as a metastatic niche, a small number of existing studies using static ex vivo imaging have begun to provide some insight.[72,73] The use of a variant of the lung slice imaging method enabled long term imaging of tumor cells (injected immediately prior to harvesting the lungs) within the microenvironment of the lung.[73] Static images of these lung slices using a fluorescent inverted microscope were taken weekly for 21 days on individual lesions, recapitulating in vivo data on the metastatic proliferation and progression of various tumor lines, the potential contribution of differing microenvironments to metastatic growth, and the anti-metastatic role of several therapeutic agents. In this system, however, the immune constituents of the lung microenvironment were rapidly lost, thus limiting its utility in the interrogation of the impact of immune cells in the metastatic environment.
Using an alternate methodology in intact, perfused ex vivo lungs,[72] an additional study found evidence, in both intravenous injection models and a spontaneous pulmonary metastasis model of breast cancer[74], that metastatic tumor cells arrest and proliferate within the microvasculature of the lung, prior to extravasation. Imaging of these lungs with epifluorescent microscopy hinted at the distinction of the lung as a metastatic site from other organs such as the liver, where early proliferation of tumor cells occurs only following extravasation.[75] But this ex vivo model isolates the lung as a metastatic site from the remainder of the animal. The application of advanced lung imaging would allow for the visualization of the arrival of metastases within the lung and with the dynamics of progression of metastatic disease within the organ, in real time. This process, is, as yet, poorly understood, but could potentially inform future directions in anti-tumor therapies.
Concluding Remarks
Though scientists and physicians have, for centuries, attempted to understand how the lung works, within a lung in a living animal, recent advances in microscopy and lung stabilization techniques have allowed us to now appropriately ask questions about the origins of pathology. Translating this technology to clinical samples in order to understand diseases of humans rather than only animal models presents an important future goal. We propose that, as the field of lung imaging develops, it will usher in a new era of therapy for lung disease by targeting more precisely the dynamics of specific populations that support and protect the lung.
Highlights.
Live imaging of the lung presents numerous challenges due to its anatomy and physiology
Advances in imaging have allowed for real time imaging despite some these challenges
Behaviors of immune cells at steady state and in response to insult have been imaged.
Advances in live imaging could reveal targets for new therapies in lung pathology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young J. Malpighi’s “De Pulmonibus”. Proc R Soc Med. 1929;23:1–11. doi: 10.1177/003591572902300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olkon DM, Joannides M. Capillaroscopic Appearance of the Pulmonary Alveoli in the Living Dog. The Anatomical Record 1930;45 [Google Scholar]
- 3.Wearn JT, et al. The normal behavior of the pulmonary blood vessels wiht observations on the intermittence of the flow of blood in the arterioles and capillaries. American Journal of Physiology 1934;109 [Google Scholar]
- 4.Terry RJ. A THORACIC WINDOW FOR OBSERVATION OF THE LUNG IN A LIVING ANIMAL. Science. 1939;90:43–44. doi: 10.1126/science.90.2324.43. [DOI] [PubMed] [Google Scholar]
- 5.Wagner WW. Pulmonary microcirculatory observations in vivo under physiological conditions. J Appl Physiol. 1969;26:375–377. doi: 10.1152/jappl.1969.26.3.375. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya J, et al. Effect of edema on pulmonary blood flow in the isolated perfused dog lung lobe. J Appl Physiol. 1980;48:444–449. doi: 10.1152/jappl.1980.48.3.444. [DOI] [PubMed] [Google Scholar]
- 7.Kuhnle GE, et al. Distribution of microvascular pressure in arteriolar vessel trees of ventilated rabbit lungs. Am J Physiol. 1993;265:H1510–5. doi: 10.1152/ajpheart.1993.265.5.H1510. [DOI] [PubMed] [Google Scholar]
- 8.Tabuchi A, et al. Intravital microscopy of the murine pulmonary microcirculation. J Appl Physiol. 2008;104:338–346. doi: 10.1152/japplphysiol.00348.2007. [DOI] [PubMed] [Google Scholar]
