Abstract
AIM: To investigate the severity of acute pancreatitis (AP) is associated to the intensity of leukocyte activation, inflammatory up-regulation and microcirculatory disruption associated to ischemia-reperfusion injury. Microvascular integrity and inhibition of pro-inflammatory mediators are key-factors in the evolution of AP. Relaxin is an insulin-like hormone that has been attributed vasorelaxant properties via the nitric oxide pathway while behaving as a glucocorticoid receptor agonist.
METHODS: AP was induced by the bilio-pancreatic duct-outlet-exclusion closed-duodenal-loops model. Treatment with relaxin was done at different time-points. Nitric oxide synthase inhibition by L-NAME and glucocorticoid receptor (GR) blockage by mifepristone was considered. AP severity was assessed by biochemical and histopathological analyses.
RESULTS: Treatment with relaxin reduced serum amylase, lipase, C-reactive protein, IL-6, IL-10, hsp72, LDH and 8-isoprostane as well as pancreatic and lung myeloperoxidase. Acinar and fat necrosis, hemorrhage and neutrophil infiltrate were also decreased. ATP depletion and ADP/ATP ratio were reduced while caspases 2-3-8 and 9 activities were increased. L-NAME and mifepristone decreased the efficiency of relaxin.
CONCLUSION: Relaxin resulted beneficial in the treatment of AP combining the properties of a GR agonist while preserving the microcirculation and favoring apoptosis over necrosis.
Keywords: Acute pancreatitis, Relaxin, Nitric oxide, Glucocorticoid receptor, Necrosis, Apoptosis
INTRODUCTION
Acute pancreatitis (AP) is an acute inflammatory disorder associated with premature activation and increase in pancreatic enzymes in blood and urine that may progress to implicate other organs such as kidney, lungs and liver[1]. Inflammatory mediators induce complications that can range from local to systemic evolving to a severe form with high morbi-mortality[2].
The perivaterian duodenum (PV-D) has an extremely rich autonomic duodenum-pancreatic innervation therefore endoscopic maneuvers, gallstones, biliary sludge and/or hemobilia migrating and impacting on the peri-vaterian duodenum stimulate primary sensory neurons to unchain autonomous-arc-reflexes (AARs) responsible for the initial acute pancreatic neurogenic inflammation that may range from local and edematous to regional and necrotizing pancreatitis[3-6]. These autonomic nerve fibers are the starting point of autonomic-arc-reflexes which may integrate in the celiac ganglia complex or in the bulbar-hypothalamic autonomous nucleus to end in the intrapancreatic ganglion[6]. Therefore irritative stimuli induce impulses that travel through thin, unmyelinated, capsaicin-sensitive (type C) nerve fibers to generate in the somata of the afferent neurons vasoactive peptides such as substance P, neurokinin-A, VIP, SOM and DYN[7]. These sensory peptides synthesized in the somata are then transported to the nerve peripheral endings where they are released. Substance P and other sensory peptides can activate mast-cells that will liberate histamine and pro-inflammatory factors thus recruiting leukocytes that will exacerbate the inflammatory response associated to vasodialtation, edema, increased vascular permeability and neutrophil infiltration associated to this neuroimmune-inflammatory interaction observed in the neurogenic inflammation[7].
Over-stimulation of receptors in the peri-vaterian duodenum trigger autonomic-arc-reflexes that induce a sympathetic hypertone followed by cathecolaminic discharges from nerve endings in the pancreas that affect capillaries and blood vessels leading to microcirculatory disturbances responsible for ischemia/ reperfusion injury associated to synthesis and release of pro-inflammatory factors together with leukocyte recruitment and activation that correlate with intracellular enzyme activation[7]. Under these circumstances the systemic inflammatory response syndrome (SIRS) may end in a multiorgan dysfunction syndrome (MODS) characterized by metabolic and homodynamic alterations, multifocal organic dysfunction and pluriparenchymatose failure[8]. The bilio-pancreatic-duct outlet exclusion closed duodenal loops (BPDOE-CDLs) model, that we have previously described and validated, mimics these circumstances[6].
Relaxin is a peptide hormone of ovarian origin that belongs to the insulin superfamily[9]. It has been reported that relaxin offers protection for reperfusion-induced cardiac injury increasing coronary blood flow favoring blood supply to the ischemic myocardium while it exerts a relaxant action on smooth muscle cells[10]. It has been shown that relaxin up regulates nitric oxide synthase (NOS) II expression thus inducing nitric oxide mediated vasodilatation by a controlled activation of endogenous nitric oxide biosynthesis[11]. By regulating nitric oxide relaxin inhibits neutrophil extravasation, adhesion, recruitment and activation[12]; decreases the expression of chemokines and adhesion molecules[13]; inhibits histamine release by mast cells[14]; depresses platelet aggregation[15]; alters Ik-B phosphorylation and degradation diminishing NF-kB activation and binding to DNA and enhances the activation of the glucocorticoid receptor (GR)[16].Therefore, via the nitric oxide pathway relaxin exerts vaso-relaxant, anti-thrombotic and anti-inflammatory properties.
Relaxin has also been shown to bind and stimulate the glucocorticoid receptor that when activated will translocate to the nucleus where it inhibits NF-kB and AP-1 by transrepression and stimulates transcription of glucocorticoid response elements (GREs) of DNA inducing transcription of responsive genes by transcativation[16] . As an agonist of the glucocorticoid receptor relaxin will then possess anti-inflammatory properties inhibiting pro-inflammatory molecules.
Our aim was to investigate the influence of relaxin on the evolution of acute pancreatitis considering the relevance of the nitric oxide pathway and glucocorticoid receptor stimulation. Pancreatic, metabolic, kidney, liver and severity indicators were assessed.
MATERIALS AND METHODS
General concepts
The Bilio-pancreatic-duct-outlet-exclusion closed-duodenal-loops model (BPDOE-CDL) model was carried out in male inbred adult Wistar rats, weighing 250 - 350 g, free of pathogens, under controlled environmental conditions (room-temperature, 20-25 °C; 12:12 light-dark cycle), with free access to standard rodent chow and water. The study was performed in accordance with national guidelines for the use and care of laboratory animals and was approved by the local animal care and use committee. Rats were randomly assigned to one of the three series: basal, control or experimental. Each of these series was divided into groups of 16 rats.
The animal series comprised eleven groups. Group 1 (reference baseline values), consisted of un-fasted, healthy animals that did not undergo any surgery or treatment. Group 2 (surgery control), only underwent the surgical maneuvers involved in triggering AP. Group 3 (AP, BPDOE-CDLs model) acute pancreatitis was triggered by filling the closed-duodenal-loops at constant pressure with 8% sodium taurocholate [SIGMA, USA] and 200 uL of methylene blue to corroborate duodenal-reflux absence[6]. Group 4 (PBS vehicle control and BPDOE-CDLs) acute pancreatitis was induced as for Group 3 and 100 uL PBS were injected sc 1h before and 4 h after the surgery. Group 5 (LN+RLX, non-selective NOS inhibitor and relaxin) L-NAME (NG-nitro-L-arginine methyl ester [SIGMA, USA]) at 10 mg/kg and 5 mg/kg and relaxin [Phoenix Pharmaceuticals Inc, USA] at 2.5ug/kg were administered sc 1h before and 4h after triggering acute pancreatitis. Group 6 (MIF+RLX, GC receptor antagonist and relaxin) received mifepristone [SIGMA, USA] at 3 mg/kg and relaxin at 2.5 ug/kg sc 1h before and 4h after unchaining acute pancreatitis. Group 7 (LN+MIF+RLX, non-selective NOS inhibitor associated to GC receptor antagonist and relaxin) L-NAME at 10 mg/kg and 5 mg/kg, mifepristone at 3 mg/kg and relaxin at 2.5ug/kg were administered sc 1h before and 4h after triggering acute pancreatitis. Group 8 (RLX 1h pre BPDOE-CDLs) received 5ug/kg sc of relaxin 1h before the surgical maneuvers. Group 9 (RLX 1h post BPDOE-CDLs) was injected with 5 ug/kg sc of relaxin 1h after the procedure. Group 10 (RLX 1h pre and 4h post BPDOE-CDLs) was administered Relaxin at 2.5 ug/kg sc 1h before and 4h after unchaining acute pancreatitis. Group 11 (RLX 4h post BPDOE-CDLs) was treated with 5 ug/kg sc of relaxin 4h after triggering acute pancreatitis. L-NAME and mifepristone were dissolved in saline solution, relaxin was dissolved in PBS. Group 12 (RLX 12h &1h pre and 4h post BPDOE-CDLs) received relaxin at 5 ug/kg sc 12 h before the surgery and 2.5 ug/kg sc 1h prior to the procedure and a second administration 4h after it.
