Abstract
AIM: To investigate the possible mechanism of the protective effects of a bioactive fraction, Ganoderma lucidum proteoglycan (GLPG) isolated from Ganoderma lucidum mycelia, against carbon tetrachloride-induced liver injury.
METHODS: A liver injury model was induced by carbon tetrachloride. Cytotoxicity was measured by MTT assay. The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined with an automatic multifunction-biochemical analyzer and the levels of superoxide dismutase (SOD) and TNF-α were determined following the instructions of SOD kit and TNF radioimmunoassay kit. Liver sections were stained with hematoxylin and eosin (H&E) for histological evaluation and examined under light microscope.
RESULTS: We found that GLPG can alleviate the L-02 liver cells injury induced by carbon tetrachloride (CCl4) through the measurements of ALT and AST activities and the administration of GLPG to L-02 cells did not display any toxicity. Furthermore, histological analysis of mice liver injury induced by CCl4 with or without GLPG pretreatment indicated that GLPG can significantly suppress the toxicity induced by CCl4 in mice liver. We also found that GLPG reduced TNF-α level induced by CCl4 in the plasma of mice, whereas increased SOD activity in the rat serum.
CONCLUSION: GLPG has hepatic protective activity against CCl4-induced injury both in vitro and in vivo. The possible anti-hepatotoxic mechanisms may be related to the suppression of TNF-α level and the free radical scavenging activity.
Keywords: Ganoderma lucidum proteoglycan (GLPG), Carbon tetrachloride (CCl4), Liver injury, Hepatic protective activity
INTRODUCTION
The mainstream pharmaceutical industry makes use of many plant products[1]. In traditional Chinese medicine (TCM) many products from plants are used in the treatment of a wide range of disorders including cancer[2]. Moreover, the researches on the bioactive ingredients and the investigation of the functional mechanism of natural products used in TCM are becoming increasingly important. Several substances which are successful in tumor therapy, such as betulinic acid and indirubin, have been elucidated by molecular biological and cell biological methods[3,4].
Fungi are an important source of materials in TCM. Extracts from about 200 species of fungi have been shown to stimulate immunoactivity and inhibit the growth of different kinds of tumors[5-8]. Ganoderma lucidum (Leyss. ex Fr.) Karst is a medicinal mushroom belonging to the polyporaceae of aphyllophorales. Its fruiting body is called “Lingzhi” in China and “Reishi” in Japan. For hundreds of years, this mushroom has been regarded as a TCM or a folk medicine used in the prevention and treatment of various human diseases, such as chronic bronchitis, hepatitis, hypertension, hypercholesterolemia, tumorigenic disease and immunological disorders in China and other Asian countries[9].
Carbon tetrachloride (CCl4) is a xenobiotic producing hepatotoxicity in human beings and animals[10-11]. In fact it has been shown that the trichloromethyl radical formed in the metabolism of CCl4 via the liver microsomal cytochrome P450 system, reacts rapidly with molecular oxygen to produce trichloromethyl peroxy radicals. These radicals react with unsaturated fatty acids of phospholipids present in cell membranes, initiating lipid peroxidation in liver cells[12]. Hydrogen atoms are removed from unsaturated fatty acids by such radical-created carbon-centered lipid radicals[13]. These lipid radicals quickly add molecular oxygen to form lipid peroxyl radicals which in turn abstract hydrogen atoms from other lipid molecules, thereby propagating the process of lipid peroxidation[14]. Transition metals such as copper and iron can catalyze oxygen free radical reactions that lead to peroxidation of membrane lipids or inactivation of antioxidant defense[15]. Previous studies have also reported involvement of iron as a mediator of CCl4-hepatotoxicity[16]. When the amount of reactive oxygen species (ROS) production exceeds the capacity of the endogenous cellular antioxidant system, significant cellular injury can occur[17,18]. Treatment of animals with different antioxidants such as vitamin E[19], vitamin E-like compounds[20], 5-methylthioadenosine[21], colchicines[22] and desferrioxamine[23] can significantly improve hepatic conditions by reducing CCl4-induced damage.
Ganoderma lucidum contains many biologically active components[24,25]. Previous studies suggested that Ganoderma lucidum polysaccharide (Gl-PS), one of the main efficacious ingredients of Ganoderma lucidum Karst, has been under modern pharmacological research in recent 30 years and is effective in modulating immune functions, inhibiting tumor growth, resisting invasion of various viruses[9,26,27]. Miyazaki and Nishijima[28] have separated a heteroglycan from the fruit body of Ganoderma lucidum. Moreover, Some researchers have isolated several hypoglycemic glycans from another fraction of the same crude polysaccharide[29].
