Abstract
Helicobacter pylori (H pylori) has been etiologically linked to gastric cancer. H pylori infection is more frequent in less developed Asian countries like India, Bangladesh, Pakistan, and Thailand and is acquired at early age than in more developed Asian countries like Japan and China. Frequency of gastric cancer, however, is very low in India, Bangladesh, Pakistan and Thailand compared to that in Japan and China. Similar enigma has been reported from Africa as compared to the West. Seroprevalence of H pylori infection in adult populations of India, Bangladesh, Pakistan and Thailand varies from 55% to 92%. In contrast, seroprevalence of H pylori in Chinese and Japanese adults is 44% and 55%, respectively. Annual incidence rate of gastric cancer in India, Bangladesh, and Thailand is 10.6, 1.3, 7.1 per 100 000 populations, respectively; in contrast, that in China and Japan is 32-59 and 80-115 per 100 000 populations, respectively. Several studies from India failed to show higher frequency of H pylori infection in patients with gastric cancer than controls. Available evidences did not support difference in H pylori strains as an explanation for this enigma. Despite established etiological role of H pylori, situation is somewhat enigmatic in Asian countries because in countries with higher frequency of infection, there is lower rate of gastric cancer. Host’s genetic make-up and dietary and environmental factors might explain this enigma. Studies are urgently needed to solve this issue.
Keywords: Stomach cancer, Helicobacter pylori, Tropical countries, Carcinogenesis, Infectious diseases
INTRODUCTION
Helicobacter pylori (H pylori) is a major cause of gastroduodenal diseases like peptic ulcer and an important risk factor for gastric carcinoma (GC) and primary gastric lymphoma (PGL). Evidences supporting the etiological role of H pylori in GC and PGL include higher frequency of isolation of H pylori in patients with GC and PGL[1,2], regression or lower rate of occurrence or recurrence of the tumor in patients in whom the infection is eradicated[3-6], occasional reports of recurrence following re-infection[7,8] and development of tumor in patients[5,9] or animals[10,11] infected with the organism. Several meta-analyses also revealed a strong relationship between H pylori and GC and PGL[12,13]. Though the evidences available in literature support causal relationship between H pylori and GC and PGL, some interesting observations from the Asian countries make such causal relationship somewhat enigmatic[14-15]. Similar enigmatic situation has also been reported from Africa[16]. Here we have reviewed the available evidences on this issue and attempted to explain possible reasons for such an enigma.
FREQUENCY OF H PYLORI INFECTION IN DIFFERENT ASIAN COUNTRIES
Figure 1 shows seroprevalence of H pylori infection in different Asian countries[4,15,17-29]. Frequency of H pylori infection differs markedly in different countries. In the developing countries like India, Bangladesh, Pakistan and Thailand, infection with H pylori is more frequent among general population and is acquired at an early age. There are several studies from India that showed that H pylori is acquired by most people in early childhood[30]. Gill et al [31] from India showed that the prevalence of IgG and IgA antibodies to H pylori was 22%, 56% and 87% in 0-4, 5-9 and 10-19 years age groups, respectively. In contrast, in more industrialized and developed regions of Asia like Japan, China and Singapore, frequency of H pylori infection has been reported to be somewhat lower[14]. The prevalence of H pylori in the United States has decreased to approximately 10% in the white middle and upper class population of 50 years of age or younger[32]. As H pylori is transmitted by feco-oral route, overcrowding, poor sanitation, lower socioeconomic status and poor water supply are some of the major factors that result in higher frequency and lower age of acquisition of H pylori in less developed Asian countries[30,33] . Though frequency of H pylori as shown in the Figure 1 is not age-standardized, all these data are in adults. As H pylori is acquired in early childhood in most developing countries and later in life in most developed countries and as average life expectancy is higher in developed countries as compared to developing countries, age standardization of frequency of infection is unlikely to alter our conclusions.
Figure 1.
