Abstract
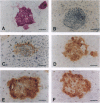
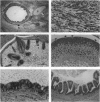
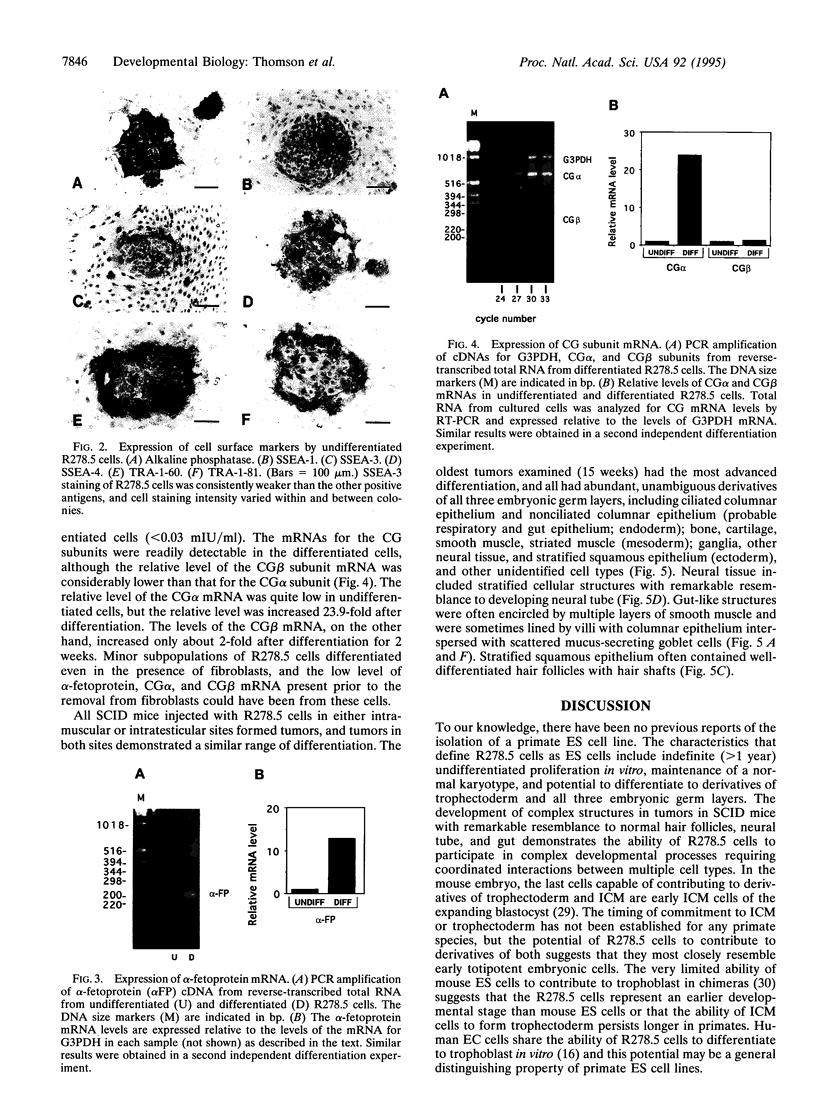
Embryonic stem cells have the ability to remain undifferentiated and proliferate indefinitely in vitro while maintaining the potential to differentiate into derivatives of all three embryonic germ layers. Here we report the derivation of a cloned cell line (R278.5) from a rhesus monkey blastocyst that remains undifferentiated in continuous passage for > 1 year, maintains a normal XY karyotype, and expresses the cell surface markers (alkaline phosphatase, stage-specific embryonic antigen 3, stage-specific embryonic antigen 4, TRA-1-60, and TRA-1-81) that are characteristic of human embryonal carcinoma cells. R278.5 cells remain undifferentiated when grown on mouse embryonic fibroblast feeder layers but differentiate or die in the absence of fibroblasts, despite the presence of recombinant human leukemia inhibitory factor. R278.5 cells allowed to differentiate in vitro secrete bioactive chorionic gonadotropin into the medium, express chorionic gonadotropin alpha- and beta-subunit mRNAs, and express alpha-fetoprotein mRNA, indicating trophoblast and endoderm differentiation. When injected into severe combined immunodeficient mice, R278.5 cells consistently differentiate into derivatives of all three embryonic germ layers. These results define R278.5 cells as an embryonic stem cell line, to our knowledge, the first to be derived from any primate species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Banting G., Damjanov I., Arnaud D., Avner P. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984 Winter;3(4):347–361. doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Damjanov I., Simon D., Banting G. S., Carlin C., Dracopoli N. C., Føgh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2. Differentiation in vivo and in vitro. Lab Invest. 1984 Feb;50(2):147–162. [PubMed] [Google Scholar]
- Babury R. A., Moscovic E. A. Selective immunolabeling of early gestational cytotrophoblast and its neoplastic counterpart by the monoclonal antibody Ber-EP4. Histol Histopathol. 1993 Apr;8(2):323–328. [PubMed] [Google Scholar]
- Beddington R. S., Robertson E. J. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989 Apr;105(4):733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- Bonduelle M. L., Dodd R., Liebaers I., Van Steirteghem A., Williamson R., Akhurst R. Chorionic gonadotrophin-beta mRNA, a trophoblast marker, is expressed in human 8-cell embryos derived from tripronucleate zygotes. Hum Reprod. 1988 Oct;3(7):909–914. doi: 10.1093/oxfordjournals.humrep.a136808. [DOI] [PubMed] [Google Scholar]