Though the fruit body and the spores of Ganoderma lucidum have been used as medicines for a long time, no data is available about the protective activity of extracts from mycelium on CCl4-induced liver injury. The main aim of this study was to investigate the effects of GLPG isolated from mycelium of Ganoderma lucidum on CCl4-induced liver injury in vivo and in vitro, and the possible mechanism of the hepatic protective activity of GLPG.
MATERIALS AND METHODS
Materials and reagents
Ganoderma lucidum (FR.) Karst (Ganodermataceae) was preserved in our laboratory. RPMI-1640, trypsin, penicillin and streptomycin were purchased from Gibco BRL (Grand Island, NY, USA). SOD kit was purchased from Jiancheng Bioengineering Institute of Nanjing. TNF radioimmunoassay kit was purchased from Jiuding Corporation (Tianjin, China). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), crystal violet and trypan blue were from Sigma (St. Louis, MO). L-02 cells were obtained from China Center for Type Culture Collection (CCTCC, Wuhan, Hubei). All the other commercially available chemicals used were of the highest grade.
Extraction and purification of GLPG
Ganoderma lucidum (Fr.) Karst was extracted as previously described[30]. In brief, fungal mycelia were collected by filtration, dried and disrupted, and then the residue was extracted with 30-40 fold boiling water for 30 min. After centrifugation, the supernatant solution was concentrated under reduced pressure and intensively dialyzed. The retentate was added to three volumes of ice cold EtOH to precipitate the crude extracts. Then the sample was allowed to stand overnight at 4 °C and then centrifuged. The precipitate obtained was lyophilized. The lyophilized products were a dark brownish powder of water-soluble substance.
To purify the crude products, a portion of crude polysaccharide fraction was dissolved in double-distilled water and centrifuged to remove the insoluble materials. The supernatant was applied onto the DEAE-cellulose column and eluted with 0.1 N NaCl. Each perk eluent was separately pooled, concentrated, dialyzed, and three volumes of ice cold EtOH was added to precipitate the polysaccharides. The polysaccharide content in each fraction was determined by phenol-sulfuric acid method [31]. The GLPG fraction was lyophilized and further dissolved to indicate the concentrations for subsequent experiments.
Animal treatment and CCl4-induced hepatotoxicity
Male BALB/c mice weighing 18-22 g (6-8 wk old), were provided by Experimental Animal Center, Wuhan University, and fed with standard diet and tap water. The animals were housed in cages (4-5 each cage) and maintained at 24 ± 2 °C, under 50-60 % relative humidity in a 12 h light/ dark cycle throughout the experiment.
GLPG was diluted with saline and given orally by gavage for 20 d, at daily doses of 300, 600 and 900 mg/kg as aqueous extract. The saline control group received equal amounts of saline given orally for 20 d. During this treatment, carbon tetrachloride was introduced to the mice orally (1600 mg/kg CCl4, mixed with corn oil) for the last 3 d. The same volume of corn oil was given to vehicle control mice orally for the last 3 d. After 3 d, the mice were euthanized under ether anaesthesia, blood and liver samples were collected.
Cell culture and liver cells injury induction
L-02 cells were cultured in RPMI-1640 medium supplemented with 10 % heat inactived fetal bovine serum (FBS), 100 IU /mL penicillin, 100 μg/mL streptomycin and 250 U/L insulin. The cells were maintained at 37 °C in a humidified atmosphere containing 5 mL/L CO2 and subcultured 2-3 times a week.
Semi-confluent L-02 cells in 24-well culture plate (Falcon, NJ, USA) were divided into control group, dimethyl sulfoxide (DMSO) vehicle group, GLPG group, and CCl4-induced hepatotoxicity group (20 mmol/L final concentration) with or without GLPG at various concentrations. After 4 h the culture supernatant was collected and stored at -20 °C.
