Abstract
AIM: To investigate the protective effect and mechanism of alanyl-glutamine dipeptide (Ala-Gln) against hepatic ischemia-reperfusion injury in rats.
METHODS: Rats were divided into group C as normal control Group (n=16) and group G as alanyl-glutamine pretreatment (n=16). Rats were intravenously infused with 0.9% saline solution in group C and Ala-Gln -enriched (2% glutamine) 0.9% saline solution in group G via central venous catheter for three days. Then all rats underwent hepatic warm ischemia for 30 min followed by different periods of reperfusion. Changes in biochemical parameters, the content of glutathione (GSH) and the activity of superoxide dismutase (SOD) in liver tissue, Bcl-2 and Bax protein expression and morphological changes of liver tissue were compared between both groups.
RESULTS: One hour after reperfusion, the levels of liver enzymes in group G were significantly lower than those in group C (P<0.05). Twenty-four hours after reperfusion, the levels of liver enzymes in both groups were markedly recovered and the levels of liver enzyme in group G were also significantly lower than those in group C (P <0.01). One and 24 h after reperfusion, GSH content in group G was significantly higher than that in group C (P <0.05). There was no statistical difference in activities of SOD between the two groups. One and 24 h after reperfusion, the positive expression rate of Bcl-2 protein was higher in group G than in group C (P <0.05) and the positive expression rate of Bax protein was lower in group G than in group C (P < 0.05). Histological and ultrastructural changes of liver tissue were inhibited in group C compared to group G.
CONCLUSION: Our results suggest that Ala-Gln pretreatment provides the rat liver with significant tolerance to warm ischemia-reperfusion injury, which may be mediated partially by enhancing GSH content and regulating the expression of Bcl-2 and Bax proteins in the liver tissue.
Keywords: Alanyl-glutamine dipeptide, Hepatic ischemia-reperfusion injury, Glutathione, Bcl-2, Bax
INTRODUCTION
Temporary clamping of the portal triad, ie, inflow occlusion by the Pringle’s maneuver, is a common strategy to minimize bleeding during hepatic resection. Unfortunately, this method resulted in hepatic ischemia-reperfusion (I/R) injury and may cause postoperative functional disorder of the liver. Development of new pharmaceutical strategies to attenuate hepatic I/R injury is important for achieving a better clinical outcome. Hepatic I/R injury factors usually include oxygen radical species, intracellular calcium overloading, inflammatory cytokines and infiltration of neutrophils[1]. However, its mechanism has not been fully elucidated.
Glutamine (Gln) is a conditionally essential nutrient during serious injury or illness, and plays a vital role in the metabolism of tissue[2-4]. Recently, Gln has been demonstrated to protect against I/R injury of gut, heart and skeletal muscle and its possible mechanism is partially related to preservation of the content of glutathione (GSH)[5-7].
Recently, apoptosis has been indicated as an important mode of cell death during hepatic I/R injury[8]. Apoptosis is governed by a number of regulating genes, such as bcl-2 and bax and the ratio of bcl-2 to bax determines survival or death following an apoptotic stimulus[9]. Animal studies demonstrate that GSH depletion is associated with decreased bcl-2 gene expression and increased apoptosis in cholangiocytes[10]. Overexpression of bcl-2 gene by transferring this gene into hepatocytes with adenovirus is resistant to hepatic I/R injury[11].
However, there are few studies dealing with the effect of Gln on hepatic I/R injury. Consequently, we designed this experiment to study the effect of Gln on hepatic I/R injury in rats and its possible mechanisms. Dipeptide L-alanyl-L-glutamine is soluble in water and remains stable after heat sterilization[12]. Peptidase activity in all body compartments shows rapid hydrolysis and release of appropriate amino acids after intravenous infusion. Therefore, alanyl-glutamine dipeptide (Ala-Gln) was used in this experiment.
MATERIALS AND METHODS
Experimental animals
Male Wistar rats weighing 250-300 g (supplied by the Experimental Animal Center of Shengjing Hospital) were housed in a standard animal laboratory with free activity and access to water and chow. They were kept under constant environment conditions in a 12 h light-dark cycle. All operations were performed under clean conditions.