Experimental procedures
Bilio-pancreatic-duct-outlet-exclusion closed-duodenal-loops model (BPDOE-CDLs) We described the BPDOE-CDLs model in detail Figure 1, (reproduced with permission granted by the Journal of Pancreatology and S. Karger AG, Basel)[6]. The normally fed animals were anesthetized with sodium pentothal (5 mg/100 g) (Thiopental, Abbott, USA). A median laparotomy was done to exteriorize the antrum-duodenum-pancreas, and a double-tube double-hole intra-duodenal catheter was inserted, through which saline solution (3 mL) was instilled. Two closed-duodenal-loops were prepared: first two proximal pre-pyloric ligatures and one post-pyloric just above the common-bilio-pancreatic duct outlet were placed. For the second loop, a ligature was placed just below the common-bilio-pancreatic-duct and two more ligatures were placed at the distal end of the peri-vaterian duodenum, thus excluding the common-bilio-pancreatic-duct outlet. Each closed-duodenal-loop was filled with 8% sodium taurocholate (1 mL) and 200 uL of methylene blue at constant pressure, following a 45-minute observation period. The closed-duodenal-loop content was aspirated, the ligatures removed and the laparotomy closed. The animal was then placed in an individual cage with microenvironmental temperature control and allowed to recover. After 12 h, the rat was exsanguinated under anesthesia by aortic puncture and a blood sample was obtained for biochemical tests. All procedures were done with aseptic techniques. If, despite exclusion of the common-bilio-pancreatic-duct, duodenal-content filtered through the ligatures and refluxed towards the bilio-pancreatic ductal tree, as evidenced from blue coloring of the pancreas, the rat was excluded from the study.
Figure 1.
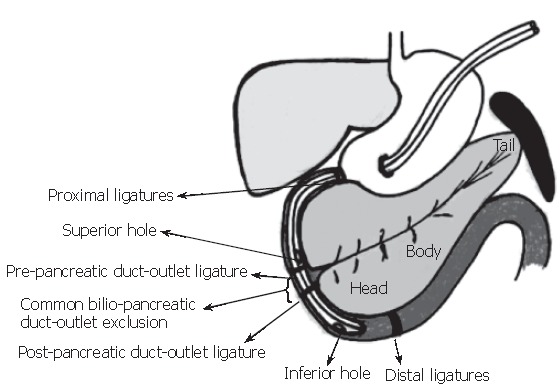
BPDOE-CDL model to trigger acute pancreatitis. Two closed-duodenal-loops were created in such a way that the entrance of the common bilio-pancreatic duct-outlet was absolutely excluded. To double check the absence of duodenal-reflux content or duodenal leakage, methylene blue was added to the sodium tauracholate which was instilled into the peri-vaterian duodenum through the superior and inferior holes (reproduced with permission granted by the Journal of Pancreatology and S. Karger AG, Basel)[6].
Macroscopic examination, dissection and weighing with a precision scale (Microgram-Series BH 300) of the pancreas were performed. The gland was subsequently divided into cephalic and splenic segments[6,17,18].
The liver, duodenum, kidneys, suprarenal glands, spleen and lungs were examined and dissected. Each organ was divided into smaller portions, one was fixed in formol-buffer and stained with hematoxylin-eosin, the others were wrapped individually in aluminum foil and preserved at -70 °C. The cephalic and splenic segments of the pancreas and the lungs were studied in more detail.
Amylase and lipase activity measurement and protein quantification in pancreatic tissue Assays were done on each segment; the tissue was homogenized on ice in 2 mL of extraction buffer (20 mmol/L Tris-HCL pH 7.6-[Sigma, USA], 0.3 mol/L NaCl-[Merck, Germany], 0.5% Triton X-100-[Sigma, USA]; distilled water). Proteins were quantified in the homogenate according to the method described by Lowry[19]. The homogenate was centrifuged at 10 000 r/min for 15 min at 4 °C. The supernatant was assayed for amylase (kinetic method at 405 nm with defined substrate at 25 °C-[Wiener, Arg.]) and lipase (turbidimetric method UV color at 25 °C-[Randox, UK]).
Myeloperoxidase (MPO) activity in pancreas and lung tissue MPO, an enzyme found in neutrophils that represents the 5% of its dry weight, catalyzes the oxidation of electron donors (eg.halides) by hydrogen peroxide[20]. MPO was measured in both pancreatic segments and in lung tissue as a biochemical indicator of neutrophil infiltrate. To determine MPO activity the tissue was homogenized on ice with 1 mL hexadecyl trim ethylammonium bromide (HTAB) (0.5 % HTAB in 50 mmol/L phosphate buffer-pH 6.0). The homogenate was sonicated during 10 s at 200 W, and then centrifuged at 40000 r/min for 15 min at 4 °C. MPO was assayed on the supernatant: 0.1 mL of the sample was combined with 2.9 mL of 5 mmol/L phosphate buffer, (pH 6.0, 0.167 mg/mL o-dianisidine hydrochloride and 0.0005% hydrogen peroxide). The change in absorbance at 460 nm was measured with a Clinicon 4010 spectrophotometer. One unit of MPO (UMPO) activity was defined as the amount that could degrade 1 µmol of peroxide per minute at 25 [21].
Caspase 2-3-8 and 9 activities in pancreas, liver and lung tissue Caspases 2-3-8 and 9 are early indicators of apoptosis in mammalian cells[22]. The CasPASETM apoptosis activity assay kit [Genotech, MO-USA] is based on detecting the chromogenic and fluorogenic resulting molecule 7-amino-4-trifluromethyl cumarin (AFC) cleaved from the synthetic substrate, Z-VAD-fluoromethyl ketone (FMK) irreversibly and selectively inhibits the reaction. The excitation and emission wavelengths of AFC in a fluorometer are 360 nm and 510 nm respectively. Frozen tissues were homogenized at 4 °C in chilled lysis buffer and the lysates were then centrifuged at 15000 g for 30 min at 4 °C, caspase activity was assessed on the supernatants. To calculate specific enzyme activity equal aliquots of supernatant were tested in presence of the Z-VAD-FMK inhibitor. Results were expressed as the specific cleavage of AFC per mg of protein per minute considering the nonspecific product formation coming from the substrate plus the inhibitor. All reagents were supplied by the kit and the assays were performed according to the manufacturer’s instructions.
ATP and ADP/ATP ratio measurements in pancreas, liver and lung tissue ATP depletion allows to detect apoptosis, and when it is associated with the percentage of ATP loss and changes in the ADP/ATP ratio apoptosis can then be conveniently differentiated from necrosis[23]. Light formed from ATP and luciferin in the presence of luciferase can be measured at 560 nm. The tissue was homogenized in TCA (trichloroacetic acid) 6% for 1 min and centrifuged at 6000 g for 5 min at 4 °C. The TCA in the supernatant was neutralized and diluted to a final concentration of 0.1% with Tris-Acetate pH 7.75 buffer. ATP concentration expressed as nmol/L per mg of protein and ADP/ATP concentration ratio were measured in the supernatant using the ENLITEN® ATP Assay System Bioluminescence Detection Kit [Promega, WI-USA] and ApoSENSORTM ADP/ATP ratio assay kit (Alexis-Corp, Switzerland) respectively according to the manufacturer’s instructions.
Extraction-purification-quantification of DNA The total mass of DNA in the pancreas, and lung tissue was used to standardize the units of enzymatic activity since it did not show significant quantitative variations in the BPDOE-CDL model.
The DNA was extracted and purified from tissue from both pancreatic segments and lung according to the manufacturer’s instructions (Wizard Genomic- [Promega, USA]). Double stranded DNA concentration was measured at 260 nm.
Histologic evaluation Pancreatic tissue was graded on a scale of 0-4 each for: edema, hemorrhage, leukocyte infiltration, acinar necrosis and fat necrosis. Lung tissue was graded using a scale of 0-4 for leukocyte infiltration. Histologic changes were graded in a blinded manner by two independent observers counting frequency of foci per field seen at 40X (absent = 0, mild = 1, moderate = 2, severe = 3, overwhelming = 4).