- 9.Lien DC, et al. Physiological neutrophil sequestration in the lung: visual evidence for localization in capillaries. J Appl Physiol. 1987;62:1236–1243. doi: 10.1152/jappl.1987.62.3.1236. [DOI] [PubMed] [Google Scholar]
- 10.Kuebler WM, et al. Leukocyte kinetics in pulmonary microcirculation: intravital fluorescence microscopic study. J Appl Physiol. 1994;76:65–71. doi: 10.1152/jappl.1994.76.1.65. [DOI] [PubMed] [Google Scholar]
- 11.Gebb SA, et al. Sites of leukocyte sequestration in the pulmonary microcirculation. J Appl Physiol. 1995;79:493–497. doi: 10.1152/jappl.1995.79.2.493. [DOI] [PubMed] [Google Scholar]
- 12.Helmchen F, Denk W. New developments in multiphoton microscopy. Curr Opin Neurobiol. 2002;12:593–601. doi: 10.1016/s0959-4388(02)00362-8. [DOI] [PubMed] [Google Scholar]
- 13.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 14.Conchello JA, Lichtman JW. Optical sectioning microscopy. Nat Methods. 2005;2:920–931. doi: 10.1038/nmeth815. [DOI] [PubMed] [Google Scholar]
- 15.Perlman CE, Bhattacharya J. Alveolar expansion imaged by optical sectioning microscopy. J Appl Physiol. 2007;103:1037–1044. doi: 10.1152/japplphysiol.00160.2007. [DOI] [PubMed] [Google Scholar]
- 16.Perlman CE, et al. Micromechanics of alveolar edema. Am J Respir Cell Mol Biol. 2011;44:34–39. doi: 10.1165/rcmb.2009-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Looney MR, et al. Stabilized imaging of immune surveillance in the mouse lung. Nat Methods. 2011;8:91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreisel D, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proceedings of the National Academy of Sciences. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton EE, et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. Journal of Experimental Medicine. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dandurand RJ, et al. Responsiveness of individual airways to methacholine in adult rat lung explants. J Appl Physiol. 1993;75:364–372. doi: 10.1152/jappl.1993.75.1.364. [DOI] [PubMed] [Google Scholar]
- 21.Held HD, et al. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol. 1999;126:1191–1199. doi: 10.1038/sj.bjp.0702394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol. 2002;119:187–198. doi: 10.1085/jgp.119.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubzick C, et al. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. Journal of Experimental Medicine. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji N, et al. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat Methods. 2010;7:141–147. doi: 10.1038/nmeth.1411. [DOI] [PubMed] [Google Scholar]
- 25.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Jakubzick C, et al. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337:121–131. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahnsen FL, et al. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol. 2006;177:5861–5867. doi: 10.4049/jimmunol.177.9.5861. [DOI] [PubMed] [Google Scholar]
- 28.Veres TZ, et al. Spatiotemporal and functional behavior of airway dendritic cells visualized by two-photon microscopy. Am J Pathol. 2011;179:603–609. doi: 10.1016/j.ajpath.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takano KI, et al. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J Histochem Cytochem. 2005;53:611–619. doi: 10.1369/jhc.4A6539.2005. [DOI] [PubMed] [Google Scholar]
- 30.Vermaelen KY, et al. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDole JR, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosmi L, et al. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 34.Halle S, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. Journal of Experimental Medicine. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyron-Quiroz JE, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Andrian UH, et al. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mempel TR, et al. Visualization of leukocyte transendothelial and interstitial migration using reflected light oblique transillumination in intravital video microscopy. J Vasc Res. 2003;40:435–441. doi: 10.1159/000073902. [DOI] [PubMed] [Google Scholar]