Intravenous drugs and other relevant chemicals
Dipeptiven (Ala-Gln solution) was from Sino-Sweden and Fresenius Pharmaceutical Corp. LTD. GSH and superoxide dismutase (SOD) detection kits were purchased from Nanjing Jiancheng Bioengineering Institute, China. Mouse anti-rat Bcl-2 and Bax monoclonal antibodies were provided by Beijing Zhongshan Biotechnology Co.Ltd, China.
Animal model and grouping
Under urethane anesthesia (intraperitoneal injection, 10 mg/kg), a silastic catheter was inserted through the right external jugular vein into the superior vena cava, tunneled subcutaneously and brought out through the skin of mid-scapular region. A flexible spring guarded the catheter, and then hooked up to an infusion pump. All rats were maintained in individual metabolic cages. After catheterization, all rats received intravenously 0.9% saline solution at a constant rate by a pump (2 mL/h) with free activity and access to water and chow for 3 d. Then, all rats were randomly divided into Group C infused with 0.9% saline solution (n =16) and group G infused with Ala-Gln-enriched (2% glutamine) 0.9% saline solution (n =16). After 3 d, under urethane anesthesia (intraperitoneal injection, 10 mg/kg) a midline laparotomy was performed and all structures in the portal triad to the liver were clamped for 30 min followed by different periods of reperfusion. Liver tissues and blood were sampled at 1 and 24 h after reperfusion for liver function tests, antioxidant enzyme measurement, Bcl-2 and Bax protein expression and morphological examination.
Liver function tests
Blood samples collected at 1 and 24 h after reperfusion were used to measure the alanine aminotransferase (ALT) and lactic dehydrogenase (LDH) levels with a standard biochemistry automatic analyzer.
Antioxidant enzyme measurement
The content of GSH and activity of SOD in rat liver tissue were measured following instructions of the commercial kit.
Immunohistochemistry
S-P immunohistochemistry was performed to detect Bcl-2 and Bax proteins following instructions of the commercial kit. Only cytoplasmic staining was evaluated and nuclear reaction was interpreted to be nonspecific staining. No positive cells was negative expression. The positive cells were considered weak positive expression when their percentage was lower and scored as (+), moderate positive expression when their percentage was between 1/3 and 2/3 and scored as (++), and strong positive expression when their percentage was higher than 2/3 and scored as (+++).
Histological and electron microscopic analysis
Liver specimens were taken at 1 and 24 h after reperfusion for hematoxylin-eosin staining and light microscopy. The specimens were immediately cut for electron microscopy. The sections were examined under a Philips CM10 electron microscope.
Statistical analysis
The data were presented as mean ± SD. Student’s t test was performed for the biochemical parameters and antioxidant enzyme levels. P < 0.05 was considered statistically significant. Software SPSS11.0 was used in all statistical analyses.
RESULTS
Hepatic serum enzyme concentration
One hour after reperfusion, the serum ALT and LDH concentrations in group G were significantly lower than those in group C (P < 0.05). Twenty-four hours after reperfusion, liver enzyme levels in both groups were markedly recovered, the serum ALT and LDH concentrations in group G were also significantly lower than those in group C (P < 0.01, Figures 1 A and 1 B)
Figure 1.
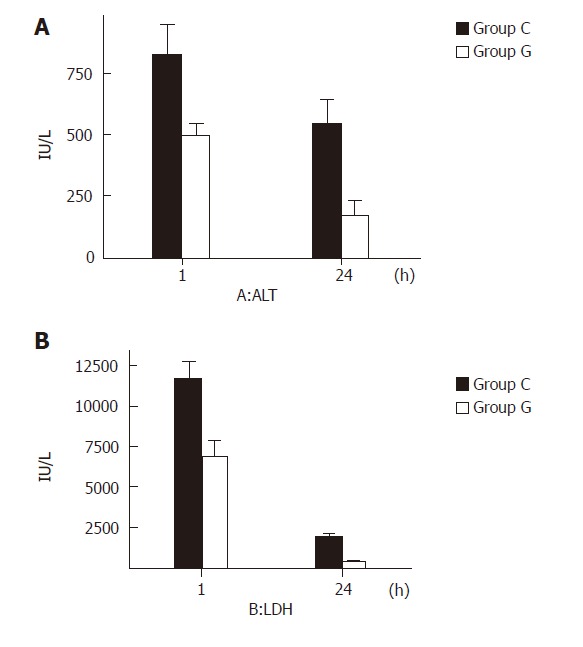
Liver function 1 and 24 h after reperfusion of ALT (A) and LDH (B) in groups C and G.