Biochemical assays in blood samples Loss of blood and hemoconcentration were evaluated by the hematocrite. The metabolic profile consisted of glucose (enzymatic method in serum at 37 °C-[Wiener, Arg.]), uric acid (enzymatic method in serum at 37 °C-[Wiener, Arg]), proteins (enzymatic method in serum at 37 °C-[Wiener, Arg.]) and calcium (direct colorimetric method in serum at 20 °C-[Wiener, Arg.]). The kidney profile comprised urea (enzymatic method in serum at 37 °C-[Wiener, Arg.]) and creatinine (end-point colorimetric method in serum at 37 °C-[Wiener, Arg]). The pancreatic profile involved amylase (kinetic method at 405 nm with defined substrate in serum at 37 °C-[Wiener, Arg.]) and lipase (UV turbidimetric method in serum at 37 °C-[Randox, UK]). The liver profile consisted of AST and ALT (IFCC optimized UV method in serum at 37 °C-[Wiener, Arg.]), bilirubin (colorimetric method to determine total and direct bilirubin in serum at 25 °C, indirect bilirubin that results from the difference between them-[Wiener, Arg.]) and alkaline phosphatase (optimized DGKC-SSCC kinetic method at 405 nm in serum at 37 °C-ALP 405-[Wiener, Arg.]) Cellular injury was assessed by LDH (DGKC optimized kinetic method in serum at 37 °C-[Wiener, Arg.]). The profile for cellular stress and inflammatory response associated to severity involved leukocyte count, quantitative C reactive protein (immunoturbidimetric method in serum at 37 °C-PCR Latex Turbitest-[Wiener, Arg.]), heat shock protein (hsp) 72 (ELISA method for rat HSP 72 in plasma and serum-[StressGen Biotechnologies, USA)], IL-6 and IL-10 (ELISA technique in serum-[Assay Designs,USA]) and 8-Isoprostane (EIA method in plasma-[Cayman, USA]).
Statistical analysis
Biochemical assays in blood and organs The ANOVA test was used to establish differences between groups. The Dunnet test compared the difference between each group in relationship to group 1 (reference baseline level in control animals). The Student-Newman-Keuls test carried out after the ANOVA compared the groups between them and in relationship to group 1, evaluating the existence of homogeneous groups. Results are expressed as mean ±standard deviation (SD). Differences were considered statistically significant with P < 0.05.
Histopathological score The ANOVA test with two fixed factors (group and place: cephalic and splenic) was followed by the Dunnet test and Student-Newman-Keuls test. Results are expressed as mean ± SD. Differences were considered statistically significant with P < 0.05.
Correlation between MPO and leukocyte infiltrate In both pancreatic segments and in lung tissue, the Spearman correlation coefficient Rho was calculated to examine the correlation between MPO and leukocyte infiltrate[24].
RESULTS
Biochemical assays in blood and other tissues together with histopathological studies evaluated the influence of relaxin on the evolution of acute pancreatitis. Association with L-NAME and/or mifepristone pondered on the weight of the nitric oxide pathway and glucocorticoid receptor stimulation involvement in acute pancreatitis modulation by relaxin. These results are described in Tables 1,2,3,4.
Table 1.
Pancreatic and general biochemical assays in blood (mean ± SD)
| Groups | Amylase | Lipase | Glucose | Urea | Creatinine | Calcium | Proteins | AST | ALT | Hto |
| IU/L | IU/L | mg/dL | mg/dL | mg/dL | mg/dL | g/L | IU/L | IU/L | % | |
| 1 Basal | 1103 ± 108 | 85 ± 18 | 92 ± 13 | 29 ± 3 | 0.35 ± 0.04 | 13.48 ± 0.39 | 85.5 ± 1.2 | 177 ± 25 | 88 ± 19 | 39 ± 5 |
| 2 Surgery control | 1198 ± 114 | 98 ± 10 | 95 ± 10 | 38 ± 5 | 0.48 ± 0.11 | 13.22 ± 0.35 | 82.7 ± 0.8 | 205 ± 21 | 99 ± 21 | 31 ± 4 |
| 3 AP | 7915 ± 159 | 5619 ± 194 | 338 ± 24 | 219 ± 19 | 1.82 ± 0.21 | 8.92 ± 0.16 | 61.1 ± 1.3 | 589 ± 26 | 295 ± 28 | 49 ± 9 |
| 4 AP+PBS | 7921 ± 137 | 5628 ± 201 | 341 ± 19 | 217 ± 15 | 1.81 ± 0.16 | 8.94 ± 0.20 | 61.2 ± 2.2 | 591 ± 25 | 299 ± 28 | 49 ± 8 |
| 5 Rlx+LN | 7154 ± 165 | 4982 ± 203 | 246 ± 23 | 183 ± 22 | 1.58 ± 0.22 | 9.37 ± 0.18 | 65.5 ± 1.8 | 544 ± 30 | 268 ± 19 | 47 ± 6 |
| 6 Rlx+MIF | 7162 ± 171 | 4997 ± 207 | 249 ± 27 | 185 ± 24 | 1.60 ± 0.25 | 9.35 ± 0.14 | 65.1 ± 1.9 | 552 ± 28 | 270 ± 22 | 47 ± 8 |
| 7 Rlx+LN+MIF | 7548 ± 175 | 5327 ± 214 | 262 ± 33 | 194 ± 21 | 1.69 ± 0.22 | 9.11 ± 0.21 | 62.4 ± 1.4 | 563 ± 34 | 281 ± 27 | 48 ± 5 |
| 8 Rlx 1h pre | 6052 ± 169 | 3618 ± 155 | 190 ± 16 | 125 ± 11 | 1.13 ± 0.14 | 11.19 ± 0.22 | 74.5 ± 2.3 | 405 ± 24 | 196 ± 23 | 40 ± 4 |
| 9 Rlx 1h post | 6427 ± 185 | 3910 ± 183 | 218 ± 15 | 146 ± 15 | 1.24 ± 0.19 | 10.91 ± 0.19 | 71.1 ± 1.9 | 452 ± 37 | 211 ± 27 | 45 ± 4 |
| 10 Rlx 1h pre+4h post | 5589 ± 129 | 3161 ± 142 | 166 ± 18 | 97 ± 8 | 1.02 ± 0.17 | 11.98 ± 0.24 | 79.4 ± 1.6 | 382 ± 29 | 178 ± 23 | 42 ± 5 |
| 11 Rlx 4h post | 6896 ± 192 | 4533 ± 178 | 235 ± 29 | 165 ± 19 | 1.39 ± 0.20 | 10.21 ± 0.19 | 69.9 ± 2.1 | 497 ± 33 | 235 ± 25 | 46 ± 5 |
| 12 Rlx 12h&1h pre+4h post | 5535 ± 144 | 3153 ± 121 | 162 ± 22 | 95 ± 10 | 1.00 ± 0.15 | 12.03 ± 0.29 | 79.8 ± 1.4 | 395 ± 33 | 171 ± 25 | 43 ± 5 |
In relationship to Reference Basal-Group 1, BPDOE-CDLs AP-Group 3 presents a significant difference in all the assays evaluated P<0.05. The pancreatic and general biochemical profile significantly improved with relaxin treatment particularly for Groups 10 &12 that corresponded to administration times 12h and/or 1h pre and 4h post surgery P<0.05. Homogenous groups for amylase and lipase:1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P<0.05. HTO: hematocrite, Rlx: relaxin, LN: L-NAME, MIF: mifepristone.
Pancreatic and general biochemical assays in blood
To evaluate if relaxin helped to maintain pancreatic, metabolic, kidney and liver function after the initiation of acute pancreatitis, pancreatic and general biochemical assays in blood were performed (Table 1).
Cholestasis was absent in all groups. Uric acid, alkaline phosphatase, total - direct and indirect bilirubin showed no significant differences among the mean values for all groups, the rest of the assays did.
Amylase and lipase showed a significant increase for homogenous groups (3-4) that developed a severe acute pancreatitis, group 7 also exhibited a marked raise although not to the same extent and the values of the enzymes for homogenous groups (5-6) were slightly lower than for group 7. Relaxin treatment 12& 1h before and 4h after BPDOE-CDLs (group 12) was similar to the one corresponding to 1h pre and 4h post pancreatic maneuvers (group 10). These treatments resulted in the least increase in pancreatic enzymes. These same trend was observed for glucose, urea, creatinine, AST and ALT. The abnormal reduction profile in calcium and serum proteins presented the same course as the pathological augmentation observed for pancreatic enzymes. The hematocrite value, considering group 2 as the reference group due to blood loss during surgery, indicated that homogenous groups (10-12), developing mild acute pancreatitis did not manifest the hemoconcentration observed for homogenous groups (3-4).