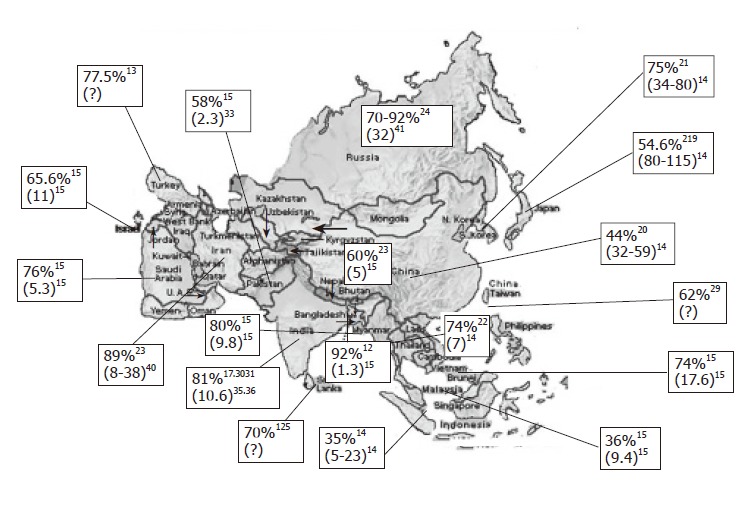
Map of Asia showing frequency of Helicobacter pylori infection in each country, crude annual incidence of gastric cancer per 100 000 populations (shown within parenthesis, where data are available). The reference from where data are obtained is shown in superscript. A: frequency in the year 1984; B: lower annual incidence of gastric cancer for Indians and higher values for Chinese living in Singapore; C: studies using PCR among patients with dyspepsia; #: annual-age standardized incidence rates; $: annual age-standardized death certification rate estimated from the graph that appeared in the report (year 1998-99).
FREQUENCY OF GASTRIC CANCER IN DIFFERENT ASIAN COUNTRIES
Gastric cancer is the world’s second commonest malignancy, having been overtaken only by lung cancer in 1980's[34]. There is a marked international variation in gastric cancer incidence with highest rates reported from Japan. Figure 1 shows annual incidence of gastric cancer per 100 000 populations from Asian countries[14,15,35-41]. It is interesting to note that despite Japan being a developed country with a lower frequency of H pylori infection, it has highest frequency of gastric cancer. Similarly, frequency of gastric cancer is quite high in China despite a lower frequency of H pylori infection. In contrast, people living in less developed countries of Asia with high frequency of H pylori infection[17,18,22,30,31,42-44] that is acquired at an earlier age have the lowest risk of developing gastric cancer[14]. It has also been observed that frequency of gastric cancer differs in different parts within many countries; for example, in Japan[45], variation in gastric cancer risk has been well-documented in different regions and has been presumed to be related to variation in nutrient consumption. In China[46], gastric cancer mortality in Changle county is about 10-fold higher than that in Hong Kong and has been attributed to variation in frequency of H pylori infection in the two regions. In India, southern[35] and eastern parts (personal observation) of the country experience somewhat higher frequency of gastric cancer than the northern parts of the country. Interestingly, similar epidemiological observations were made long ago in India in respect of another H pylori-related gastroduodenal ailment, i.e., peptic ulcer disease[47].
STUDIES ON ASSOCIATION BETWEEN H PYLORI INFECTION AND GASTRIC CANCER IN ASIA
Studies from India failed to show an association between H pylori infection and gastric cancer[48-51]. In a study on 50 patients with gastric cancer and 50 controls with non-ulcer dyspepsia, H pylori infection was detected less frequently in gastric cancer (38%, 19/50) than those with non-ulcer dyspepsia (68%, 34/50)[48]. An another study demonstrated that 64.7% (33/51) patients with gastric carcinoma and 74.4% (32/43) with non-ulcer dyspepsia had infection with H pylori [49]. These studies can be criticized due to small sample size with a consequent type II statistical error. Also, in most of these studies, endoscopy-based tests were used to diagnose H pylori infection. Endoscopy-based tests can be false negative in patients with gastric cancer due to gastric atrophy and intestinal metaplasia[52]. However, a recently completed large study from our center in which 279 patients with gastric neoplasms (263 gastric cancer and 16 primary gastric lymphoma) failed to show a higher frequency of H pylori infection in patients with gastric neoplasms as compared with the controls (101 non-ulcer dyspepsia and 355 healthy subjects)[53]. In contrast, studies from China and Japan showed association between H pylori infection and gastric cancer[54,55].