Antioxidant enzyme level
One and 24 hours after reperfusion, the GSH content in the liver tissue of group G was significantly higher than that in group C (P < 0.05). There was no statistical difference in the activity of SOD between the two groups (P >0.05, Figures 2 A and 2 B)
Figure 2.
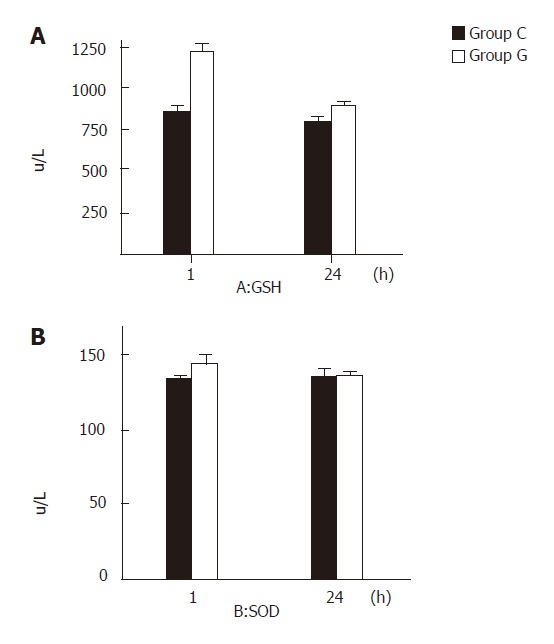
GSH content (A) and SOD activity (B) 1 and 24 h after reperfusion in groups C and G.
Bcl-2 and Bax expression
One and 24 h after reperfusion, the positive expression rate of Bcl-2 was higher in group G than in group C (P <0.05) and the positive expression rate of Bax was lower in group G than in group C (P < 0.05) (Table 1, Figures 3 A-3D).
Table 1.
Positive expression of Bcl-2 and Bax proteins in liver tissue
| Bcl-2 protein | Bax protein | |||||||||||
| Group | Positive expression | total number | positive expression percentage(%) | positive expression | total number | Positive expression percentage(%) | ||||||
| - | + | ++ | +++ | - | + | ++ | +++ | |||||
| group C(1h) | 5 | 0 | 0 | 3 | 3 | 37.5a | 0 | 1 | 3 | 4 | 7 | 87.5c |
| group G(1h) | 0 | 0 | 0 | 8 | 8 | 100.0 | 4 | 2 | 1 | 1 | 2 | 25.0 |
| group C(24h) | 5 | 1 | 1 | 1 | 2 | 25.0e | 0 | 1 | 4 | 3 | 7 | 87.5g |
| group G(24h) | 1 | 0 | 0 | 7 | 7 | 87.5 | 5 | 1 | 1 | 1 | 2 | 25.0 |
P < 0.05,
P < 0.05,
P < 0.05,
P < 0.05 vs group G.
Figure 3.