Cytotoxicity assay
Cytotoxicity was measured by MTT assay. The cells were seeded in 96-well culture plate (Falcon, NJ, USA) at the concentration of 4×103 cells per well in 100 μL medium. After incubation of the cells for 12 h at 37 °C, various concentrations of GLPG were added and the incubation was continued for 48 h, or at a given concentration of GLPG (200 μg/mL final concentration) the incubation time was prolonged for 72 h. Then the viable cells were determined by MTT reduction assay. In brief, MTT was dissolved in phosphate-buffered saline (PBS) at 5 mg/mL and sterilized by filtration to remove insoluble reside present in some batches of MTT. At the indicated time, the MTT solution (20 μL) was added to each well. After incubation for 5 h, cell culture medium was removed carefully, and 150 μL dimethyl sulfoxide (DMSO) was added to each well and mixed thoroughly to dissolve the dark purple crystals. The plate was incubated for 10 min at room temperature, to ensure that all formazane were dissolved. The plate was thenread on a Perkin-Elmer ELISA reader (HTS7000 plus) at a wavelength of 570 nm.
The effects of GLPG on cell proliferation and viability were compared according to the commonly accepted method of staining cells with trypan blue. L-02 cells were seeded in 96-well plate at the concentration of 2×103 cells per well in 100 μL of RPMI-1640 medium. The cells were incubated with or without various concentrations of GLPG for 48 h. Then the cells were trypsinized and collected. The number of cells was determined in a Neubauer hemacytometer using the trypan blue exclusion method, and the mean values were calculated.
Assay of ALT and AST activities in vivo and in vitro
As a marker of hepatocyte necrosis, the activities of ALT and AST were determined with an automatic multifunction-biochemical analyzer (Beckman, USA) in serum and cell culture medium.
Superoxide dismutase (SOD) determination
Wistar rats weighing 80-120 g (7 wk old) were divided into 3 groups, 22 rats each group. GLPG was diluted with saline and given orally for 10 wk at daily doses of 1 000 and 3 000 mg/kg as an aqueous extract. The saline control group received equal amount of saline given orally for 10 wk. After 10 wk the rats were sacrificed by decapitation and the serum was collected to determine the activity of SOD, according to the instructions of the kit used.
SOD measurement method was based on the principle in which xanthine reacts with xanthine oxidase to generate superoxide radicals reacting with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The SOD activity was then measured as previously described [32].
Histopathological examination
Liver was removed, fixed overnight in 10% buffered formalin and paraffin-embedded. The sections were stained with hematoxylin and eosin (H&E) for histological evaluation and examined under light microscope. In brief, 4-µm thick sections of paraffin-embedded mice liver were dewaxed in xylene, rehydrated in graded alcohol series, and washed with distilled water for 2 min. Subsequently, the sections were stained with hematoxylin for 5 min at room temperature. After 15 min, the sections were counterstained with eosin for 2 min, dehydrated in graded alcohol series, washed with xylene, and blocked by rosin. H&E- stained slides were observed under microscope at × 40 magnifications.
Measurement of plasma TNF-ɑ level
Plasma TNF-ɑ level was determined by TNF radio-immunoassay kit. All the mice were introduced orally with CCl4 with or without GLPG, and the mice in control group were euthanized, then the blood was collected in the tubes with previous addition of 25 μL of heparin solution (4 000 IU). The plasma samples obtained after centrifugation at 3 000 r/min for 10 min at 4 °C were stored at -70 °C until assay.
The TNF-ɑ levels of plasma samples were measured by sequential-saturation-type assay as previously described [33]. In brief, 200 μL of standard sample, the plasma samples, and the control were added to each tube, then 100 μL anti-TNF-ɑ antibody was added to each tube and mixed thoroughly. After 18 h of incubation at 4 °C, 100 μL of solution containing radioactive label was added to each tube and mixed. After a further incubation for 3 h at 37 °C, 1 000 μL of the secondary antibody was added for 15 min. the tubes were centrifuged at 3 600 r/min for 20 min, the supernatant was discarded carefully. The gamma cpm of deposition was measured with a gamma counter, and the plasma TNF-α levels were measured by standard sample curve diagram. TNF-α values were expressed as ng/mL.
Statistical analysis
Data were expressed as mean ± SD. The difference between the means of two groups was evaluated by ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Effects of GLPG on cytotoxicity
Compared with the L-02 cells administrated with GLPG and the control cells, there was no obvious change occurred in cell growth and morphology (Figures 1A and 1B). Trypan blue exclusion method showed that the total cell number of L-02 cells treated with GLPG was approximately 96 % as compared to that of the control cells Even at the GLPG concentration was 1 024 μg/mL. And we also found that no toxic response and death was occurred in vivo experiment (data not shown). And we also found that no toxic response and death was occurred in vivo experiment (data not shown).