WHAT IS ASIAN ENIGMA
Oxford dictionary describes the term “enigma” as a mysterious or puzzling thing. What is puzzling about gastric cancer and H pylori infection in Asia The countries with highest frequency of H pylori infection have the lowest risk of gastric cancer in contrast to the countries like Japan and China where gastric cancer risk is highest in the world despite a lower occurrence of H pylori infection. This casts major objection to some of the simplified model of gastric carcinogenesis resulting from H pylori infection that stated that if the infection is acquired at an early age particularly in presence of malnutrition, it may reduce gastric acid secretion, pangastritis and gastric cancer may be the likely outcome. In contrast, infection acquired later in life and in person with good nutritional status and normal gastric acid secretion would result in hyperchlorhydria and duodenal ulcer disease[56]. It is well documented in the literature that patients with duodenal ulcer infrequently or never develop gastric cancer[5,57]. If this simplified model of gastric carcinogenesis would have been true, India, Bangladesh, Pakistan would have higher frequency of gastric cancer than Japan and China.
WHAT ARE THE POSSIBLE EXPLANATIONS FOR THE ASIAN ENIGMA
Agent factors
All strains of H pylori are not pathogenic. Is it possible that people living in countries with lower frequency of gastric cancer are infected with non-pathogenic strains of H pylori than people living in China or Japan However, available evidences do not support this hypothesis. Peptic ulcer disease, which is associated with infection by pathogenic strains of H pylori, has been reported a common problem in India and Bangladesh[47]. Genotypic analysis of H pylori strains from India showed pathogenic strains to be present in more than 80% of adults and children with gastroduodenal diseases as well as in control population[58-59]. Studies that used CagA antibody in patients with non-ulcer dyspepsia have shown that CagA antibody is detected in sera of most patients[60]. From our center, a recently completed large study on 279 patients with gastric neoplasms (263 gastric cancer and 16 primary gastric lymphoma) and controls (101 non-ulcer dyspepsia and 355 healthy subjects) showed that frequency of CagA IgG antibody was similar among the patients with gastric carcinoma and the controls, suggesting that difference in virulence factor of H pylori, at least CagA, is unlikely to explain the variation in outcome of H pylori infection[53]. In a study from US, Korea and Colombia[61] in which the first-degree relatives of patients with gastric cancer were evaluated to know whether similar strains of H pylori or similar environmental factors are responsible for pattern of gastritis. However, this study failed to show any relationship between specific virulence factors or H pylori strains and specific histologic pattern or outcome even among those sharing the same environment in childhood[61]. However, several studies from Japan and China[62,63] showed that virulence factors of H pylori are strongly associated with gastric carcinoma. Based on the available evidences, one can not conclude that in Asian countries, despite high frequency of H pylori infection, low frequency of gastric cancer is related to infection with non-pathogenic strains. Though a study from Africa showed that virulence-associated genes of H pylori may partially explain the African enigma[64], the same corollary may not hold well to explain the Asian enigma.
Host’s genetic factors
Host’s genetic make-up determines in a major way response to any infection, including that to H pylori. This is evidenced by the fact that relatives of patients with gastric cancer infected with H pylori developed precancerous abnormalities like gastric atrophy and hypochlorhydria more often than those with non-ulcer dyspepsia[65]. Patients with duodenal ulcer, which is also caused by H pylori, do not develop gastric cancer in contrast to other conditions associated with H pylori infection, such as gastric ulcer, non-ulcer dyspepsia and hyperplastic gastric polyp[5]. These also depict variations in host’s response despite infection with the same organism. Japanese immigrants to the United States have higher gastric cancer risk than native-born Americans, though lesser than Japanese living in Japan[66]; this suggests importance of the genetic factors with additive effects of environmental factors.
Difference in carcinogenic risk in people living in different geographical areas might be related to variation in genetic make-up among different races. Specific allelic variation of different genes (polymorphism) present in a proportion of general population may determine variation in carcinogenic potential in different populations in response to environmental carcinogenic exposure, including that to H pylori infection[67]. Genetic susceptibility of a person may be important in a number of carcinogenic processes that include: (1) mucosal protection against H pylori infection and injury by other carcinogens; (2) mucosal inflammatory response to infection with H pylori; (3) degree of apoptotic cell death[68]; (4) carcinogen activation and detoxification by various enzyme systems of the hosts; (5) variability in the repair of mutated DNA; and (6) ability of the cell to proliferate in a controlled manner to repair the damage.
Several studies have been carried out on single nucleotide polymorphism in relation to gastric carcinogenesis[67]. However, many of these studies did not take into account the role of H pylori infection and dietary factors in addition to the genetic factors. Therefore, there is need of more data on genetic polymorphism in relation to H pylori infection and dietary factors. In fact, genetic studies comparing Asian population with high gastric cancer risk like Japan and China and low cancer risk despite a very high prevalence of H pylori infection like India are needed to understand the explanation for the Asian enigma at a molecular level.