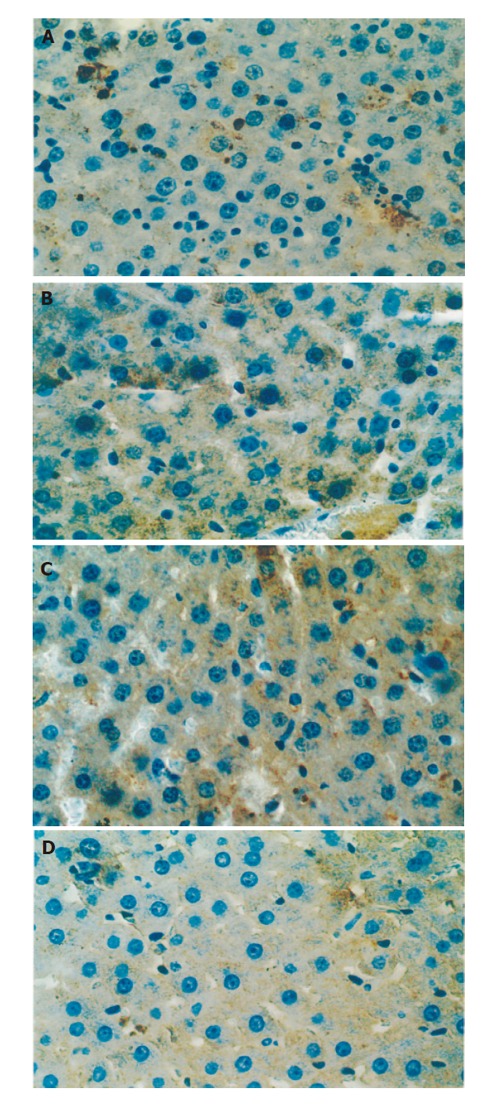
Bcl-2 expression in group C (A) and group G (B) (post-reperfusion 1 h) (×400), and Bax expression in group C (C) and group G (D).
Histological changes
One hour after reperfusion, only slight swelling and less fatty and vacuolation degeneration were found in hepatocytes of group G. However, severe swelling was observed in group C along with more fatty and vacuolization degeneration in hepatocytes. Compartment of hepatic sinus became narrow or disappeared. Infiltration of neutrophils and hemorrhage were also observed. Twenty-four h after reperfusion, the swelling and vacuolization degeneration in group G became worse, but the hepatic cellular structure was still clear and only single-cell necroses were observed. However, derangement of constitution was observed in group C along with a few necroses of hepatocytes.
Ultrastructural changes
One h after reperfusion, mitochondria and glycogen granules were observed in hepatic cytoplasm of group G. Rough endoplasmic reticulum was arrayed as lamellar. Mitochondria and rough endoplasmic reticulum were slightly swollen. More primary lysosomes were found. In group C, mitochondria were severe swollen and had a reduction in the number of cristae. Rough endoplasmic reticulum was dilated and ruptured in different degrees. Smooth endoplasmic reticulum increased and glycogen granules decreased. More secondary lysosomes were ob-served. Twenty-four h after reperfusion, more mitoch-ondria, endoplasmic reticulum and a few of glycogen granules and secondary lysosomes were observed in cytoplasm of group G, along with slightly focal swelling. In group C, hepatocytes only were observed with cellular contour. Severe edema, deformation and necrosis were also observed. The structure of organelles in cytoplasm was not clear. (Figures 4A-4D)
Figure 4.
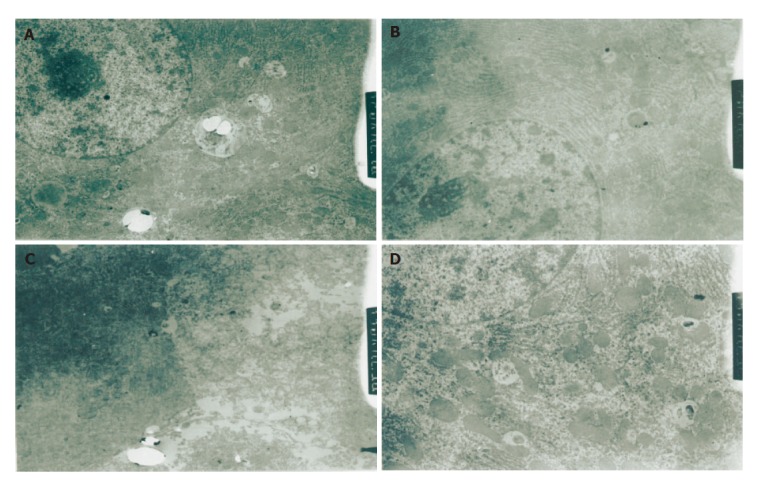
Hepatic ultrastructure 1 h after reperfusion (×1500) in group C (A) and group G (B), 24 h after reperfusion in group C (C) and group G (D).