Figure 1.
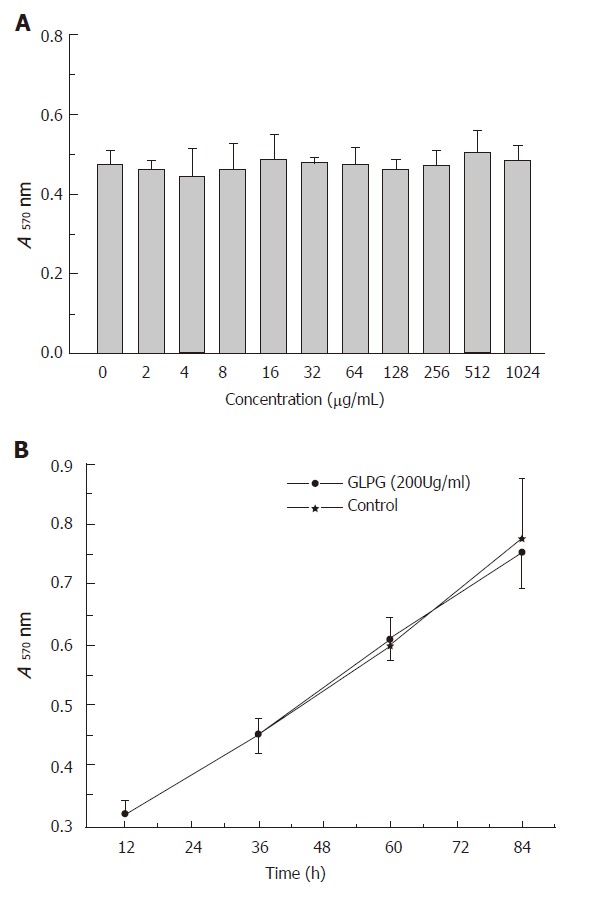
Effects of GLPG on proliferation of L-02 cells treated with GLPG at the concentration of 2-1024 μg/mL for 2 d (A) and at the concentration of 200 μg/mL for 3 d (B). Viable cells were detected every 24 h. The cells untreated with GLPG were used as controls in experiment. Results shown represent the mean ± SD for at least three separate experiments.
Detection of liver weight and assay of ALT and AST activities in vivo and in vitro
Compared to the control and GLPG groups, GLPG (300, 600 and 900 mg/kg) markedly ameliorated hepatic injury induced by CCl4. The liver weight (Table 1) and serum ALT and AST activities (Table 2) were observed in mice 24 and 72 h after CCl4 administration. The liver weight did not increase compared to CCl4-treated group. Furthermore, the serum ALT and AST activities in GLPG pretreatment groups induced by CCl4 were lower than those in CCl4-treated group. Simultaneously, GLPG treatment reduced the serum ALT and AST activities in a dose-dependent manner within the similar range of concentrations, indicating that GLPG showed anti-hepatotoxic activities on CCl4-induced liver injury.
Table 1.
Effect of GLPG on liver weight in mice with CCl4-induced hepatic injury after 72 h (mean ± SD)
| Group | Liver weight (g) |
| Control | 1.77 ± 0.26 |
| GLPG (500 mg/kg) | 1.74 ± 0.21 |
| CCl4 (1600 mg/kg) | 2.22 ± 0.34 |
| CCl4 (1600 mg/kg) + GLPG(300 mg/kg) | 1.67 ± 0.17 |
| CCl4 (1600 mg/kg) + GLPG(600 mg/kg) | 1.57 ± 0.18 |
| CCl4 (1600 mg/kg) + GLPG(900 mg/kg) | 1.52 ± 0.14 |
aP<0.05 vs CCl4-treatment group; bP<0.01 vs control group; n=10 mice.
Table 2.