2- Severity indicators in blood
We next wanted to assess if the severity of acute pancreatitis was ameliorated by relaxin treatment measuring blood parameters (Table 2 & Figure 2A). The raise in leukocyte count correlated (R = 803, P < 0.05) with the expression of inflammatory IL-6 and liver synthesized acute phase protein CRP. The significant increase observed for homogenous groups (3-4) was followed in order by that developed in group 7 and then by homogenous groups (5-6); these values were markedly diminished for homogenous groups (10-12). Inhibition of either the nitric oxide pathway or glucocorticoid receptor stimulation reduced the efficiency of relaxin treatment; inhibiting simultaneously both of them although leading to a more severe manifestation of the disease still showed better prognosis than that observed for homogenous groups (3-4) suggesting that there may be another mechanism by which relaxin may be also acting. Treatment with a single dose of relaxin 1h before, 1h after or 4h after unchaining acute pancreatitis although still beneficial was not as effective. Pro-inflammatory IL-6 and anti-inflammatory IL-10 exhibited the same profile as a consequence of the induction of the inflammatory response and attempt to counter-act it. Lipid peroxidation leading to elevated 8-isoprostane and synthesis of hsp72 aiming to protect from ongoing cellular stress followed the same trend.
Table 2.
Severity indicators assays in blood (mean ± SD)
| Groups | Leukocytes | CRP | HSP 72 | IL-6 | IL-10 | 8-isoprostane |
| nr/mL | mg/dL | pg/mL | pg/mL | pg/mL | pg/mL | |
| 1 Basal | 6100 ± 224 | 0 ± 0 | 739 ± 32 | below detection limit | below detection limit | 0.11 ± 0.005 |
| 2 Surgery control | 9856 ± 272 | 3.3 ± 1.02 | 1221 ± 21 | 207 ± 26 | 382 ± 59 | 0.13 ± 0.006 |
| 3 AP | 26162 ± 258 | 30.9 ± 1.15 | 3396 ± 64 | 1962 ± 27 | 2216 ± 94 | 0.55 ± 0.011 |
| 4 AP+PBS | 26145 ± 294 | 30.6 ± 1.21 | 3378 ± 56 | 1956 ± 32 | 2201 ± 85 | 0.56 ± 0.010 |
| 5 Rlx+LN | 21223 ± 207 | 21.7 ± 1.24 | 2727 ± 48 | 1792 ± 28 | 2063 ± 90 | 0.49 ± 0.010 |
| 6 Rlx+MIF | 21207 ± 236 | 21.4 ± 1.31 | 2765 ± 52 | 1784 ± 35 | 2052 ± 78 | 0.48 ± 0.009 |
| 7 Rlx+LN+MIF | 23239 ± 273 | 27.3 ± 1.12 | 3023 ± 39 | 1849 ± 29 | 2113 ± 122 | 0.50 ± 0.008 |
| 8 Rlx 1h pre | 17438 ± 266 | 13.9 ± 1.22 | 1924 ± 49 | 1473 ± 33 | 1695 ± 72 | 0.37 ± 0.010 |
| 9 Rlx 1h post | 18776 ± 248 | 16.4 ± 1.52 | 2258 ± 42 | 1601 ± 25 | 1809 ± 87 | 0.42 ± 0.009 |
| 10 Rlx 1h pre+4h post | 14527 ± 285 | 10.8 ± 1.44 | 1711 ± 39 | 1098 ± 32 | 1451 ± 68 | 0.31 ± 0.010 |
| 11 Rlx 4h post | 19349 ± 225 | 19.5 ± 1.46 | 2506 ± 38 | 1689 ± 23 | 1975 ± 84 | 0.46 ± 0.008 |
| 12 Rlx 12h&1h pre+4h post | 14542 ± 295 | 10.9 ± 1.27 | 1721 ± 43 | 1105 ± 28 | 1460 ± 76 | 0.32 ± 0.007 |
Severe AP was developed in groups 3 & 4. This severity was ameliorated by treatment with relaxin. In relationship to reference basal-group 1, BPDOE-CDLs AP in group 3 presented a significant difference in all the assays evaluated while treated groups 10 & 12 were the ones to exhibit the best prognosis with a significant improvement for all markers P<0.05.Homogenous groups for severity indicators :1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P<0.05. Rlx: relaxin, LN: L-NAME, MIF: mifepristone.
Figure 2.
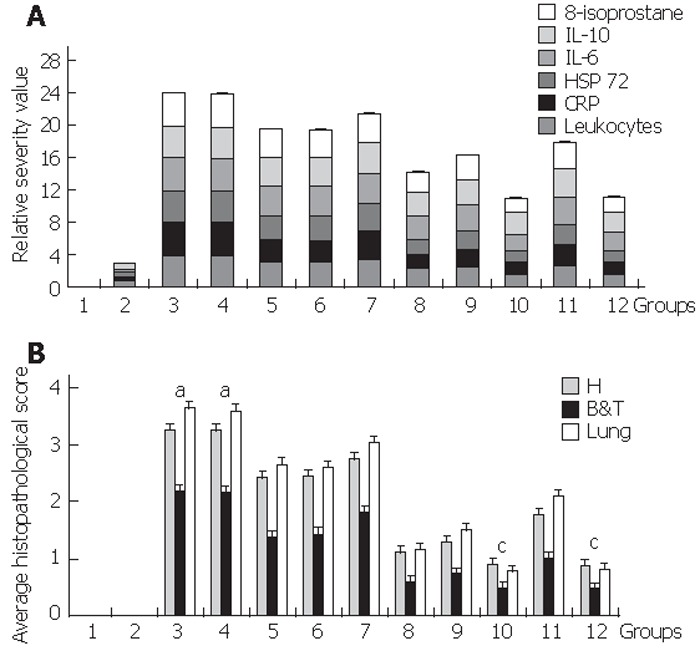
Relative severity value in blood and average histopathological score. A: Relative severity value in blood. To visualize the severity degree based on biochemical assays in blood, we arbitrarily established a single “relative severity value” of zero to group 1 (reference baseline value) and of four to group 3 (AP) per assay. To calculate the single “relative severity value” for the rest of the groups we used the following formula: “relative severity value” = [( X – reference baseline value) / ( AP value – reference baseline value )] x 4. Introducing each mean value from table 2 (referred to in the above formula as X) we obtained six different single “relative severity values” for each group, whose contribution to the total “relative severity value” is shown in this graph. For those values in table 2 that were below the detection limit, the value assigned to X corresponded to that of the assay’s sensitivity (IL-6: 112 pg/mL; IL-10: 12 pg/mL). Homogenous Groups are 1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P<0.05. Results are shown as mean ± SD. B: Average histopathological score. To visualize the severity degree based on the score-associated to histopathological lesions, we averaged the means of the six parameters used in table 4 for each group. Homogenous groups are 1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P < 0.05. There was a significant increase in the assessed parameters for Groups 3&4 that developed severe AP aP < 0.03 while groups 10& 12 exhibited a significant improvement cP < 0.05 Results shown as mean ± SD.
Assays in pancreatic and lung tissue
We then wanted to investigate the enzymatic and inflammatory pancreatic and lung response (Table 3).
Table 3.
