Abstract
A 79-year-old female patient with hepatitis C virus-related liver cirrhosis was diagnosed as having hepatocellular carcinoma (HCC) with a diameter of 2.0 cm. She refused therapy for HCC. Nine months after the diagnosis, she developed dermatomyositis when the HCC enlarged to a diameter of 6.0 cm. She underwent therapy for dermatomyositis, and then transcatheter arterial chemoembolization for HCC. Although the manifestations of dermatomyositis improved and entire tumor necrosis was achieved, she died of pneumonia 2 mo after the treatment of HCC. HCC and/or chronic hepatitis C virus infection might be involved in the pathogenesis of dermatomyositis.
Keywords: Dermatomyositis, Hepatocellular carcinoma, Hepatitis C virus
INTRODUCTION
The cause of dermatomyositis remains unknown. However, some factors have been implicated in the pathogenesis of dermatomyositis. Many reports on the relationship between dermatomyositis and cancer or viral infection have been published[1-17]. We report herein a rare case of dermatomyositis that occurred during the natural course of hepatocellular carcinoma (HCC) in an elderly female patient with hepatitis C virus (HCV)-related liver cirrhosis.
CASE REPORT
A 79-year-old woman was treated for HCV-related liver cirrhosis at our hospital. She received medication for the liver disease (ursodeoxycholic acid) and for epilepsy (clonazepam) due to a past head injury. There was no family history of autoimmune disease or malignancy. In October 2003, a hepatic tumor with a diameter of 2.0 cm in the right anterosuperior segment was detected on dynamic computed tomography (CT): the tumor was shown to be a hypodense lesion in the basal study which was enhanced during the arterial phase and became hypodense during the delayed phase. The serum alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) levels were 3.4 ng/mL (normal, <10 ng/mL) and 431 mAU/mL (normal, <40 mAU/mL), respectively. Based on these findings, the tumor was diagnosed as HCC. However, the HCC was not treated at that time because the patient refused therapy.
In July 2004, she began to develop progressive muscle weakness in the extremities and systemic erythema. She was admitted to the Department of Dermatology at our hospital. On admission, her height was 146 cm and her body weight was 60 kg. A heliotrope rash in the periorbital skin, Gottron papules on the metacarpophalangeal joints, and poikiloderma on the anterior neck and upper chest were observed. There was no skin thickening of the extremities or joint swelling. Muscle examination revealed symmetrical proximal weakness: she could not rise from a chair or raise her hands up to the level of her shoulders. Table 1 shows her laboratory data on admission. Elevated serum muscle enzymes were observed. HBsAg (enzyme immunoassay [EIA], Dinabot, Tokyo, Japan) was negative. Anti-HCV antibody (second generation, EIA, Dinabot) was positive with a high titer, and HCV RNA (reverse transcription-PCR, Sionogi, Osaka, Japan) was also positive. Hypergammaglobulinemia was observed. Antinuclear antibodies were positive (1:640, homogeneous, speckled), while rheumatoid factor, myeloperoxidase antineutrophil cytoplasmic antibodies, anti-DNA, anti-Jo-1, anti-RNP, anti-SSA, and anti-SSB were all negative. Electromyography of the right biceps brachii muscle showed fibrillation and low amplitude voltage of a short duration (less than 200 mV). Skin biopsy of the left forearm showed flattened epidermis, partial liquefaction of the basal layer, scattered inflammatory cell infiltration around the small vessels, and degeneration of collagen fiber of the dermis (Figure 1). Muscle biopsy of the left triceps failed to show remarkable findings. On the basis of these findings, the diagnosis of dermatomyositis was made. Initially, 50 mg of prednisolone per day was administered, and then the dosage was gradually decreased to 15 mg/d. Skin eruption almost disappeared completely and muscle power slowly improved. Muscle enzymes also improved to the normal range. During the treatment of dermatomyositis, the patient decided to undergo therapy for the HCC.
Table 1.