Effects of GLPG on ALT and AST activities in mice with CCl4-induced hepatic injury after 24 h and 72 h (mean±SD)
| Group | ALT(U/L) | AST(U/L) | ||
| 24h | 72h | 24h | 72h | |
| Control | 54.38 ± 6.20 | - | 44.50 ± 34.36 | - |
| GLPG | 74.00 ± 17.14 | - | 51.75 ± 29.43 | - |
| (500mg/kg) | ||||
| CCl4 | 642.50 ± 225.33 | 105.00 ± 39.64 | 147.50 ± 58.80 | 180.25 ± 45.51 |
| (1600mg/kg) | ||||
| CCl4 | 433.38 ± 133.97b | 96.56 ± 27.84 | 99.88 ± 40.47a | 161.13 ± 18.29 |
| (1600mg/kg)+GLPG (300mg/kg) | ||||
| CCl4 | 374.00 ± 107.34c | 87.30 ± 15.60 | 61.25 ± 26.50c | 151.63 ± 32.67 |
| (1600mg/kg)+GLPG(600mg/kg) | ||||
| CCl4 | 316.50 ± 98.76c | 79 .70 ± 17.28a | 56.75 ± 36.58c | 114.75 ± 67.13a |
| (1600mg/kg)+GLPG (900mg/kg) | ||||
P < 0.05 vs CCl4 treatment group;
P< 0.01,
P< 0.001 vs control group; n = 20 mice.
Administration of GLPG also showed anti-hepatotoxic activity in L-02 cells injury induced by CCl4. GLPG at concentrations of 100, 250 and 500 μg/mL suppressed the activities of ALT and AST in a dose-dependent manner (Figure 2).
Figure 2.
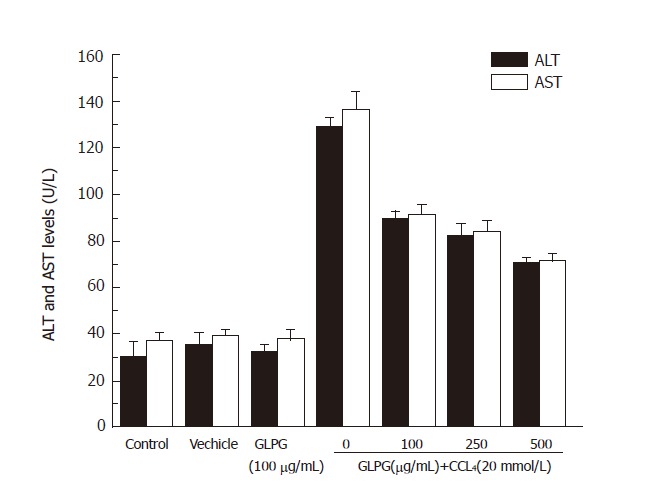
Effects of GLPG on ALT and AST activities in CCl4-induced L-02 cell (n = 3) injury. Compared to control group, GLPG suppressed the activities of ALT and AST in the other groups. Results shown represent the mean ± SD from three separate experiments.
Superoxide dismutase (SOD) assay
According to the results of SOD assay, the activity of SOD in the serum of rats pretreated with GLPG increased significantly compared with that of control group. As shown in Figure 3, compared to the control group, the activities of SOD in the group administered orally with GLPG at the concentrations of 1 000 mg/kg and 3 000mg/kg increased by 57.2 % and 70.6 %, respectively.
Figure 3.
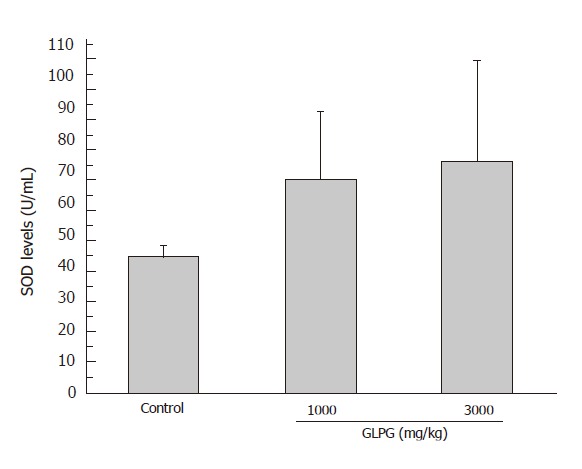
Effect of GLPG on SOD activity in serum of rats. The rats were administered orally with or without GLPG at 1000 mg/kg and 3000 mg/kg. The increment of SOD activities was 57.2 % and 70.6 %, respectively.