Assays in pancreatic and lung tissue (mean ± SD)
| Groups | Panc. Amylase IU/mg DNA ds | Panc. Lipase IU/mg DNA ds | Panc. Proteins mg/mg DNA ds | Pancreatic MPO UMPO/mg DNA ds | Lung MPO UMPO/mg DNA ds | Panc. Wet Weight mg/100 g body wt | ||||
| H |
B&T |
H |
B&T |
H |
B&T |
H |
B&T |
|||
| 1 Basal |
995 ± 24 |
603 ± 27 |
98 ± 10 |
75 ± 11 |
185 ± 17 |
103 ± 12 |
0 ± 0 |
0 ± 0 |
0 ± 0 |
1310 ± 19 |
| 2 Surgery control |
927 ± 19 |
576 ± 22 |
81 ± 11 |
66 ± 10 |
206 ± 19 |
122 ± 14 |
0 ± 0 |
0 ± 0 |
0 ± 0 |
1385 ± 24 |
| 3 AP |
263 ± 17 |
181 ± 14 |
26 ± 9 |
16 ± 8 |
115 ± 10 |
57 ± 10 |
10.64 ± 0.39 |
7.15 ± 0.11 |
3.85 ± 0.13 |
2335 ± 38 |
| 4 AP + PBS |
261 ± 20 |
185 ± 26 |
25 ± 9 |
15 ± 9 |
114 ± 11 |
58 ± 10 |
10.59 ± 0.31 |
7.22 ± 0.09 |
3.83 ± 0.12 |
2321 ± 43 |
| 5 Rlx + LN |
392 ± 18 |
216 ± 21 |
34 ± 5 |
24 ± 6 |
129 ± 14 |
64 ± 8 |
8.12 ± 0.32 |
5.21 ± 0.12 |
2.93 ± 0.17 |
2159 ± 40 |
| 6 Rlx + MIF |
389 ± 19 |
219 ± 23 |
35 ± 7 |
23 ± 5 |
130 ± 12 |
64 ± 13 |
8.09 ± 0.36 |
5.27 ± 0.15 |
2.96 ± 0.15 |
2142 ± 48 |
| 7 Rlx+ LN + MIF |
315 ± 15 |
202 ± 12 |
32 ± 8 |
19 ± 4 |
121 ± 10 |
60 ± 8 |
9.01 ± 0.42 |
6.16 ± 0.10 |
3.10 ± 0.14 |
2198 ± 39 |
| 8 Rlx 1h pre |
590 ± 29 |
342 ± 16 |
50 ± 12 |
37 ± 7 |
154 ± 18 |
81 ± 16 |
5.94 ± 0.22 |
3.68 ± 0.08 |
2.21 ± 0.08 |
1798 ± 33 |
| 9 Rlx 1h post |
505 ± 33 |
307 ± 35 |
45 ± 10 |
32 ± 4 |
147 ± 20 |
70 ± 10 |
6.23 ± 0.25 |
3.95 ± 0.11 |
2.43 ± 0.09 |
1967 ± 39 |
| 10 Rlx 1h pre + 4h post |
679 ± 31 |
387 ± 18 |
60 ± 9 |
43 ± 8 |
165 ± 23 |
90 ± 11 |
5.79 ± 0.19 |
3.59 ± 0.09 |
2.06 ± 0.10 |
1688 ± 29 |
| 11 Rlx 4h post |
439 ± 22 |
273 ± 24 |
39 ± 9 |
27 ± 9 |
135 ± 15 |
66 ± 7 |
7.05 ± 0.28 |
4.45 ± 0.09 |
2.64 ± 0.14 |
2087 ± 42 |
| 12 Rlx 12h&1h pre + 4h post | 683 ± 26 | 392 ± 17 | 59 ± 8 | 44 ± 9 | 167 ± 21 | 89 ± 10 | 5.78 ± 0.21 | 3.54 ± 0.03 | 2.07 ± 0.09 | 1693 ± 25 |
Pancreatic amylase and lipase decreased significantly in severe AP group 3, P<0.05. Treatment with relaxin induced a significant improvement with an increase in pancreatic enzymes and proteins P < 0.05, while there was a decrease in UMPO. This amelioration of the disease was even more remarkable for groups 10 & 12 indicating that treatment 12 and/or 1h pre and 4h post BPDOE-CDLs procedure had a protective role. The increase in pancreatic wet weight in groups with severe AP as a result of edema was significant P<0.01. Homogenous groups for pancreatic amylase-lipase and MPO: :1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P<0.05; lung MPO: :1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P<0.05. Rlx: relaxin, LN: L-NAME, MIF: mifepristone.
There were no significant differences between the mean value of the groups for DNA of the lung and the same pancreatic segment, but the mean values for the cephalic and splenic segments for each group were significantly different (data shown previously[6]).
The decrease in pancreatic amylase and lipase was inverse to their increase in blood. Pancreatic proteins showed a similar expression profile to that of pancreatic enzymes, the elevation for group 2 may result from the synthesis of acute phase pancreatic proteins.
Pancreatic and lung MPO activity reflecting neutrophil infiltrate, was significantly higher for homogenous groups (3-4) while homogenous Groups (10-12) that had been treated with relaxin before and after triggering acute pancreatitis presented lesser UMPO values. The Spearman Correlation Coefficient between MPO activity and leukocyte infiltrate was significant for the pancreatic cephalic segment (R = 0.943, P < 0.01); for the pancreatic splenic segment (R = 0.831, P < 0.001); and for the lung (R = 0.871, P < 0.01).
The increase in pancreatic wet weight due to edema was more significant for homogenous groups (5-6)-7-(3-4) than for homogenous groups (10-12).
Histopathological scores in pancreas and lung tissue
Homogenous groups (10-12) that developed a mild acute pancreatitis exhibited edema as the main histopathological feature and a lower value for pancreatic and lung leukocyte infiltrate that correlated with MPO activity. Homogenous groups (5-6)-7-(3-4) were characterized by high scores for leukocyte infiltrate that correlated with MPO activity, pancreatic hemorrhage as well as acinar and fat necrosis. Necrotic foci presented a spotted pattern with higher frequency in the cephalic segment. group 11 that received relaxin 4h after triggering acute pancreatitis were characterized by hemorrhage. The average of pancreatic histopathological scores presented a significant lower value for homogenous groups (10-12) when comparing them to the average values obtained for homogenous groups (5-6)-7-(3-4). These results are described in Table 4 and Figure 2B.
Table 4.
Histopathological scores in pancreas and lung tissue (mean ± SD)
| Pancreatic Edema | Pancreatic emorrhage | Panc. Leuk. Infiltrate | Panc. Acinar Necrosis | Panc. Fat Necrosis | Lung Leuk | ||||||
| H | B&T | H | B&T | H | B&T | H | B&T | H | B&T | Infiltrate | |
| Groups | |||||||||||
| 1 Basal | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2 Surgery control | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 3 AP | 1.31 ± 0.09 | 1.14 ± 0.08 | 3.58 ± 0.08 | 1.84 ± 0.06 | 3.61 ± 0.10 | 2.47 ± 0.08 | 3.87 ± 0.10 | 2.90 ± 0.08 | 3.98 ± 0.12 | 2.60 ± 0.08 | 3.65 ± 0.15 |
| 4 AP+PBS | 1.33 ± 0.07 | 1.12 ± 0.10 | 3.55 ± 0.10 | 1.82 ± 0.07 | 3.64 ± 0.09 | 2.46 ± 0.11 | 3.83 ± 0.09 | 2.86 ± 0.12 | 3.95 ± 0.10 | 2.66 ± 0.11 | 3.61 ± 0.10 |
| 5 Rl x+LN | 1.01 ± 0.06 | 0.73 ± 0.10 | 2.46 ± 0.10 | 1.08 ± 0.06 | 2.93 ± 0.14 | 1.53 ± 0.07 | 2.96 ± 0.10 | 2.03 ± 0.05 | 2.84 ± 0.11 | 1.57 ± 0.08 | 2.66 ± 0.10 |
| 6 Rlx+MIF | 0.99 ± 0.10 | 0.75 ± 0.09 | 2.49 ± 0.06 | 1.27 ± 0.05 | 2.97 ± 0.11 | 1.58 ± 0.09 | 2.99 ± 0.09 | 2.00 ± 0.07 | 2.80 ± 0.07 | 1.61 ± 0.09 | 2.62 ± 0.07 |
| 7 Rlx+LN+MIF | 1.26 ± 0.10 | 0.92 ± 0.08 | 2.97 ± 0.09 | 1.42 ± 0.05 | 3.23 ± 0.08 | 2.25 ± 0.06 | 3.35 ± 0.05 | 2.45 ± 0.08 | 3.00 ± 0.09 | 2.08 ± 0.10 | 3.04 ± 0.08 |
| 8 Rlx 1h pre | 0.51 ± 0.08 | 0.42 ± 0.08 | 1.37 ± 0.07 | 0.59 ± 0.05 | 0.86 ± 0.08 | 0.45 ± 0.05 | 1.39 ± 0.11 | 0.85 ± 0.08 | 1.43 ± 0.10 | 0.75 ± 0.08 | 1.17 ± 0.09 |
| 9 Rlx 1h post | 0.62 ± 0.07 | 0.50 ± 0.08 | 1.50 ± 0.08 | 0.71 ± 0.08 | 1.03 ± 0.03 | 0.62 ± 0.06 | 1.64 ± 0.07 | 1.01 ± 0.06 | 1.71 ± 0.08 | 0.98 ± 0.05 | 1.52 ± 0.10 |
| 10 Rlx 1h pre+4h post | 0.42 ± 0.07 | 0.37 ± 0.06 | 1.26 ± 0.10 | 0.43 ± 0.07 | 0.72 ± 0.07 | 0.37 ± 0.09 | 0.92 ± 0.09 | 0.59 ± 0.05 | 1.19 ± 0.09 | 0.63 ± 0.09 | 0.80 ± 0.08 |
| 11 Rlx 4h post | 0.77 ± 0.08 | 0.59 ± 0.07 | 1.97 ± 0.05 | 0.89 ± 0.09 | 1.67 ± 0.05 | 0.89 ± 0.08 | 2.28 ± 0.13 | 1.45 ± 0.08 | 2.23 ± 0.09 | 1.19 ± 0.07 | 2.11 ± 0.05 |
| 12 Rlx 12h&1h pre+4h post | 0.45 ± 0.05 | 0.36 ± 0.05 | 1.24 ± 0.08 | 0.45 ± 0.04 | 0.70 ± 0.05 | 0.38 ± 0.06 | 0.90 ± 0.05 | 0.60 ± 0.05 | 1.20 ± 0.05 | 0.61 ± 0.07 | 0.83 ± 0.06 |
Groups 3&4 that developed severe AP presented as main features leukocyte infiltrate, hemorrhage and acinar and fat necrosis and the histopathological scores were higher. Groups 10 & 12 that received relaxin 12 and/or 1h pre and 4h post surgery developed a mild AP and exhibited the lowest scores being the main feature edema. Homogenous groups for pancreatic edema, hemorrhage, acinar and fat necrosis and pancreatic and lung leukocyte infiltrate: :1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P < 0.05. Rlx: relaxin, LN: L-NAME, MIF: mifepristone.