- Bongso A., Fong C. Y., Ng S. C., Ratnam S. Isolation and culture of inner cell mass cells from human blastocysts. Hum Reprod. 1994 Nov;9(11):2110–2117. doi: 10.1093/oxfordjournals.humrep.a138401. [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M. H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984 May 17;309(5965):255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Braude P., Bolton V., Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988 Mar 31;332(6163):459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- Doetschman T., Williams P., Maeda N. Establishment of hamster blastocyst-derived embryonic stem (ES) cells. Dev Biol. 1988 May;127(1):224–227. doi: 10.1016/0012-1606(88)90204-7. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Golos T. G., Durning M., Fisher J. M., Fowler P. D. Cloning of four growth hormone/chorionic somatomammotropin-related complementary deoxyribonucleic acids differentially expressed during pregnancy in the rhesus monkey placenta. Endocrinology. 1993 Oct;133(4):1744–1752. doi: 10.1210/endo.133.4.8404617. [DOI] [PubMed] [Google Scholar]
- Golos T. G., Durning M., Fisher J. M. Molecular cloning of the rhesus glycoprotein hormone alpha-subunit gene. DNA Cell Biol. 1991 Jun;10(5):367–380. doi: 10.1089/dna.1991.10.367. [DOI] [PubMed] [Google Scholar]
- Graves K. H., Moreadith R. W. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol Reprod Dev. 1993 Dec;36(4):424–433. doi: 10.1002/mrd.1080360404. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M., Taborn G. U., Garton R. L., Caplice M. D., Brenin D. R. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev Biol. 1994 May;163(1):288–292. doi: 10.1006/dbio.1994.1146. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Hollenberg A. N. Regulation of chorionic gonadotropin gene expression. Endocr Rev. 1993 Apr;14(2):203–221. doi: 10.1210/edrv-14-2-203. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y., Zsebo K., Hogan B. L. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992 Sep 4;70(5):841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Notarianni E., Galli C., Laurie S., Moor R. M., Evans M. J. Derivation of pluripotent, embryonic cell lines from the pig and sheep. J Reprod Fertil Suppl. 1991;43:255–260. [PubMed] [Google Scholar]
- Pera M. F., Blasco Lafita M. J., Mills J. Cultured stem-cells from human testicular teratomas: the nature of human embryonal carcinoma, and its comparison with two types of yolk-sac carcinoma. Int J Cancer. 1987 Sep 15;40(3):334–343. doi: 10.1002/ijc.2910400309. [DOI] [PubMed] [Google Scholar]
- Ramírez-Solis R., Davis A. C., Bradley A. Gene targeting in embryonic stem cells. Methods Enzymol. 1993;225:855–878. doi: 10.1016/0076-6879(93)25054-6. [DOI] [PubMed] [Google Scholar]
- Resnick J. L., Bixler L. S., Cheng L., Donovan P. J. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992 Oct 8;359(6395):550–551. doi: 10.1038/359550a0. [DOI] [PubMed] [Google Scholar]