Dietary and environmental factors
Diet may play a major role in gastric carcinogenesis. In India, southern[35] and eastern parts (personal observation) of the country experience somewhat higher frequency of gastric cancer than the northern parts of the country. Rice is the staple cereal in eastern India. Non-vegetarian foods, particularly fish, are very common in eastern Indian diet, which is also spicy with more salts. Diet in southern India is somewhat similar to that in eastern India with rice, fish, excess spice and salt being commonly eaten. In contrast, northern Indian diet is mainly wheat-based and a greater proportion of people are vegetarian. Tobacco smoking, high-temperature food intake, spicy food and rice eating have been shown to be risk factors for gastric cancer in India[69,70]. In another study, consumption of dry fish has been shown to be a risk factor for gastric cancer in India[71]. Diet has been considered to be a major factor for increased frequency of gastric and esophageal cancer in Kashmir province of India[72]. Similar observations have also been made in several countries, including Japan where northern districts have reported a higher frequency of gastric cancer than southern district and this has been related to increased dietary intake of salts in northern districts[14]. Tobacco use and alcohol consumption are the other factors that may influence the international variation in frequency of gastric cancer[15]. Possible explanation of Asian enigma might be related, at least in part, to difference in diet between different countries.
CONCLUSIONS AND FUTURE DIRECTIONS
The available evidences clearly show that H pylori alone is not the only independent factor in gastric carcinogenesis. Host’s genetic make-up and dietary factors play a major role in determining whether or not a person infected with H pylori will develop gastric atrophy, intestinal metaplasia and gastric cancer. This has major importance in preventive strategies of gastric cancer. Despite H pylori being an important agent for causing gastric cancer, a recent randomized controlled trial from high risk region of gastric cancer in China failed to show benefit of eradicating H pylori in preventing gastric cancer[73]. This might be related to the fact that only 1-2% people infected with H pylori develop atrophic gastritis per year, which is a precancerous lesion[74]. Racial and genetic factors are also important as evidenced by difference in gastric cancer risk in different populations, and a recent study, though not from Asia, showed differences in IgG subclass responses between subjects from Gambia and United Kingdom[75]. Unless randomized controlled trials of eradication of H pylori among people who are not only infected with H pylori but also carry multiple genetic factors which increase their predisposition to developing gastric cancer are undertaken, it is difficult to obtain meaningful conclusions about how useful H pylori eradication would be to prevent gastric carcinoma. In fact, in foreseeable future, a day may come when an individual infected with H pylori may be able to know, using mathematical modeling and his genetic make-up, as to what would be his risk of developing gastric cancer, and based on that his physician may advise him whether he should undergo H pylori eradication treatment and/or modification of his diet to reduce risk of gastric cancer. Since gastric cancer is likely to be a multifactorial disease, which include genetic, dietary and environmental factors and not H pylori alone, all these factors need to be considered while constructing the model.
Footnotes
Supported by grants from the Indian Council of Medical Research, No. 5/4/3-5/03/99-NCD-II
S- Editor Guo SY L- Editor Kumar M E- Editor Ma WH
References
- 1.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura S, Yao T, Aoyagi K, Iida M, Fujishima M, Tsuneyoshi M. Helicobacter pylori and primary gastric lymphoma. A histopathologic and immunohistochemical analysis of 237 patients. Cancer. 1997;79:3–11. [PubMed] [Google Scholar]