General AP histopathology Groups with severe AP presented parenchymal necrosis with neutrophil infiltrate, fat necrosis, congestive blood vessels in peri-pancreatic fat, and intense hemorrhage. The duodenum exhibited lysis in the mucosa, subserosal blood vessel congestion and severe neutrophil infiltrate but there was no evidence of possible leakage towards the cephalic segment. These groups exhibited alterations in the liver, lung, kidneys, spleen and suprarenal glands. The liver showed necrosis, hemorrhage, blood vessel congestion, neutrophil infiltrate and steatosis. The lungs were highly compromised exhibiting congestion of big blood vessels and alveolar capillaries with neutrophil infiltrate; the bronchial wall had reactive lymphoid hyperplasia and aspirative hemorrhage. The main features observed in the kidneys were congestion of cortical and medullar blood vessels and glomerular capillaries jointly with tubular and medullar acute necrosis. Suprarenal glands were characterized by edema and partial lipid loss while the spleen presented edema.
Caspases activity (2-3-8-9)
We next examined if treatment of pancreatic injury was associated to the activation of caspases 2-3-8-9 in the pancreas as primary site of injury and in the lung and the liver as relevant affected distant organs. Homogenous groups (10-12) that developed a mild acute pancreatitis manifested a greater activation of caspases 2-3-8-9 suggesting that the activity of both the initiator caspase 8 and the executor caspase 3 together with caspases 9 and 2 involved in the apoptosome or intrinsic pathways are stimulated by relaxin treatment suggesting that apoptosis is more significant in these groups than in homogenous groups (5-6)-7-(3-4) that developed a severe necrotizing acute pancreatitis (Figure 3).
Figure 3.
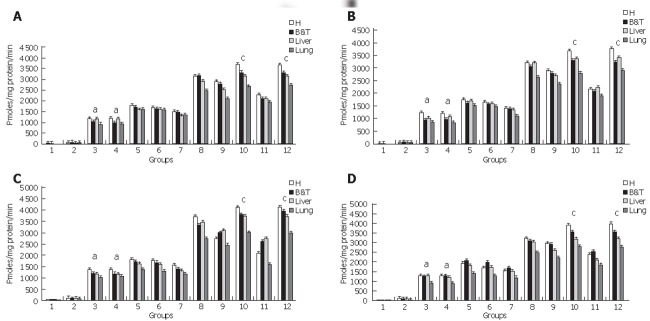
Caspase Activities. A: Caspase 2 activity was measured to evaluate the upstream activation of the intrinsic apoptotic pathway. Groups 10&12 presented a significant activation cP < 0.05 which was reduced for groups 3&4 aP < 0.05. Homogenous groups for caspase 2 activity are: 1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P < 0.05. H: pancreatic head, B&T: pancreatic body and tail. B: Caspase 3 Activity was measured to evaluate the activation of the executor caspase 3. Groups 10&12 presented a significant activation cP < 0.05 which was reduced for groups 3&4 aP < 0.05. Homogenous groups for caspase 2 activity are: 1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P < 0.05. H: pancreatic head, B&T: pancreatic body and tail. C: Caspase 8 Activity was measured to evaluate the activation of the initiator caspase 8. groups 10&12 presented a significant activation cP < 0.05 which was reduced for groups 3&4 aP < 0.05. Homogenous groups for caspase 8 activity are: 1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P < 0.05. H: pancreatic head, B&T: pancreatic body and tail. Results shown as mean ± SD. D: Caspase 9 Activity was measured to evaluate the activation of the intrinsic pathway .Groups 10&12 presented a significant activation cP < 0.05 which was reduced for groups 3&4 aP < 0.05. Homogenous groups for caspase 9 activity are: 1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P < 0.05. H: pancreatic head, B&T: pancreatic body and tail. Results are shown as mean ± SD.
Death due to apoptosis or necrosis
The next step was to elucidate whether relaxin treatment favored death by apoptosis over necrosis. Blood lactate dehydrogenase (LDH) is a marker of cellular injury and death. The raise in blood LDH due to acute pancreatitis was ameliorated in homogenous groups (10-12) (Figure 4A). To evaluate if the undergoing death process in the pancreas as primary site of injury and in the lung and the liver as relevant affected distant organs corresponded to apoptosis or necrosis we measured ATP concentration (Figure 4B) and then calculated the % of loss of ATP (Figure 4C) given the fact that the death process involves ATP consumption being an 85% or more ATP depletion associated to necrosis. Also the ADP/ATP ratio (Figure 4D) helped to differentiate between these two death mechanisms as a higher increase is indicative of necrosis while a lower one points towards apoptosis.
Figure 4.
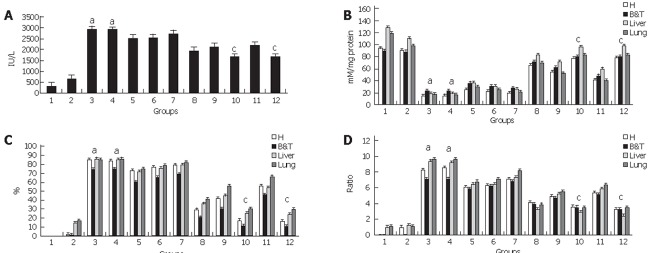
Apoptosis versus necrosis. A: Blood lactate dehydrogenase. As a marker of cellular injury and death significant high blood levels of LDH for groups 3&4 aP < 0.005 reflected the undergoing severe AP while groups 10&12 presented a significant reduction in the enzyme value cP < 0.005. Homogenous. Groups for LDH are:1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P < 0.005. B-D: ATP concentration. The significant decrease in ATP concentration that surpassed the 80% loss for groups 3&4 aP<0.005,is the hallmark of the necrotic process that is being developed while groups 10&12 that underwent apoptosis did not manifest such extreme values cP<0.005. This expression in the nucleotide levels is translated into the ADP/ATP ratio that is significantly increased aP<0.01 for groups 3&4 that underwent necrosis while groups 10&12 that underwent apoptosis had significantly lower values cP<0.05. Homogenous Groups are: 1-2-(12-10)-8-9-11-(5-6-7)-(3-4), P<0.005. Results are shown as mean ± SD.
ATP depletion for homogenous groups (5-6)-7-(3-4) associated to a higher % of ATP loss and ADP/ATP ratio correlated (R = 0.842; P < 0.05) with the higher frequency observed in necrotic foci and severity indicators for these groups together with the elevated LDH value suggesting that for groups undergoing severe acute pancreatitis necrosis was the main form of cell death.