Laboratory data on admission
| Range | Range | ||||||
| WBC | 3190 | /mm3 | 3500-8500 | CPK | 2092 | IU/L | 46-160 |
| RBC | 401×104 | /mm3 | 380×104-480×104 | aldolase | 13 | IU/L | 2.5-6.2 |
| Hb | 13.7 | g/dL | 11.8-15.0 | myoglobin | 293 | ng/mL | ≤ 60 |
| Plt | 9.0×104 | /mm3 | 13.0-30.0×104 | ||||
| PT | 11.6 | s | 10.0-13.0 | IgG | 2027 | mg/dL | 870-1700 |
| CRP | 0.08 | mg/dL | 0-0.4 | ||||
| AST | 150 | IU/L | ≤ 35 | ||||
| ALT | 39 | IU/L | ≤ 35 | HBsAg | (-) | ||
| LDH | 583 | IU/L | ≤ 230 | anti-HCV | (+) | ||
| ALP | 200 | IU/L | ≤ 340 | HCV RNA | (+) | ||
| Γ-GTP | 20 | IU/L | ≤ 55 | ||||
| T-Bil | 0.8 | mg/dL | 0.2-1.2 | ANA | × 640 | ||
| ChE | 192 | IU/L | 185-431 | HO, SP | |||
| T-CHO | 170 | mg/dL | ≤ 220 | RF | (-) | ||
| TP | 5.7 | g/dL | 6.5-8.0 | MPO-ANCA | (-) | ||
| Alb | 2.7 | g/dL | 4.2-5.0 | anti-Jo-1 | (-) | ||
| BUN | 15.3 | mg/dL | 9.0-20.0 | anti-DNA | (-) | ||
| Cr | 0.6 | mg/dL | 0.4-1.1 | anti-RNP | (-) | ||
| FBS | 96 | mg/dL | ≤ 110 | anti-SSA | (-) | ||
| Ca | 8 | mg/dL | 8.5-10.2 | anti-SSB | (-) |
Figure 1.

Hematoxylin-eosin staining of skin biopsy of the left forearm showing flattened epidermis and scattered inflammatory cell infiltration around the small vessels of the dermis (200 × magnifications).
When she was referred to the Department of Internal Medicine in August 2004, the HCC nodule was enlarged to a maximal diameter of 6.0 cm, and ascites was observed (Figure 2). The serum AFP and DCP levels also increased to 2 130.2 ng/mL and 7 423 mAU/mL, respectively. Other serum tumor markers, including carcinoembryonic antigen, carbohydrate antigen 19-9, and carbohydrate antigen 125, were all negative. No other malignancies were identified by imaging studies, such as ultrasonography and CT or esophagogastroduodenal endoscopy. In August 2004, she underwent transcatheter arterial chemoembolization for the treatment of the HCC after the ascites was controlled. After transcatheter arterial chemoembolization, entire tumor necrosis was achieved; the serum AFP and DCP levels decreased to 46.7 ng/mL and 54 mAU/mL, respectively. The clinical course of dermatomyositis was not affected by the embolization. Unfortunately, the patient died of bacterial pneumonia in October 2004.
Figure 2.
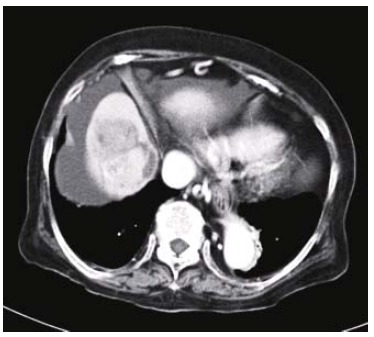
Contrast-enhanced computed tomography showing a hypervascular, in part, necrotic tumor in the right anterosuperior segment of the liver and ascites.
DISCUSSION
The relationship between cancer and dermatomyositis has been widely reported in the literature[1-9]. Recent population-based studies have demonstrated that 15-32% of patients with dermatomyositis are associated with various types of cancers, such as ovarian, lung, pancreatic, stomach, and colorectal cancers[1,2]. However, there have been few reports on the association between dermatomyositis and HCC[3-9]. Although the rare association might be fortuitous, a recent report described the improvement of dermatomyositis without the need of corticosteroids after the resection of HCC, supporting the hypothesis that HCC can cause dermatomyositis through a paraneoplastic mechanism[8]. In the present case, dermatomyositis occurred when the HCC increased from a baseline diameter of 2.0 cm to 6.0 cm at 10 mo. In most other reported cases, advanced HCC, more than 6.0 cm in diameter or multiple, has been found at the diagnosis of dermatomyositis[4-9]. Those reports indicate that advanced HCC might induce an autoimmune response as the trigger of dermatomyositis.
