Abstract
A 61-year-old male presents to the emergency room with complaints of fatigue, dizziness and bright red blood per rectum (BRBPR) for 2 days. Past medical history was significant for gastroesophageal reflux disease, non-steroidal anti-inflammatory drug (NSAID) induced ulcer, and end-stage renal disease (GFR < 30) status post 2 failed renal grafts. Pertinent medications include pantoprazole and sodium polystyrene sulfonate in sorbitol (Kayexalate 30 g/d orally). On esophagogastroduodenoscopy (EGD) there was a single shallow, flat, non-bleeding gastric ulcer (3 mm) in the pre-pyloric region of the stomach with no stigmata of bleeding. A colonoscopy was performed showing evidence of colitis and localized ulcerations in the cecum which were biopsied. Histopathology revealed basophilic, nonpolarizable, rhomboid-like crystals without evidence of necrosis.
Keywords: Kayexalate, medication, side effect, gastrointestinal bleed
CASE PRESENTATION
A 61-year-old male presented to the emergency room with complaints of fatigue, dizziness and bright red blood per rectum (BRBPR) for two days. On presentation, his vital signs were unstable (heart rate: 110 bpm; blood pressure: 90/64 mmHg), with a hemoglobin level of 9 g/dL (baseline 10–11 g/dL). His blood pressure responded appropriately to fluid resuscitation with a 2 L normal saline bolus. Past medical history was significant for gastro-esophageal reflux disease, non-steroidal anti-inflammatory drug (NSAID) induced ulcer, and end-stage renal disease (GFR <30) status following two failed renal grafts. Pertinent medications include pantoprazole and sodium polystyrene sulfonate in sorbitol (Kayexalate 30 g orally).
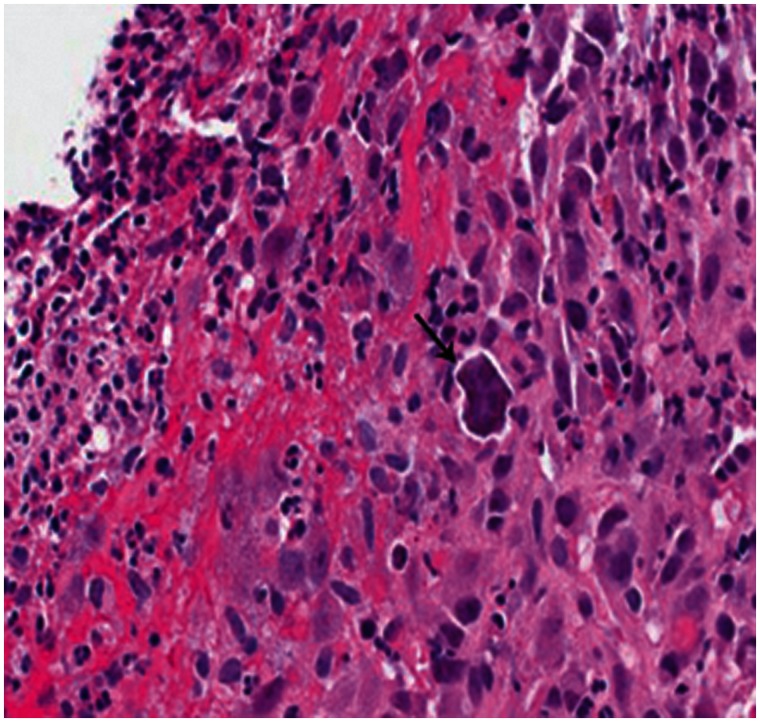
On esophago-gastroduodenoscopy (EGD), there was a single shallow, flat, non-bleeding gastric ulcer (3 mm) in the pre-pyloric region of the stomach, with no stigmata of bleeding. However, the patient continued to have intermittent episodes of BRBPR, necessitating multiple blood transfusions during his hospitalization. Subsequently, a colonoscopy was performed showing evidence of colitis and localized ulcerations in the cecum, which were biopsied. Histopathology revealed basophilic, non-polarizable, rhomboid-like crystals without evidence of necrosis (Figure 1).
Figure 1.
Cecal biopsy revealing crystal in ulcer (indicated by arrow).
DISCUSSION
Kayexalate is a cation-exchange resin that acts in the large intestine by exchanging sodium ions for potassium ions. It is used in the treatment of hyperkalemia and can be administered orally or as an enema. The mechanism of mucosal damage is not clear, and reported to be more common in patients with uremia. It is speculated that it is caused by its osmotic action and vasospasm of the intestinal vasculature [1]. Previous studies have suggested that Kayexalate in sorbitol may be associated with intestinal necrosis and inflammation in uremic patients [2, 3]. Abraham and colleagues focused on endoscopic and histological findings in patients with Kayexalate crystals from upper gastrointestinal biopsies [4]. Kayexalate-induced intestinal injury reveals rhomboid or triangular basophilic crystals adherent to the surface epithelium [5]. Our study highlights Kayexalate as a cause of lower gastrointestinal mucosal injury and ulceration with no evidence of necrosis, as confirmed histologically by evidence of Kayexalate crystalline resin in a patient with end-stage renal disease. Although Kayexalate-induced colonic injury is rare, recognition of Kayexalate crystals as a marker can be instrumental in providing the correct diagnosis and should be considered in patients with end-stage renal failure with mucosal injury.
Conflict of interest: none declared.
REFERENCES
- 1.Parfitt JR, Driman DK. Pathological effects of drugs on the gastrointestinal tract: a review. Hum Pathol. 2007;38:527–36. doi: 10.1016/j.humpath.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Gardiner GW. Kayexalate (sodium polystyrene sulphonate) in sorbitol associated with intestinal necrosis in uremic patients. Can J Gastroenterol. 1997;11:573–77. doi: 10.1155/1997/370814. [DOI] [PubMed] [Google Scholar]
- 3.Rashid A, Hamilton SR. Necrosis of the gastrointestinal tract in uremic patients as a result of sodium polystyrene sulfonate (Kayexalate) in sorbitol: an under-recognized condition. Am J Surg Pathol. 1997;21:60–69. doi: 10.1097/00000478-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Abraham SC, Bhagavan BS, Lee LA, et al. Upper gastrointestinal tract injury in patients receiving Kayexalate (sodium polystyrene sulfonate) in sorbitol: clinical, endoscopic, and histopathologic findings. Am J Surg Pathol. 2001;25:637–44. doi: 10.1097/00000478-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Schiere S, Karrenbeld A, Tulleken JE, et al. Sodium polystyrene sulfonate (resonium a) as possible cause of rectal blood loss. Ned Tijdschr Geneeskd. 1997;141:2127–29. [PubMed] [Google Scholar]