- 3.Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A. Helicobacter and gastric MALT lymphoma. Gut. 2002;50 Suppl 3:III19–III24. doi: 10.1136/gut.50.suppl_3.iii19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thiede C, Morgner A, Alpen B, Wündisch T, Herrmann J, Ritter M, Ehninger G, Stolte M, Bayerdörffer E, Neubauer A. What role does Helicobacter pylori eradication play in gastric MALT and gastric MALT lymphoma. Gastroenterology. 1997;113:S61–S64. doi: 10.1016/s0016-5085(97)80014-5. [DOI] [PubMed] [Google Scholar]
- 5.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 6.Uemura N, Okamoto S. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer in Japan. Gastroenterol Clin North Am. 2000;29:819–827. doi: 10.1016/s0889-8553(05)70149-7. [DOI] [PubMed] [Google Scholar]
- 7.Cammarota G, Montalto M, Tursi A, Vecchio FM, Fedeli G, Gasbarrini G. Helicobacter pylori reinfection and rapid relapse of low-grade B-cell gastric lymphoma. Lancet. 1995;345:192. doi: 10.1016/s0140-6736(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 8.Ghoshal UC, Guha D, Bandyopadhyay S, Pal C, Chakraborty S, Ghoshal U, Ghosh TK, Pal BB, Banerjee PK. Gastric adenocarcinoma in a patient re-infected with H. pylori after regression of MALT lymphoma with successful anti-H. pylori therapy and gastric resection: a case report. BMC Gastroenterol. 2002;2:6. doi: 10.1186/1471-230X-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zucca E, Bertoni F, Roggero E, Bosshard G, Cazzaniga G, Pedrinis E, Biondi A, Cavalli F. Molecular analysis of the progression from Helicobacter pylori-associated chronic gastritis to mucosa-associated lymphoid-tissue lymphoma of the stomach. N Engl J Med. 1998;338:804–810. doi: 10.1056/NEJM199803193381205. [DOI] [PubMed] [Google Scholar]
- 10.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 11.Erdman SE, Correa P, Coleman LA, Schrenzel MD, Li X, Fox JG. Helicobacter mustelae-associated gastric MALT lymphoma in ferrets. Am J Pathol. 1997;151:273–280. [PMC free article] [PubMed] [Google Scholar]
- 12.Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H. pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801–804. doi: 10.3748/wjg.v7.i6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373–2379. doi: 10.1111/j.1572-0241.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 14.Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106–1112. doi: 10.1111/j.1572-0241.2002.05663.x. [DOI] [PubMed] [Google Scholar]
- 15.Lunet N, Barros H. Helicobacter pylori infection and gastric cancer: facing the enigmas. Int J Cancer. 2003;106:953–960. doi: 10.1002/ijc.11306. [DOI] [PubMed] [Google Scholar]
- 16.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429–431. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham DY, Adam E, Reddy GT, Agarwal JP, Agarwal R, Evans DJ Jr, Malaty HM, Evans DG. Seroepidemiology of Helicobacter pylori infection in India. Comparison of developing and developed countries. Dig Dis Sci. 1991;36:1084–1088. doi: 10.1007/BF01297451. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad MM, Rahman M, Rumi AK, Islam S, Huq F, Chowdhury MF, Jinnah F, Morshed MG, Hassan MS, Khan AK, et al. Prevalence of Helicobacter pylori in asymptomatic population--a pilot serological study in Bangladesh. J Epidemiol. 1997;7:251–254. doi: 10.2188/jea.7.251. [DOI] [PubMed] [Google Scholar]
- 19.Fujisawa T, Kumagai T, Akamatsu T, Kiyosawa K, Matsunaga Y. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol. 1999;94:2094–2099. doi: 10.1111/j.1572-0241.1999.01283.x. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell HM, Li YY, Hu PJ, Liu Q, Chen M, Du GG, Wang ZJ, Lee A, Hazell SL. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J Infect Dis. 1992;166:149–153. doi: 10.1093/infdis/166.1.149. [DOI] [PubMed] [Google Scholar]