Recent studies have demonstrated that antibodies to Mi-2, a component of the nucleosome remodeling-deacetylase complex, are strongly associated with dermatomyositis (frequencies up to 31%)[18,19]. An in vitro study has shown that a 169-bp cDNA product, which is 88.8% homologous to the human Mi-2beta antigen, is identified in H4IIE rat hepatoma cells[20]; and 100% homology is found at the protein level. Based on these findings, anti-Mi-2 antibodies might be cross-reactive with HCC. Unfortunately, anti-Mi-2 antibodies were not examined in the present case. Further studies on autoantibodies cross-reacting with HCC should be undertaken to shed light on the pathogenesis of dermatomyositis associated with HCC.
Occasional associations between dermatomyositis and HCV infection have been reported[6,7,9-14]. Thus far, there has been no direct evidence that HCV infection can cause dermatomyositis. A recent preliminary study has shown no significant higher incidence of HCV in patients with dermatomyositis as compared with the control population[10]. However, chronic HCV infection has been known to be associated with the presence of autoantibodies and various types of autoimmune diseases, leading to the hypothesis that chronic HCV infection could cause dermatomyositis through an autoimmune mechanism[21]. In the present case, antinuclear antibodies were strongly positive, suggesting that there might be some immune disorders related to the development of dermatomyositis. We speculate that antibodies against HCV or HCV-enzyme complex might cross-react with homologous area of host proteins and thus cause autoimmune diseases including dermatomyositis. Crowson et al[22] have reported that HCV RNA is expressed in a focal, weak fashion in endothelia and perivascular inflammatory cells in some HCV-infected patients associated with cutaneous eruptions. They postulated that parasitism by HCV can render endothelia autoantigenic through exposure of cryptic antigens via virally induced endothelia cell injury. Histopathologic examinations of HCV expression using skin biopsy specimens from patients with dermatomyositis associated with HCV infection might provide information on the role of HCV in the pathogenesis of dermatomyositis.
In the present case, other infectious agents including echovirus, adenovirus, and coxsackievirus, which are known to cause myositis, were not examined[15-17]. Therefore, there remains the possibility that dermatomyositis of the present case might be caused by such viruses.
In summary, this report suggests that HCC, in particular advanced HCC, and/or chronic HCV infection might be factors in the pathogenesis of dermatomyositis. The mechanisms of autoimmune responses induced by such factors should be studied in the future.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Liu WF
References
- 1.Sigurgeirsson B, Lindelöf B, Edhag O, Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–367. doi: 10.1056/NEJM199202063260602. [DOI] [PubMed] [Google Scholar]
- 2.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, Evans SR, Felson DT. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 3.Wong KO. Dermatomyositis: a clinical investigation of twenty-three cases in Hong Kong. Br J Dermatol. 1969;81:544–547. doi: 10.1111/j.1365-2133.1969.tb16031.x. [DOI] [PubMed] [Google Scholar]
- 4.Gray RG, Altman RD, Gottlieb NL. Aberrant serum enzyme patterns in dermatomyositis associated with hepatoma. J Rheumatol. 1976;3:227–232. [PubMed] [Google Scholar]
- 5.Horie Y, Yamada M, Nakai K, Kawasaki H, Hirayama C, Matsui K, Kambe N, Shimao S. Combined hepatocellular-cholangiocarcinoma associated with dermatomyositis. J Gastroenterol Hepatol. 1989;4:101–104. doi: 10.1111/j.1440-1746.1989.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 6.Gomez A, Solans R, Simeon CP, Selva A, Garcia F, Fonollosa V, Vilardell M. Dermatomyositis, hepatocarcinoma, and hepatitis C: comment on the article by Weidensaul et al. Arthritis Rheum. 1997;40:394–395. doi: 10.1002/art.1780400232. [DOI] [PubMed] [Google Scholar]