- 39.Khandoga AG, et al. In vivo imaging and quantitative analysis of leukocyte directional migration and polarization in inflamed tissue. PLoS ONE. 2009;4:e4693. doi: 10.1371/journal.pone.0004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, et al. LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration. J Exp Med. 2005;201:409–418. doi: 10.1084/jem.20040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillipson M, et al. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreisel D, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118:6172–6182. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.BIERMAN HR, et al. The sequestration and visceral circulation of leukocytes in man. Ann N Y Acad Sci. 1955;59:850–862. doi: 10.1111/j.1749-6632.1955.tb45987.x. [DOI] [PubMed] [Google Scholar]
- 45.Staub NC, et al. Leucocytes and pulmonary microvascular injury. Ann N Y Acad Sci. 1982;384:332–343. doi: 10.1111/j.1749-6632.1982.tb21382.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmid-Schönbein GW, et al. The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvasc Res. 1980;19:45–70. doi: 10.1016/0026-2862(80)90083-7. [DOI] [PubMed] [Google Scholar]
- 47.Megens RTA, et al. Intravital imaging of phagocyte recruitment. Thromb Haemost. 2011;105:802–810. doi: 10.1160/TH10-11-0735. [DOI] [PubMed] [Google Scholar]
- 48.Matthay MA, et al. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caudrillier A, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 51.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 52.Ma Y, et al. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair. 2013;6:11. doi: 10.1186/1755-1536-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Alessio FR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 55.Rock JR, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barkauskas CE, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar PA, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warburton D, et al. Do lung remodeling, repair, and regeneration recapitulate respiratory ontogeny? Am J Respir Crit Care Med. 2001;164:S59–62. doi: 10.1164/ajrccm.164.supplement_2.2106064. [DOI] [PubMed] [Google Scholar]
- 59.Kenzaki K, et al. Lung regeneration: implantation of fetal rat lung fragments into adult rat lung parenchyma. J Thorac Cardiovasc Surg. 2006;131:1148–1153. doi: 10.1016/j.jtcvs.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 60.Varanou A, et al. Human embryonic stem cells and lung regeneration. Br J Pharmacol. 2008;155:316–325. doi: 10.1038/bjp.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roszell B, et al. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schnatwinkel C, Niswander L. Multiparametric image analysis of lung-branching morphogenesis. Dev Dyn. 2013;242:622–637. doi: 10.1002/dvdy.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mund SI, et al. Developmental alveolarization of the mouse lung. Dev Dyn. 2008;237:2108–2116. doi: 10.1002/dvdy.21633. [DOI] [PubMed] [Google Scholar]
- 64.Chamoto K, et al. Migration of CD11b+ accessory cells during murine lung regeneration. Stem Cell Res. 2013;10:267–277. doi: 10.1016/j.scr.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melero-Martin JM, et al. Host myeloid cells are necessary for creating bioengineered human vascular networks in vivo. Tissue Eng Part A. 2010;16:2457–2466. doi: 10.1089/ten.tea.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kedrin D, et al. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Engelhardt JJ, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21:402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fein MR, Egeblad M. Caught in the act: revealing the metastatic process by live imaging. Dis Model Mech. 2013;6:580–593. doi: 10.1242/dmm.009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sceneay J, et al. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013 doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 70.Perlikos F, et al. Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review. Crit Rev Oncol Hematol. 2013;87:1–11. doi: 10.1016/j.critrevonc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 72.Al-Mehdi AB, et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 73.Mendoza A, et al. Modeling metastasis biology and therapy in real time in the mouse lung. J Clin Invest. 2010;120:2979–2988. doi: 10.1172/JCI40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong CW, et al. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol. 2002;161:749–753. doi: 10.1016/S0002-9440(10)64233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ritsma L, et al. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Sci Transl Med. 2012;4:158ra145. doi: 10.1126/scitranslmed.3004394. [DOI] [PubMed] [Google Scholar]