- 21.Malaty HM, Kim JG, Kim SD, Graham DY. Prevalence of Helicobacter pylori infection in Korean children: inverse relation to socioeconomic status despite a uniformly high prevalence in adults. Am J Epidemiol. 1996;143:257–262. doi: 10.1093/oxfordjournals.aje.a008736. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Perez GI, Taylor DN, Bodhidatta L, Wongsrichanalai J, Baze WB, Dunn BE, Echeverria PD, Blaser MJ. Seroprevalence of Helicobacter pylori infections in Thailand. J Infect Dis. 1990;161:1237–1241. doi: 10.1093/infdis/161.6.1237. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki M, Kawasaki T, Ogaki T, Itoh K, Kobayashi S, Yoshimizu Y, Aoyagi K, Iwakawa A, Takahashi S, Sharma S, et al. Seroprevalence of Helicobacter pylori infection in Nepal: low prevalence in an isolated rural village. Eur J Gastroenterol Hepatol. 1998;10:47–50. doi: 10.1097/00042737-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Reshetnikov OV, Häivä VM, Granberg C, Kurilovich SA, Babin VP. Seroprevalence of Helicobacter pylori infection in Siberia. Helicobacter. 2001;6:331–336. doi: 10.1046/j.1523-5378.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 25.Abbas Z, Jafri W, Khan AH, Shah MA. Prevalence of Helicobacter pylori antibodies in endoscopy personnel and non-medical volunteers of Karachi. J Pak Med Assoc. 1998;48:201–203. [PubMed] [Google Scholar]
- 26.Fernando N, Holton J, Vaira D, DeSilva M, Fernando D. Prevalence of Helicobacter pylori in Sri Lanka as determined by PCR. J Clin Microbiol. 2002;40:2675–2676. doi: 10.1128/JCM.40.7.2675-2676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akin L, Tezcan S, Hascelik G, Cakir B. Seroprevalence and some correlates of Helicobacter pylori at adult ages in Gülveren Health District, Ankara, Turkey. Epidemiol Infect. 2004;132:847–856. doi: 10.1017/s0950268804002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malekzadeh R, Sotoudeh M, Derakhshan MH, Mikaeli J, Yazdanbod A, Merat S, Yoonessi A, Tavangar M, Abedi BA, Sotoudehmanesh R, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin JT, Wang JT, Wang TH, Wu MS, Lee TK, Chen CJ. Helicobacter pylori infection in a randomly selected population, healthy volunteers, and patients with gastric ulcer and gastric adenocarcinoma. A seroprevalence study in Taiwan. Scand J Gastroenterol. 1993;28:1067–1072. doi: 10.3109/00365529309098311. [DOI] [PubMed] [Google Scholar]
- 30.Mazumder DN, Ghoshal UC. Epidemiology of Helicobacter pylori in India. Indian J Gastroenterol. 1997;16 Suppl 1:S3–S5. [PubMed] [Google Scholar]
- 31.Gill HH, Majmudar P, Shankaran K, Desai HG. Age-related prevalence of Helicobacter pylori antibodies in Indian subjects. Indian J Gastroenterol. 1994;13:92–94. [PubMed] [Google Scholar]
- 32.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559–578. doi: 10.1016/s0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 33.Sarker SA, Rahman MM, Mahalanabis D, Bardhan PK, Hildebrand P, Beglinger C, Gyr K. Prevalence of Helicobacter pylori infection in infants and family contacts in a poor Bangladesh community. Dig Dis Sci. 1995;40:2669–2672. doi: 10.1007/BF02220458. [DOI] [PubMed] [Google Scholar]
- 34.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 35.Mohandas KM, Nagral A. Epidemiology of digestive tract cancers in India. II. Stomach, and gastrointestinal lymphomas. Indian J Gastroenterol. 1998;17:24–27. [PubMed] [Google Scholar]
- 36.Malhotra SL. Geographical distribution of gastrointestinal cancers in India with special reference to causation. Gut. 1967;8:361–372. doi: 10.1136/gut.8.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkin DM, Muir CS. Cancer Incidence in Five Continents. Comparability and quality of data. IARC Sci Publ. 1992;120:45–173. [PubMed] [Google Scholar]
- 38.World Health Statistics Manual. WHO databank. Geneva: WHO. Available from URL: http: //www-dep.iarc.fr/who/menu.htm. Accessed August, 11; 2005. [Google Scholar]
- 39.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000; cancer incidence, mortality and prevalence worldwide. Version 1.0. IARC CancerBase No. 5. Lyon: IARC Press;; 2001. [Google Scholar]