- Roach S., Cooper S., Bennett W., Pera M. F. Cultured cell lines from human teratomas: windows into tumour growth and differentiation and early human development. Eur Urol. 1993;23(1):82–88. doi: 10.1159/000474574. [DOI] [PubMed] [Google Scholar]
- Rose T. M., Weiford D. M., Gunderson N. L., Bruce A. G. Oncostatin M (OSM) inhibits the differentiation of pluripotent embryonic stem cells in vitro. Cytokine. 1994 Jan;6(1):48–54. doi: 10.1016/1043-4666(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5099–5102. doi: 10.1073/pnas.72.12.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger D. J., Büscher M., Hecht J. H., Mellon P. L. Coordinate control of the alpha- and beta-subunit genes of human chorionic gonadotropin by trophoblast-specific element-binding protein. Mol Endocrinol. 1993 Dec;7(12):1579–1588. doi: 10.1210/mend.7.12.7511787. [DOI] [PubMed] [Google Scholar]
- Sukoyan M. A., Golubitsa A. N., Zhelezova A. I., Shilov A. G., Vatolin S. Y., Maximovsky L. P., Andreeva L. E., McWhir J., Pack S. D., Bayborodin S. I. Isolation and cultivation of blastocyst-derived stem cell lines from American mink (Mustela vison). Mol Reprod Dev. 1992 Dec;33(4):418–431. doi: 10.1002/mrd.1080330408. [DOI] [PubMed] [Google Scholar]
- Terasawa E., Bridson W. E., Nass T. E., Noonan J. J., Dierschke D. J. Developmental changes in the luteinizing hormone secretory pattern in peripubertal female rhesus monkeys: comparisons between gonadally intact and ovariectomized animals. Endocrinology. 1984 Dec;115(6):2233–2240. doi: 10.1210/endo-115-6-2233. [DOI] [PubMed] [Google Scholar]
- Wenk J., Andrews P. W., Casper J., Hata J., Pera M. F., von Keitz A., Damjanov I., Fenderson B. A. Glycolipids of germ cell tumors: extended globo-series glycolipids are a hallmark of human embryonal carcinoma cells. Int J Cancer. 1994 Jul 1;58(1):108–115. doi: 10.1002/ijc.2910580118. [DOI] [PubMed] [Google Scholar]
- Williams R. L., Hilton D. J., Pease S., Willson T. A., Stewart C. L., Gearing D. P., Wagner E. F., Metcalf D., Nicola N. A., Gough N. M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988 Dec 15;336(6200):684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- Winkel G. K., Pedersen R. A. Fate of the inner cell mass in mouse embryos as studied by microinjection of lineage tracers. Dev Biol. 1988 May;127(1):143–156. doi: 10.1016/0012-1606(88)90196-0. [DOI] [PubMed] [Google Scholar]
- Wolf E., Kramer R., Polejaeva I., Thoenen H., Brem G. Efficient generation of chimaeric mice using embryonic stem cells after long-term culture in the presence of ciliary neurotrophic factor. Transgenic Res. 1994 May;3(3):152–158. doi: 10.1007/BF01973982. [DOI] [PubMed] [Google Scholar]