Histological analysis
We examined the effects of GLPG at various doses (300, 600 and 900 mg/kg) on histopathological changes of CCl4-induced liver injury in mice in vivo. GLPG showed strong hepatic protective activity. Significant changes of liver tissue were observed in carbon tetrachloride-induced group after 24 h and 72 h (Figures 4C and 4D) as compared to the control group (Figure 4A) and the GLPG pretreated group (Figure 4B). No obvious histopathological change was observed in control and GLPG groups. and Both the control group and the GLPG group had no infiltrations and hemorrhagic signs. Figure 4C shows the histological changes after 24 h of CCl4 post-administration with a marked liver injury. After 72 h of CCl4 post-administration, liver lobule structures disappeared and necrotic hepatic tissues induced by CCl4 with pretreatment GLPG (300 mg/kg) after 24 h (Figure 4E) and 72 h (Figure 4F) were had no evident recovery. liver injury and necrotic hepatic tissue recovered markedly. As shown in Figure 4G-J, basophilic granules and many double-nuclear regenerative liver cells appeared 24 and 72 h after GLPG pretreatment at different doses (600 mg/kg and 900 mg/kg). These results showed that GLPG could alleviate CCl4-induced liver injury in vivo in mice.
Figure 4.
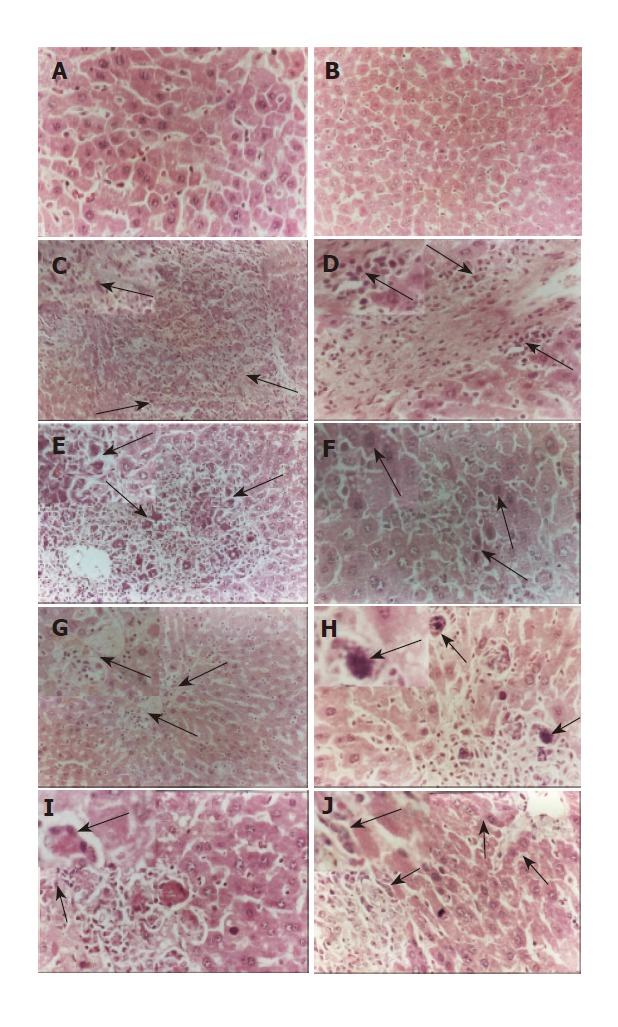
Histological changes (marked by arrows) of CCl4-induced hepatic injury in the presence or absence of GLPG in mice by hematoxylin-eosin (H&E) staining in control group (A), GLPG group (B), and CCl4-induced liver injury group (C-J) 24 and 72 h after GLPG pretreatment at different doses(0, 300, 600 and 900 mg/kg).
Measurement of plasma TNF-α level
Figure 5 showed that the changes of TNF-ɑ levels in the plasma of experimental groups 48 h after CCl4 treatment with or without GLPG at different doses. Low TNF-α levels were detected in the plasma of the control group and GPLG group. In contrast, a marked rise of TNF-ɑ level was found in the plasma of CCl4-pretreatment group. Simultaneously, GLPG (500 mg/kg) can decrease the level of TNF-α in the plasma of mice treated with CCl4 (data not shown). Administration of GLPG at 300, 600 and 900 mg/kg significantly suppressed the levels of TNF-ɑ in dose-dependent manner after 48 h. The mean values of TNF-α levels at 48 h were 34 %, 51.3 % and 64.2 % at 300, 600 and 900 mg/kg respectively.
Figure 5.