- 40.Sadjadi A, Malekzadeh R, Derakhshan MH, Sepehr A, Nouraie M, Sotoudeh M, Yazdanbod A, Shokoohi B, Mashayekhi A, Arshi S, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer. 2003;107:113–118. doi: 10.1002/ijc.11359. [DOI] [PubMed] [Google Scholar]
- 41.Levi F, Lucchini F, Negri E, Zatonski W, Boyle P, La Vecchia C. Trends in cancer mortality in the European Union and accession countries, 1980-2000. Ann Oncol. 2004;15:1425–1431. doi: 10.1093/annonc/mdh346. [DOI] [PubMed] [Google Scholar]
- 42.Abasiyanik MF, Tunc M, Salih BA. Enzyme immunoassay and immunoblotting analysis of Helicobacter pylori infection in Turkish asymptomatic subjects. Diagn Microbiol Infect Dis. 2004;50:173–177. doi: 10.1016/j.diagmicrobio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Reshetnikov OV, Denisova DV, Zavyalova LG, Häivä VM, Granberg C. Helicobacter pylori seropositivity among adolescents in Novosibirsk, Russia: prevalence and associated factors. J Pediatr Gastroenterol Nutr. 2003;36:72–76. doi: 10.1097/00005176-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Singh V, Trikha B, Nain CK, Singh K, Vaiphei K. Epidemiology of Helicobacter pylori and peptic ulcer in India. J Gastroenterol Hepatol. 2002;17:659–665. doi: 10.1046/j.1440-1746.2002.02746.x. [DOI] [PubMed] [Google Scholar]
- 45.Tsubono Y, Takahashi T, Iwase Y, Iitoi Y, Akabane M, Tsugane S. Nutrient consumption and gastric cancer mortality in five regions of Japan. Nutr Cancer. 1997;27:310–315. doi: 10.1080/01635589709514542. [DOI] [PubMed] [Google Scholar]
- 46.Wong BC, Lam SK, Ching CK, Hu WH, Kwok E, Ho J, Yuen ST, Gao Z, Chen JS, Lai KC, et al. Differential Helicobacter pylori infection rates in two contrasting gastric cancer risk regions of South China. China Gastric Cancer Study Group. J Gastroenterol Hepatol. 1999;14:120–125. doi: 10.1046/j.1440-1746.1999.01823.x. [DOI] [PubMed] [Google Scholar]
- 47.Tovey F. Peptic ulcer in India and Bangladesh. Gut. 1979;20:329–347. doi: 10.1136/gut.20.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kate V, Ananthakrishnan N. Helicobacter pylori and gastric carcinoma: evidence for the link. Natl Med J India. 2000;13:329. [PubMed] [Google Scholar]
- 49.Kate V, Ananthakrishnan N, Badrinath S, Ratnakar C. Prevalence of Helicobacter pylori infection in disorders of the upper gastrointestinal tract in south India. Natl Med J India. 1998;11:5–8. [PubMed] [Google Scholar]
- 50.Khanna AK, Seth P, Nath G, Dixit VK, Kumar M. Correlation of Helicobacter pylori and gastric carcinoma. J Postgrad Med. 2002;48:27–28. [PubMed] [Google Scholar]
- 51.Sivaprakash R, Rao UA, Thyagarajan SP, Ramathilakam B, Jayanthi V. Investigation for the prevalence of Helicobacter pylori infection in patients with gastric carcinoma in Madras, India. Jpn J Med Sci Biol. 1996;49:49–56. doi: 10.7883/yoken1952.49.49. [DOI] [PubMed] [Google Scholar]
- 52.Karnes WE Jr, Samloff IM, Siurala M, Kekki M, Sipponen P, Kim SW, Walsh JH. Positive serum antibody and negative tissue staining for Helicobacter pylori in subjects with atrophic body gastritis. Gastroenterology. 1991;101:167–174. doi: 10.1016/0016-5085(91)90474-y. [DOI] [PubMed] [Google Scholar]
- 53.Ghoshal UC, Tiwari S, Pandey R, Dhingra S, Ghoshal U, Singh H, Nagpal AK, Gupta VK, Naik S, Ayyagari A. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: The Indian enigma. Am J Gastroenterol. 2005;100:S64. doi: 10.1007/s10620-008-0229-7. [DOI] [PubMed] [Google Scholar]
- 54.Asaka M, Kato M, Kudo M, Katagiri M, Nishikawa K, Yoshida J, Takeda H, Miki K. Relationship between Helicobacter pylori infection, atrophic gastritis and gastric carcinoma in a Japanese population. Eur J Gastroenterol Hepatol. 1995;7 Suppl 1:S7–10. [PubMed] [Google Scholar]
- 55.Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374–376. doi: 10.3748/wjg.v6.i3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983–1991. doi: 10.1016/s0016-5085(97)70019-2. [DOI] [PubMed] [Google Scholar]