DISCUSSION
Gln is one of the most abundant amino acid in plasma and plays an important role in the metabolism of body. The circulating and tissue concentrations of Gln diminish precipitously after stress. When Gln is exhausted to a certain extent, a large amount of Gln released from skeletal muscle and a small amount from lung are transported to liver and gut. Recently, Gln has been demonstrated to protect against ischemia-reperfusion injury of gut, heart and skeletal muscle[5-7].
The results of our study indicated that after 30 min ischemia, liver function and morphology were impaired and Gln pretreatment could protect against liver damage.
The exact mechanisms of the protective effect of Gln against ischemia and reperfusion injury of organs and tissues are still incompletely understood. Gln protects against ischemia-reperfusion injury of gut, heart and skeletal muscle by preserving the GSH content in the tissues[5-7]. GSH is an important endogenous antioxidant that protects against oxygen free radical injuries and intravenous GSH administration during reperfusion of ischemic liver can prevent reperfusion injury in rats[13]. The content of GSH is useful for determination of the degree of tissue damage[14]. In this study, Gln pretreatment could obviously increase the GSH content in rat liver tissue, indicating that Gln can protect against hepatic I/R injury by maintaining the relatively high content of GSH in tissue and relieving the cellular oxidant injuries[15].
In addition, Gln pretreatment can also protect against hepatic I/R injury by participating in energy metabolism, increasing the cellular energy metabolism, protecting the structure and function of mitochondria and decreasing the production of oxygen free radicals, leading to less consumption of GSH in liver tissue. A complete inhibition of α-ketoglutarate dehydrogenase activity in the TCA cycle has been observed in hyperoxia-exposed glutamine-deprived cells and Gln could protect α-ketoglutarate dehydrogenase from inactivation under oxidative stress as well as mitochondria from oxidative stress and increase cellular ATP levels[16]. Gln could stimulate the synthesis of glycogen and increase the glycogen store in hepatocytes, reinforcing the ability of liver against cellular oxidant injuries[17]. In this study, mitochondria and glycogen granules were observed in hepatic cytoplasm, mitochondria and rough endoplasmic reticulum were protected.
Gln increases the activity of SOD in liver tissue after stress[18], which was not confirmed in our study due to the model of rat, the number of samples and the dosage of Gln in this experiment.
It was reported that portal triad clamping produces not only ischemic injury of the liver but also portal venous congestion[19]. Acute portal venous congestion for a long period may impair the intestinal mucosal barrier and increase intestinal permeability, causing endotoxemia, bacterial translocation, activation of reactive oxygen radicals and inflammatory cytokines, such as tumor necrosis factor-α (TNF-α)[20-23]. Reperfusion of stagnant portal venous blood with deleterious chemical mediators into the ischemic liver aggravates the liver injury, leading to intra-abdominal sepsis and abscess formation, which is the major cause of postoperative septic complications induced by hepatic I/R injury[24-26]. However, attempts to protect against hepatic I/R injury by alleviating the possible detrimental effects of portal venous congestion have not achieved satisfactory results.
Gln is a precursor for synthesis of nucleic acids and glutathione. It is the main fuel for rapid proliferating and dividing cells such as enterocytes and lymphocytes. It can maintain the metabolism of intestinal mucosal cells directly or indirectly, promote hyperplasia of epithelial cells of ileum and colon, maintain the structure and function of small intestinal mucosal and reduce the increment of intestina permeability[27-30].
Gln can enhance the function of gut-associated lymphoid tissue (GALT) and gut[31]. Gln administration can prevent the depletion of GSH in Peyer’s patches of endotoxaemic mice[32] and promote secretion of mucosal secretory immunoglobulin A (S-IgA), a major effector of the gut-associated lymphoid tissue in the intestine, which can bind to bacteria and prevent their adherence to the epithelium and bacterial translocation[33,34].