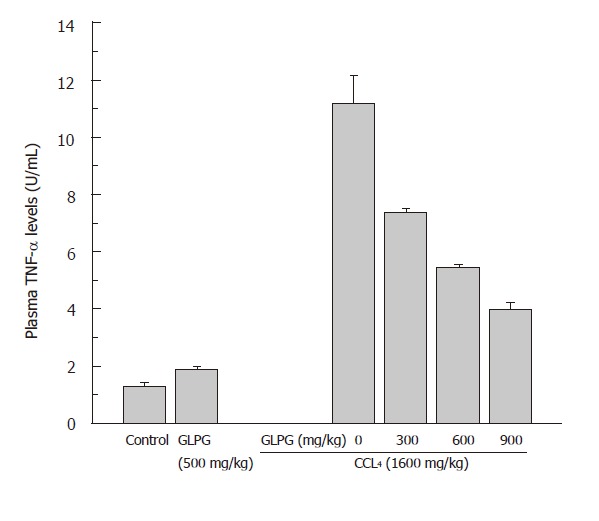
Effect of GLPG on the levels of TNF-ɑ in the plasma of mice, as determined by TNF-ɑ radioimmunoassay kit. Compared with control group, administration of GLPG at various doses could significantly suppress the level of TNF-ɑ in a dose-dependent manner after 48 h. Data are the mean ± SD from 4 determinations.
DISCUSSION
Rapid lipid peroxidation of the membrane structural lipids has been proposed as the basis of CCl4 liver toxicity[34-35]. In fact the first step in liver injury induced by CCl4 is the formation of reactive oxygen species (ROS) that may further lead to membrane peroxidation or cell injury. There is evidence that Kupffer cells as well as hepatocytes have inducible cytochrome P-450 and are capable of metabolizing CCl4 or other toxic species[36]. Moreover, it was reported that Kupffer cell activation is a crucial step in hepatocyte injury induced by CCl4 or other toxic agents[37-38]. Transition metal ions like Cu+2 and Fe+3 play an important role as components of proteins essential for biological functions. However, these metals can also initiate Haber-Weis and Fenton reactions where superoxide anions are transformed into detrimental hydroxyl radicals which are in turn responsible for attack of the membrane polyunsaturated fatty acids[15]. The use of metal chelating agents may have therapeutic effect by reducing the oxidative burst and the consequent membrane lipid peroxidation[39-40]. Previous findings have shown that transition metals can mediate free radical production after CCl4 administration in rats[16,41-42]. Treatment of these animals with agents capable of chelating these metal ions can protect hepatocytes against damage by reducing oxidative burst and lipid peroxidation[16,41-42].
In this study, a water soluble substance, Ganoderma lucidum proteoglycan was isolated from mycelia of Ganoderma lucidum by EtOH precipitation and DEAD-cellulose column chromatography. GLPG is a proteoglycan consisting of about 86.4 % carbohydrates and has antioxidant activity against CCl4-induced liver injury. Treatment with GLPG could ameliorate hepatic injury induced by CCl4. Administration of GLPG significantly decreased ALT and AST activities in CCl4-induced liver injury in vivo and in vitro (Table 2 and Figure 2). Histological changes observed in GLPG-pretreatment groups were less than in CCl4-induced group. Some histological changes, such as hemorrhage, inflammatory infiltration and necrosis in hepatic tissue, were simultaneously improved after pretreatment with GLPG. These results suggested that the hepatic protective activity of GLPG against CCl4-induced liver injury was in a dose-dependent manner.
Though the scavenging effect of Ganoderma lucidum polysaccharide (Gl-PS) has been reported [25,43-46], no report is availabe about the hepatic protective activity of GLPG. In our study, the activity of SOD after pretreatment with GLPG increased significantly in the serum of rats, while SOD could eliminate peroxide in vivo, suggesting that GLPG might scavenge the free radicals induced by CCl4.
Although it is generally believed that the hepatic protective activity of GLPG is mainly due to its ability to scavenge free radicals induced by CCl4. However, there may be other mechanisms. A large amount of this cytokine may be interpreted as a progression of hepatic damage[47]. TNF-α induced by CCl4 may contribute to cellular damage in liver injury. The antioxidant activity of GLPG can reduce TNF-α level in the plasma of mice, thus inhibit inflammation occurrence. Though the mechanism of GLPG down-regulates CCl4-induced TNF-α level in vivo in mice remains unknown, the suppression of GLPG on TNF-α level might play an important role in its hepatic protective activity against CCl4-induced liver injury.
In conclusion, GLPG exhibits strong hepatic protective activity against CCl4-induced liver injury both in vivo and in vitro. And the possible anti-hepatotoxic mechanisms may be related to the suppression of TNF-α level and the free radical scavenging activity.