Homogenous Groups (10-12) that manifested a mild acute pancreatitis evidenced lesser ATP depletion with a % of ATP loss lower than 40% together with a lower ADP/ATP ratio. These results correlated with lower values for LDH (R = 0.798, P < 0.05) while edema was the principal histopathological feature. This suggested that for these groups apoptosis was the main form of cell death resulting in a more controlled process with less pro-inflammatory stimulation ameliorating the aggressiveness of the pathology.
Increased degree of severity
For each variable with significant differences between the groups’ mean values, the relationship of these results were compared to the reference baseline level established in group 1. We then integrated this information to rank the groups in order of “increased degree of severity” (Table 5) showing that the severe acute pancreatitis developed by homogenous groups (3-4) was significantly ameliorated for homogenous groups (10-12).
Table 5.
Order of groups according to the increase in the severity degree
| GROUP NR. | GROUP DESCRIPTION |
| 1 | Basal Reference Control |
| 2 | Surgery Control |
| 12 | Relaxin administered 12h & 1h pre- BPDOE-CDLs and 4h post-BPDOE-CDLs |
| 10 | Relaxin administered 1h pre- BPDOE-CDLs and 4h post-BPDOE-CDLs |
| 8 | Relaxin administered 1h pre-BPDOE-CDLs |
| 9 | Relaxin administeres 1h post-BPDOE-CDLs |
| 11 | Relaxin administered 4h post-BPDOE-CDLs |
| 5 | Relaxin and L-NAME administered 1h pre- BPDOE-CDLs and 4h post-BPDOE-CDLs |
| 6 | Relaxin and Mifepristone administered 1h pre- BPDOE-CDLs and 4h post-BPDOE-CDLs |
| 7 | Relaxin, L-NAME & Mifepristone administered 1h pre- BPDOE-CDLs and 4h post-BPDOE-CDLs |
| 3 | BPDOE-CDLs AP model |
| 4 | Vehicle (PBS) control |
The BPDOE-CDLs model induces a severe AP (group 3).Prophylactic treatment with relaxin, as shown for groups10&12, markedly reduces the severity of the pathology, being still useful although in a lesser degree even after AP was triggered.
DISCUSSION
According to the severity degree exhibited by acute pancreatitis will be the complexity of the pathology involving other organs apart from the pancreas that will lead to metabolic, liver, kidney and lung alterations reflected in the histopathology and numerous biochemical parameters that require to be assessed in order to arrive to a proper classification of the disease.
It has been reported that relaxin can stimulate the nitric oxide pathway or the glucocorticoid receptor[7,14]. In our work we have evaluated the combined vasorelaxing and anti-inflammatory properties of relaxin on the evolution of acute pancreatitis, our results show that relaxin plays a highly beneficial role when administered within the first hour of the initiation of the noxa being this even more pronounced when the drug was given 1h pre and 4h post triggering acute pancreatitis, this would be particularly relevant for the prevention of post-ERCP pancreatitis providing basis for future studies where combined drug treatments can be considered. When relaxin was received 4h after unchaining acute pancreatitis although still beneficial the efficiency of the treatment was reduced the reason probably being that additional supplemental therapies are required at this stage in order to restore the already altered hemodynamic and metabolic balance. The positive effect exerted by relaxin was significantly diminished by L-NAME and by mifepristone indicating that the nitric oxide pathway and glucocorticoid receptor stimulation are involved. The association of both inhibitors produced an even stronger reduction in the effect induced by relaxin however this group still presented less severe results than those observed for non-treated acute pancreatitis suggesting that there may be other pathways implicated.
While apoptosis is a programmed and controlled mechanism of cell death that is associated to reduced inflammation, necrosis, caused by irreparable cell injury causing membrane alterations that favor the release of enzymes that are responsible for degrading cellular structures and thus activating the inflammatory response[25]. Pancreatic hemorrhage and necrosis characterize severe acute pancreatitis being the consequence of dysfunctional tissue irrigation due to an initial hypoxia leading to an ischemia-reperfusion process due to alternative vasoconstriction followed by vasodilatation. These microcirculatory disturbances are linked to neutrophil activation and inflammation together with cellular stress that will convert cell death by apoptosis to necrosis, downregulating these factors by preserving pancreatic microvascular blood flow to ensure adequate tissue oxygenation and inhibiting pro-inflammatory mediators reduces the risk of necrotizing pancreatitis by favoring cell death by apoptosis over necrosis.
The fact that the severity of acute pancreatitis is inversely related to the degree of apoptosis suggests that this type of cell death may be a beneficial response to acinar cell injury particularly in acute pancreatitis. Clearly, the degree of inflammation and, therefore, the severity of pancreatitis are less in situations associated with acinar cell apoptosis than under conditions associated with acinar cell necrosis. The group of Steer[25] emphasizes then the notion that medications or other interventions that favor the development of apoptosis may minimize the severity of pancreatitis, and they could, therefore be of substantial clinical value. Together with Jones and Gores[26] they remark the concept that pharmacological induction of pancreatic apoptosis during the early stages of acute pancreatitis provides a relevant therapeutic strategy for the treatment of this disease.
The high density of neurons embedded in the pancreatic cephalic segment associated to the intimate relation between the peri-vaterian duodenum and the pancreas linked by the gastroduodenal plexus[27-29] emphasizes the relevance of the rich population of receptors in the peri-vaterian duodenum that when activated by irritative stimuli such as endoscopic maneuvers will unchain duodenopancreatic and duodenogastric autonomic-arc-reflexes. The BPDOE-CDLs model mimics this situation in which severe acute pancreatitis results from a sympathetic neurogenic overstimlaution that causes adrenergic hyper-reactivity responsible for the initial vasoconstriction and the subsequent ischemia/reperfusion injury. We propose that relaxin would, in first instance, counteract vasoconstriction arising from sympathetic-adrenergic stimulation by inducing vasodilatation through the nitric oxide pathway and exert anti-inflammatory properties by glucocorticoid receptor stimulation while favoring apoptosis over necrosis.
Severe acute pancreatitis induced by the BPDOE-CDLs model manifested kidney failure translated by the significant increase in urea and creatinine in blood associated to acute tubular necrosis; metabolic and liver alterations. The raise in severity factors indicating an intense inflammatory response defined by the increase in CRP and leukocyte count that correlated with neutrophil infiltrate in pancreatic and lung tissue associated to high activity values for MPO in these tissues suggested a SIRS in which the activation of neutrophils converted cellular death by apoptosis to necrosis as suggested by the high necrosis score in pancreatic tissue and the elevation of blood LDH describing organ injury. For this group caspases 2-3-8 and 9 activities were significantly reduced when comparing them to treated homogenous groups (10-12). The dramatic depletion in ATP concentration, correlated with a loss of ATP that surpassed the 80 % thus inhibiting the initiation of the apoptotic pathway, and the increase in ADP/ATP ratio would be indicating a strong necrotic mechanism associated to a pro-inflammatory process as corroborated by the histopathology.
Groups that were treated with relaxin showed an improvement in blood metabolic parameters with reduced values for leukocyte count and CRP together with lower leukocyte infiltrate and MPO activity in pancreatic and lung tissue pointing to a milder inflammatory response. The increase in the activity of caspases 2-3-8 and 9 manifesting that both the intrinsic and the effector apoptotic pathways are activated is associated to a less drastic depletion of ATP that would not interfere with apoptosis and thus exhibit a reduced inflammatory reaction. Treatment with relaxin 4h after unchaining acute pancreatitis was beneficial however the best results were observed when relaxin was given within the first hour being optimal when administered 1h before and 4h after triggering acute pancreatitis as quite probably in this circumstance it could preserve microcirculation ensuring tissue oxygenation while inhibiting the expression of pro-inflammatory mediators associated with pro-apoptotic molecules, situation that is kept-up by the second dose, these results are relevant for the prevention of post-ERCP acute pancreatitis. There was no significant difference between homogenous groups 10 and 12 implying that starting treatment 12h or 1h prior to triggering acute pancreatitis exerts the same effect.
To evaluate the pathways involved in treatment with relaxin we associated the administration of relaxin 1h pre and 4h post BPDOE-CDLs procedure with L-NAME (NOS inhibitor) and/or mifepristone (glucocorticoid recptor antagonist). The inhibition of either the nitric oxide pathway or glucocorticoid receptor stimulation significantly reduced the positive effects described previously for homogenous groups (10-12). Surprisingly for group 7 when we associated both inhibitors the severity that was developed although greater than for homogenous groups (6-7) was still less than that displayed by homogenous groups (3-4) suggesting that there is another pathway involved in the treatment with relaxin.