Hepatic injury caused by ischemia can be described as necrosis. It was reported that apoptosis of hepatocytes and sinusoidal endothelial cells is a critical mechanism contributing to hepatic I/R injury[8]. The mechanism of apoptosis in ischemia and reperfusion injury usually includes prodtion of oxygen radical species and intracellular calcium overloading[35]. Translocation of bacteria and endotoxin from gut, well documented in hepatic I/R injury, also contribute to the induction of hepatocyte apoptosis. TNF-α induced by endotoxin also can induce hepatocyte apoptosis[36]. A number of genes regulate the apoptotic process. The family of bcl-2-related proteins plays a key role in the regulation of apoptosis.
Bcl-2, a member of the bcl-2-related protein family, can promote cell survival through protein-protein interactions with other bcl-2-related protein family members. Recent studies indicate that overexpression of Bcl-2 protein could reduce hepatocellular apoptosis after reperfusion and protect against hepatic I/R injury[8,11]. Bax, another member of the bcl-2-related protein family, has extensive amino acid homology with Bcl-2 and may form homodimers to accelerate cell death or form heterodimers with Bcl-2 to inhibit cell death. Therefore, changes in the ratio of Bcl-2 and Bax expression may determine survival or death following apoptotic stimuli and attenuate the anti-apoptotic effect of Bcl-2 protein by reducing post-ischemic apoptosis[9,37].
In this study, up-regulation of Bcl-2 protein expression and down-regulation of Bax protein expression in rat liver were found 1 and 24 h after reperfusion, indicating that Gln can protect against hepatic I/R injury by regulating the expression of Bcl-2 and Bax proteins. Our findings suggest that Gln pretreatment could enhance GSH content and protect against oxidative stress in hepatic I/R injury. It has been proved that reduction in the cellular level of GSH increases degradation of Bcl-2 protein and apoptosis[10]. Gln pretreatment can significantly increase cellular ATP levels and synthesis of glycogen and glycogen store in hepatocytes[16,17]. Gln can decrease the diminution of liver tissue ATP content and intracellular calcium overloading maintain the activity of Na+-K+ and Ca+-ATPase, and prevent hepatocellular apoptosis. On the other hand, Gln pretreatment can protect gut barrier and decrease the release of enteric endotoxin and inflammatory cytokines (such as TNF-α), thus reducing the direct pro-apoptotic effect of endotoxin and TNF-α.
In conclusion, glutamine pretreatment can protect against hepatic I/R injury by enhancing GSH content and regulating the expression of Bcl-2 and Bax proteins in liver tissue.
Footnotes
Supported by the Natural Science Foundation of Liaoning Province, No. 20022063
Co-first-author: Chang-Jun Jia and Xu Zhang
Co-correspondence: Chang-Jun Jia
S- Editor Wang J L- Editor Wang XL E- Editor Wu M
References
- 1.Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003;10:189–194. doi: 10.1007/s00534-002-0720-z. [DOI] [PubMed] [Google Scholar]
- 2.Melis GC, ter Wengel N, Boelens PG, van Leeuwen PA. Glutamine: recent developments in research on the clinical significance of glutamine. Curr Opin Clin Nutr Metab Care. 2004;7:59–70. doi: 10.1097/00075197-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Gianotti L, Alexander JW, Gennari R, Pyles T, Babcock GF. Oral glutamine decreases bacterial translocation and improves survival in experimental gut-origin sepsis. JPEN J Parenter Enteral Nutr. 1995;19:69–74. doi: 10.1177/014860719501900169. [DOI] [PubMed] [Google Scholar]
- 4.Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, Teerlink T, Meuwissen SG, Haarman HJ, Thijs LG, et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998;352:772–776. doi: 10.1016/S0140-6736(98)02007-8. [DOI] [PubMed] [Google Scholar]