- 57.Hansson LE, Nyrén O, Hsing AW, Bergström R, Josefsson S, Chow WH, Fraumeni JF, Adami HO. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 58.Mukhopadhyay AK, Kersulyte D, Jeong JY, Datta S, Ito Y, Chowdhury A, Chowdhury S, Santra A, Bhattacharya SK, Azuma T, et al. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J Bacteriol. 2000;182:3219–3227. doi: 10.1128/jb.182.11.3219-3227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh M, Prasad KN, Yachha SK, Krishnani N. Genotypes of Helicobacter pylori in children with upper abdominal pain. J Gastroenterol Hepatol. 2003;18:1018–1023. doi: 10.1046/j.1440-1746.2003.03119.x. [DOI] [PubMed] [Google Scholar]
- 60.Kumar S, Dhar A, Srinivasan S, Jain S, Rattan A, Sharma MP. Antibodies to Cag A protein are not predictive of serious gastroduodenal disease in Indian patients. Indian J Gastroenterol. 1998;17:126–128. [PubMed] [Google Scholar]
- 61.Li L, Genta RM, Go MF, Gutierrez O, Kim JG, Graham DY. Helicobacter pylori strain and the pattern of gastritis among first-degree relatives of patients with gastric carcinoma. Helicobacter. 2002;7:349–355. doi: 10.1046/j.1523-5378.2002.00108.x. [DOI] [PubMed] [Google Scholar]
- 62.Nomura AM, Lee J, Stemmermann GN, Nomura RY, Perez-Perez GI, Blaser MJ. Helicobacter pylori CagA seropositivity and gastric carcinoma risk in a Japanese American population. J Infect Dis. 2002;186:1138–1144. doi: 10.1086/343808. [DOI] [PubMed] [Google Scholar]
- 63.Yang GF, Deng CS, Xiong YY, Gong LL, Wang BC, Luo J. Expression of nuclear factor-kappa B and target genes in gastric precancerous lesions and adenocarcinoma: association with Helicobactor pylori cagA (+) infection. World J Gastroenterol. 2004;10:491–496. doi: 10.3748/wjg.v10.i4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bravo LE, van Doom LJ, Realpe JL, Correa P. Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma. Am J Gastroenterol. 2002;97:2839–2842. doi: 10.1111/j.1572-0241.2002.07031.x. [DOI] [PubMed] [Google Scholar]
- 65.El-Omar EM, Oien K, Murray LS, El-Nujumi A, Wirz A, Gillen D, Williams C, Fullarton G, McColl KE. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology. 2000;118:22–30. doi: 10.1016/s0016-5085(00)70410-0. [DOI] [PubMed] [Google Scholar]
- 66.Haenszel W, Kurihara M. Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst. 1968;40:43–68. [PubMed] [Google Scholar]
- 67.González CA, Sala N, Capellá G. Genetic susceptibility and gastric cancer risk. Int J Cancer. 2002;100:249–260. doi: 10.1002/ijc.10466. [DOI] [PubMed] [Google Scholar]
- 68.Tiwari S, Ghoshal U, Ghoshal UC, Dhingra S, Pandey R, Singh M, Ayyagari A, Naik S. Helicobacter pylori-induced apoptosis in pathogenesis of gastric carcinoma. Indian J Gastroenterol. 2005;24:193–196. [PubMed] [Google Scholar]
- 69.Gajalakshmi CK, Shanta V. Lifestyle and risk of stomach cancer: a hospital-based case-control study. Int J Epidemiol. 1996;25:1146–1153. doi: 10.1093/ije/25.6.1146. [DOI] [PubMed] [Google Scholar]
- 70.Mathew A, Gangadharan P, Varghese C, Nair MK. Diet and stomach cancer: a case-control study in South India. Eur J Cancer Prev. 2000;9:89–97. doi: 10.1097/00008469-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Rao DN, Ganesh B, Dinshaw KA, Mohandas KM. A case-control study of stomach cancer in Mumbai, India. Int J Cancer. 2002;99:727–731. doi: 10.1002/ijc.10339. [DOI] [PubMed] [Google Scholar]
- 72.Khuroo MS, Zargar SA, Mahajan R, Banday MA. High incidence of oesophageal and gastric cancer in Kashmir in a population with special personal and dietary habits. Gut. 1992;33:11–15. doi: 10.1136/gut.33.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187–194. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 74.Correa P, Haenszel W, Cuello C, Zavala D, Fontham E, Zarama G, Tannenbaum S, Collazos T, Ruiz B. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. [PubMed] [Google Scholar]
- 75.Campbell DI, Pearce MS, Parker L, Thomas JE, Sullivan PB, Dale A. Immunoglobulin G subclass responses to Helicobacter pylori vary with age in populations with different levels of risk of gastric carcinoma. Clin Diagn Lab Immunol. 2004;11:631–633. doi: 10.1128/CDLI.11.3.631-633.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