We therefore conclude that relaxin plays a beneficial role in the evolution of acute pancreatitis triggered by the BPDOE-CDLs model as it induces an anti-inflammatory response via glucocorticoid receptor stimulation while favoring apoptosis over necrosis; simultaneously it helps to preserve pancreatic microcirculation by the nitric oxide pathway ensuring an appropriate blood flow and tissue oxygenation thus avoiding ischemia and the consequent ischemia/reperfusion injury while it reduces the expression and activation of pro-inflammatory factors and decreases neutrophil infiltration of the pancreas.
ACKNOWLEDGMENTS
We gratefully acknowledge Delia Garrido, PhD, for her technical assistance in statistics and Irene Sorin, MD for her assistance with the histopathological evaluation.
Footnotes
Supported by a research grant given to Dr Laura Iris Cosen-Binker by GlaxoSmithKline S.A.
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
References
- 1.Banks P. Acute Pancreatitis: Clinical presentation, in : Go V, Gardener J, Brooks F, Lebenthal E, Di Magno E, Scheele G - The Exocrine Pancreas: Biology, Pathobiology and Disease- 2oEdition- New York-USA: Raven Press. 1993:475–480. [Google Scholar]
- 2.Wilson C, Imrie CW, Carter DC. Fatal acute pancreatitis. Gut. 1988;29:782–788. doi: 10.1136/gut.29.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YK, Abdulian JD, Escalante-Glorsky S, Youssef AI, Foliente RL, Collen MJ. Clinical outcome of post-ERCP pancreatitis: relationship to history of previous pancreatitis. Am J Gastroenterol. 1995;90:2120–2123. [PubMed] [Google Scholar]
- 4.Van Os EC, Petersen BT. Pancreatitis secondary to percutaneous liver biopsy-associated hemobilia. Am J Gastroenterol. 1996;91:577–580. [PubMed] [Google Scholar]
- 5.Sherman S, Lehman GA. ERCP- and endoscopic sphincterotomy-induced pancreatitis. Pancreas. 1991;6:350–367. doi: 10.1097/00006676-199105000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Cosen-Binker LI, Binker MG, Negri G, Tiscornia O. Acute pancreatitis possible initial triggering mechanism and prophylaxis. Pancreatology. 2003;3:445–456. doi: 10.1159/000074972. [DOI] [PubMed] [Google Scholar]
- 7.Tiscornia OM, Hamamura S, Lehmann ES, Otero G, Waisman H, Tiscornia-Wasserman P, Bank S. Biliary acute pancreatitis: a review. World J Gastroenterol. 2000;6:157–168. doi: 10.3748/wjg.v6.i2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFadden DW. Organ failure and multiple organ system failure in pancreatitis. Pancreas. 1991;6 Suppl 1:S37–S43. doi: 10.1097/00006676-199101001-00007. [DOI] [PubMed] [Google Scholar]
- 9.Bani D. Relaxin: a pleiotropic hormone. Gen Pharmacol. 1997;28:13–22. doi: 10.1016/s0306-3623(96)00171-1. [DOI] [PubMed] [Google Scholar]
- 10.Perna AM, Masini E, Nistri S, Briganti V, Chiappini L, Stefano P, Bigazzi M, Pieroni C, Bani Sacchi T, Bani D. Novel drug development opportunity for relaxin in acute myocardial infarction: evidences from a swine model. FASEB J. 2005;19:1525–1527. doi: 10.1096/fj.04-3664fje. [DOI] [PubMed] [Google Scholar]
- 11.Failli P, Nistri S, Quattrone S, Mazzetti L, Bigazzi M, Sacchi TB, Bani D. Relaxin up-regulates inducible nitric oxide synthase expression and nitric oxide generation in rat coronary endothelial cells. FASEB J. 2002;16:252–254. doi: 10.1096/fj.01-0569fje. [DOI] [PubMed] [Google Scholar]
- 12.Masini E, Nistri S, Vannacci A, Bani Sacchi T, Novelli A, Bani D. Relaxin inhibits the activation of human neutrophils: involvement of the nitric oxide pathway. Endocrinology. 2004;145:1106–1112. doi: 10.1210/en.2003-0833. [DOI] [PubMed] [Google Scholar]
- 13.Nistri S, Chiappini L, Sassoli C, Bani D. Relaxin inhibits lipopolysaccharide-induced adhesion of neutrophils to coronary endothelial cells by a nitric oxide-mediated mechanism. FASEB J. 2003;17:2109–2111. doi: 10.1096/fj.03-0216fje. [DOI] [PubMed] [Google Scholar]
- 14.Masini E, Bani D, Bigazzi M, Mannaioni PF, Bani-Sacchi T. Effects of relaxin on mast cells. In vitro and in vivo studies in rats and guinea pigs. J Clin Invest. 1994;94:1974–1980. doi: 10.1172/JCI117549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bani D, Bigazzi M, Masini E, Bani G, Sacchi TB. Relaxin depresses platelet aggregation: in vitro studies on isolated human and rabbit platelets. Lab Invest. 1995;73:709–716. [PubMed] [Google Scholar]
- 16.Dschietzig T, Bartsch C, Greinwald M, Baumann G, Stangl K. The pregnancy hormone relaxin binds to and activates the human glucocorticoid receptor. Ann N Y Acad Sci. 2005;1041:256–271. doi: 10.1196/annals.1282.039. [DOI] [PubMed] [Google Scholar]
- 17.Tiscornia O, Celener D, Vaccaro MI, Cresta D, Waisman H. Biliary Acute Pancreatitis: Role of Autonomous Nervous System and Disruption of Entero-Pancreatic-Feedback. Pren Med Arg. 1988;85:494–503. [Google Scholar]
- 18.Ermak T, Grendell J, Brandoug L. Anatomy, Embriology and Developmental Anomalies, in: Slessinger M, Fordtram J. Gastrointestinal Disease-3� Edition - Philadelphia, Pennsylvania, USA: WB Saunders Company. 1983:1415–1425. [Google Scholar]
- 19.LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Trush MA, Egner PA, Kensler TW. Myeloperoxidase as a biomarker of skin irritation and inflammation. Food Chem Toxicol. 1994;32:143–147. doi: 10.1016/0278-6915(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 21.Negri G, Cosen-Binker LI, Bustos D, Tiscornia M. Myeloperoxidase (MPO) activity : a correlation with the inflammation degree in an experimental model of acute pancreatitis in the rat. Clin Chem Lab Med. 2001;39:Special Supplement S248. [Google Scholar]
- 22.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 23.Fortunato F, Deng X, Gates LK, McClain CJ, Bimmler D, Graf R, Whitcomb DC. Pancreatic response to endotoxin after chronic alcohol exposure: switch from apoptosis to necrosis? Am J Physiol Gastrointest Liver Physiol. 2006;290:G232–G241. doi: 10.1152/ajpgi.00040.2005. [DOI] [PubMed] [Google Scholar]
- 24.Winer B, Brown D, Michels K: Statistical principles in experimental design. Mc Graw-Hill Book Company-New York, USA. 1991 [Google Scholar]
- 25.Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 26.Jones BA, Gores GJ. Physiology and pathophysiology of apoptosis in epithelial cells of the liver, pancreas, and intestine. Am J Physiol. 1997;273:G1174–G1188. doi: 10.1152/ajpgi.1997.273.6.G1174. [DOI] [PubMed] [Google Scholar]
- 27.Salvioli B, Bovara M, Barbara G, De Ponti F, Stanghellini V, Tonini M, Guerrini S, Cremon C, Degli Esposti M, Koumandou M, et al. Neurology and neuropathology of the pancreatic innervation. JOP. 2002;3:26–33. [PubMed] [Google Scholar]
- 28.Tiscornia OM, Dreiling DA, Yacomotti J, Kurtzbart R, de La Torre A, Farache S. Neural control of the exocrine pancreas: an analysis of the cholinergic, adrenergic, and peptidergic pathways and their positive and negative components. 1: Neural mechanisms. Mt Sinai J Med. 1987;54:366–383. [PubMed] [Google Scholar]
- 29.Tiscornia OM, Dreiling DA, Yacomotti J, Kurtzbart R, de la Torre A, Farache S. Neural control of the exocrine pancreas: an analysis of the cholinergic, adrenergic, and peptidergic pathways and their positive and negative components. 2. Integration of neural and hormonal mechanisms. Mt Sinai J Med. 1988;55:126–131. [PubMed] [Google Scholar]