- 5.Harward TR, Coe D, Souba WW, Klingman N, Seeger JM. Glutamine preserves gut glutathione levels during intestinal ischemia/reperfusion. J Surg Res. 1994;56:351–355. doi: 10.1006/jsre.1994.1054. [DOI] [PubMed] [Google Scholar]
- 6.Wischmeyer PE, Jayakar D, Williams U, Singleton KD, Riehm J, Bacha EA, Jeevanandam V, Christians U, Serkova N. Single dose of glutamine enhances myocardial tissue metabolism, glutathione content, and improves myocardial function after ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr. 2003;27:396–403. doi: 10.1177/0148607103027006396. [DOI] [PubMed] [Google Scholar]
- 7.Prem JT, Eppinger M, Lemmon G, Miller S, Nolan D, Peoples J. The role of glutamine in skeletal muscle ischemia/reperfusion injury in the rat hind limb model. Am J Surg. 1999;178:147–150. doi: 10.1016/s0002-9610(99)00148-8. [DOI] [PubMed] [Google Scholar]
- 8.Sun K, Liu ZS, Sun Q. Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic postconditioning. World J Gastroenterol. 2004;10:1934–1938. doi: 10.3748/wjg.v10.i13.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 10.Celli A, Que FG, Gores GJ, LaRusso NF. Glutathione depletion is associated with decreased Bcl-2 expression and increased apoptosis in cholangiocytes. Am J Physiol. 1998;275:G749–G757. doi: 10.1152/ajpgi.1998.275.4.G749. [DOI] [PubMed] [Google Scholar]
- 11.Bilbao G, Contreras JL, Eckhoff DE, Mikheeva G, Krasnykh V, Douglas JT, Thomas FT, Thomas JM, Curiel DT. Reduction of ischemia-reperfusion injury of the liver by in vivo adenovirus-mediated gene transfer of the antiapoptotic Bcl-2 gene. Ann Surg. 1999;230:185–193. doi: 10.1097/00000658-199908000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13:295–302. [PubMed] [Google Scholar]
- 13.Schauer RJ, Kalmuk S, Gerbes AL, Leiderer R, Meissner H, Schildberg FW, Messmer K, Bilzer M. Intravenous administration of glutathione protects parenchymal and non-parenchymal liver cells against reperfusion injury following rat liver transplantation. World J Gastroenterol. 2004;10:864–870. doi: 10.3748/wjg.v10.i6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armeni T, Ghiselli R, Balercia G, Goffi L, Jassem W, Saba V, Principato G. Glutathione and ultrastructural changes in inflow occlusion of rat liver. J Surg Res. 2000;88:207–214. doi: 10.1006/jsre.1999.5781. [DOI] [PubMed] [Google Scholar]
- 15.Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DW. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992;215:114–119. doi: 10.1097/00000658-199202000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad S, White CW, Chang LY, Schneider BK, Allen CB. Glutamine protects mitochondrial structure and function in oxygen toxicity. Am J Physiol Lung Cell Mol Physiol. 2001;280:L779–L791. doi: 10.1152/ajplung.2001.280.4.L779. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Tian Fz, Tang LJ, Huang DR, Li XJ, Yin ZL. Effects of glycogen on rabbit donor liver during ischemia-reperfusion. Shijie Huaren Xiaohua Zazhi. 2001;9:255–259. [Google Scholar]
- 18.Xia JZ, Wu ZH. Metabolism of glutamine-dipeptide supplemented TPN decreasing injuries of liver and intestine in intraperitoneal chemotherapy rats. Parenteral Enteral Nutrition. 1997;4:78–82. [Google Scholar]
- 19.Dai CL, Xia ZL, Kume M, Yamamoto Y, Yamagami K, Ozaki N, Yamaoka Y. Heat shock protein 72 normothermic ischemia, and the impact of congested portal blood reperfusion on rat liver. World J Gastroenterol. 2001;7:415–418. doi: 10.3748/wjg.v7.i3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferri M, Gabriel S, Gavelli A, Franconeri P, Huguet C. Bacterial translocation during portal clamping for liver resection. A clinical study. Arch Surg. 1997;132:162–165. doi: 10.1001/archsurg.1997.01430260060013. [DOI] [PubMed] [Google Scholar]