- 7.Inuzuka M, Tomita K, Tokura Y, Takigawa M. Acquired ichthyosis associated with dermatomyositis in a patient with hepatocellular carcinoma. Br J Dermatol. 2001;144:416–417. doi: 10.1046/j.1365-2133.2001.04040.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng TI, Tsou MH, Yang PS, Sung SM, Chuang VP, Sung JL. Dermatomyositis and erythrocytosis associated with hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:1239–1240. doi: 10.1046/j.1440-1746.2002.t01-1-02851.x. [DOI] [PubMed] [Google Scholar]
- 9.Kee KM, Wang JH, Lee CM, Changchien CS, Eng HL. Chronic hepatitis C virus infection associated with dermatomyositis and hepatocellular carcinoma. Chang Gung Med J. 2004;27:834–839. [PubMed] [Google Scholar]
- 10.Nishikai M, Miyairi M, Kosaka S. Dermatomyositis following infection with hepatitis C virus. J Rheumatol. 1994;21:1584–1585. [PubMed] [Google Scholar]
- 11.Fiore G, Giacovazzo F, Giacovazzo M. HCV and dermatomyositis: report of 5 cases of dermatomyositis in patients with HCV infection. Riv Eur Sci Med Farmacol. 1996;18:197–201. [PubMed] [Google Scholar]
- 12.Moccia F. Autoimmune thrombocytopenic purpura and dermatomyositis associated with chronic hepatitis C. A therapeutic dilemma. Ann Ital Med Int. 1998;13:240–243. [PubMed] [Google Scholar]
- 13.Nakamura K, Matsumori A, Kusano KF, Banba K, Taniyama M, Nakamura Y, Morita H, Matsubara H, Yamanari H, Ohe T. Hepatitis C virus infection in a patient with dermatomyositis and left ventricular dysfunction. Jpn Circ J. 2000;64:617–618. doi: 10.1253/jcj.64.617. [DOI] [PubMed] [Google Scholar]
- 14.Germany RE, Cohen SM. Hepatitis C, collagenous colitis, and dermatomyositis occurring in the same patient. Am J Gastroenterol. 2002;97:1848–1849. doi: 10.1111/j.1572-0241.2002.05868.x. [DOI] [PubMed] [Google Scholar]
- 15.Mease PJ, Ochs HD, Wedgwood RJ. Successful treatment of echovirus meningoencephalitis and myositis-fasciitis with intravenous immune globulin therapy in a patient with X-linked agammaglobulinemia. N Engl J Med. 1981;304:1278–1281. doi: 10.1056/NEJM198105213042107. [DOI] [PubMed] [Google Scholar]
- 16.Mikol J, Felten-Papaiconomou A, Ferchal F, Perol Y, Gautier B, Haguenau M, Pepin B. Inclusion-body myositis: clinicopathological studies and isolation of an adenovirus type 2 from muscle biopsy specimen. Ann Neurol. 1982;11:576–581. doi: 10.1002/ana.410110605. [DOI] [PubMed] [Google Scholar]
- 17.Bowles NE, Dubowitz V, Sewry CA, Archard LC. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987;1:1004–1007. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- 18.Targoff IN, Reichlin M. The association between Mi-2 antibodies and dermatomyositis. Arthritis Rheum. 1985;28:796–803. doi: 10.1002/art.1780280711. [DOI] [PubMed] [Google Scholar]
- 19.Roux S, Seelig HP, Meyer O. Significance of Mi-2 autoantibodies in polymyositis and dermatomyositis. J Rheumatol. 1998;25:395–396. [PubMed] [Google Scholar]
- 20.Hammermann R, Warskulat U, Haussinger D. Anisoosmotic regulation of the Mi-2 autoantigen mRNA in H4IIE rat hepatoma cells and primary hepatocytes. FEBS Lett. 1998;435:21–24. doi: 10.1016/s0014-5793(98)01030-8. [DOI] [PubMed] [Google Scholar]
- 21.McMurray RW, Elbourne K. Hepatitis C virus infection and autoimmunity. Semin Arthritis Rheum. 1997;26:689–701. doi: 10.1016/s0049-0172(97)80005-4. [DOI] [PubMed] [Google Scholar]
- 22.Crowson AN, Nuovo G, Ferri C, Magro CM. The dermatopathologic manifestations of hepatitis C infection: a clinical, histological, and molecular assessment of 35 cases. Hum Pathol. 2003;34:573–579. doi: 10.1016/s0046-8177(03)00193-x. [DOI] [PubMed] [Google Scholar]