- 21.van Leeuwen PA, Hong RW, Rounds JD, Rodrick ML, Wilmore D. Hepatic failure and coma after liver resection is reversed by manipulation of gut contents: the role of endotoxin. Surgery. 1991;110:169–174. discussion 174-175. doi: 10.1016/0261-5614(91)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Wang XD, Pärsson H, Andersson R, Soltesz V, Johansson K, Bengmark S. Bacterial translocation, intestinal ultrastructure and cell membrane permeability early after major liver resection in the rat. Br J Surg. 1994;81:579–584. doi: 10.1002/bjs.1800810434. [DOI] [PubMed] [Google Scholar]
- 23.Colletti LM, Remick DG, Burtch GD, Kunkel SL, Strieter RM, Campbell DA. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van As AB, Lotz Z, Tyler M, Kahn D. Reperfusion injury associated with portal venous and hepatic arterial perfusion in liver transplantation. Transplantation. 2002;74:158–163. doi: 10.1097/00007890-200207270-00003. [DOI] [PubMed] [Google Scholar]
- 25.Liu DL, Jeppsson B, Hakansson CH, Odselius R. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch Surg. 1996;131:442–447. doi: 10.1001/archsurg.1996.01430160100022. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Andersson R, Soltesz V, Bengmark S. Bacterial translocation after major hepatectomy in patients and rats. Arch Surg. 1992;127:1101–1106. doi: 10.1001/archsurg.1992.01420090109016. [DOI] [PubMed] [Google Scholar]
- 27.Scheppach W, Loges C, Bartram P, Christl SU, Richter F, Dusel G, Stehle P, Fuerst P, Kasper H. Effect of free glutamine and alanyl-glutamine dipeptide on mucosal proliferation of the human ileum and colon. Gastroenterology. 1994;107:429–434. doi: 10.1016/0016-5085(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Langkamp-Henken B, Suzuki K, Stahlgren LH. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability. JPEN J Parenter Enteral Nutr. 1994;18:303–307. doi: 10.1177/014860719401800404. [DOI] [PubMed] [Google Scholar]
- 29.Yasuhara M. [L-glutamine-induced heme oxygenase-1 protects small intestine from warm ischemia and reperfusion injury in the rat] Hokkaido Igaku Zasshi. 2001;76:21–34. [PubMed] [Google Scholar]
- 30.Blikslager AT, Rhoads JM, Bristol DG, Roberts MC, Argenzio RA. Glutamine and transforming growth factor-alpha stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery. 1999;125:186–194. [PubMed] [Google Scholar]
- 31.Ikeda S, Kudsk KA, Le T, Zarzaur BL, Johnson CD. Glutamine improves impaired cellular exudation and polymorphonuclear neutrophil phagocytosis induced by total parenteral nutrition after glycogen-induced murine peritonitis. Shock. 2003;19:50–54. doi: 10.1097/00024382-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Manhart N, Vierlinger K, Spittler A, Bergmeister H, Sautner T, Roth E. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer's patches in endotoxemic mice. Ann Surg. 2001;234:92–97. doi: 10.1097/00000658-200107000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarantos P, Ockert K, Souba WW. Endotoxin stimulates lymphocyte glutaminase expression. Arch Surg. 1993;128:920–924. doi: 10.1001/archsurg.1993.01420200094017. [DOI] [PubMed] [Google Scholar]
- 34.Alverdy JA, Aoys E, Weiss-Carrington P, Burke DA. The effect of glutamine-enriched TPN on gut immune cellularity. J Surg Res. 1992;52:34–38. doi: 10.1016/0022-4804(92)90275-5. [DOI] [PubMed] [Google Scholar]
- 35.Oshimi Y, Miyazaki S. Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated cytosolic Ca2 level. J Immunol. 1995;54:599–609. [PubMed] [Google Scholar]
- 36.Gantner F, Leist M, Jilg S, Germann PG, Freudenberg MA, Tiegs G. Tumor necrosis factor-induced hepatic DNA fragmentation as an early marker of T cell-dependent liver injury in mice. Gastroenterology. 1995;109:166–176. doi: 10.1016/0016-5085(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 37.Bartling B, Holtz J, Darmer D. Contribution of myocyte apoptosis to myocardial infarction. Basic Res Cardiol. 1998;93:71–84. doi: 10.1007/s003950050065. [DOI] [PubMed] [Google Scholar]