Footnotes
Supported by a grant from the Institute of Virology, College of Life Sciences, Wuhan University
Co-first-authors: Xiao-Jun Yang
S- Editor Wang J L- Editor Wang XL E- Editor Wu M
References
- 1.Pezzuto JM. Plant-derived anticancer agents. Biochem Pharmacol. 1997;53:121–133. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Yang SL, Lian B. Exploration of clinical study of antileukemia cell drug-resistance by traditional Chinese medicine. Zhong Xi Yi Jie He Za Zhi. 1995;15:636–637. [PubMed] [Google Scholar]
- 3.Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 4.Hoessel R, Leclerc S, Endicott JA, Nobel ME, Lawrie A, Tunnah P, Leost M, Damiens E, Marie D, Marko D, et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat Cell Biol. 1999;1:60–67. doi: 10.1038/9035. [DOI] [PubMed] [Google Scholar]
- 5.Wang HX, NG TB, Liu WK, Ooi VE, Chang ST. Polysaccharide-peptide complexes from the cultured mycelia of the mushroom Coriolus versicolor and their culture medium activate mouse lymphocytes and macrophages. Int J Biochem Cell Biol. 1996;28:601–607. doi: 10.1016/1357-2725(95)00157-3. [DOI] [PubMed] [Google Scholar]
- 6.Wang HX, Liu WK, Ng TB, Ooi VE, Chang ST. The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology. 1996;31:205–211. doi: 10.1016/0162-3109(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 7.Ng TB. A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (Basidiomycetes: Polyporaceae) Gen Pharmacol. 1998;30:1–4. doi: 10.1016/s0306-3623(97)00076-1. [DOI] [PubMed] [Google Scholar]
- 8.Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: a modern perspective. Crit Rev Immunol. 1999;19:65–96. [PubMed] [Google Scholar]
- 9.Lin ZB. Modern research of Ganoderma Lucidum. Ed. Beijing: Beijing Medical University Press; 2001. pp. 219–309. [Google Scholar]
- 10.Brattin WJ, Glende EA, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- 11.Comporti M. Lipid peroxidation and cellular damage in toxic liver injury. Lab Invest. 1985;53:599–623. [PubMed] [Google Scholar]
- 12.Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 13.McCay PB, Lai EK, Poyer JL, DuBose CM, Janzen EG. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J Biol Chem. 1984;259:2135–2143. [PubMed] [Google Scholar]
- 14.Muriel P. Peroxidation of lipids and liver injury. In: Baskin SI, Salem H, eds , editors. Antioxidants, oxidants and free radicals. Washington, DC: Taylor and Francis Publications; 1987. pp. 237–257. [Google Scholar]
- 15.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes M, Siegers CP. The role of iron in the paracetamol- and CCl4-induced lipid peroxidation and hepatotoxicity. Chem Biol Interact. 1985;55:327–334. doi: 10.1016/s0009-2797(85)80139-3. [DOI] [PubMed] [Google Scholar]
- 17.Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984;222:1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 19.Södergren E, Cederberg J, Vessby B, Basu S. Vitamin E reduces lipid peroxidation in experimental hepatotoxicity in rats. Eur J Nutr. 2001;40:10–16. doi: 10.1007/pl00007381. [DOI] [PubMed] [Google Scholar]
- 20.Campo GM, Squadrito F, Ceccarelli S, Calò M, Avenoso A, Campo S, Squadrito G, Altavilla D. Reduction of carbon tetrachloride-induced rat liver injury by IRFI 042, a novel dual vitamin E-like antioxidant. Free Radic Res. 2001;34:379–393. doi: 10.1080/10715760100300321. [DOI] [PubMed] [Google Scholar]
- 21.Simile MM, Banni S, Angioni E, Carta G, De Miglio MR, Muroni MR, Calvisi DF, Carru A, Pascale RM, Feo F. 5'-Methylthioadenosine administration prevents lipid peroxidation and fibrogenesis induced in rat liver by carbon-tetrachloride intoxication. J Hepatol. 2001;34:386–394. doi: 10.1016/s0168-8278(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 22.Das D, Pemberton PW, Burrows PC, Gordon C, Smith A, McMahon RF, Warnes TW. Antioxidant properties of colchicine in acute carbon tetrachloride induced rat liver injury and its role in the resolution of established cirrhosis. Biochim Biophys Acta. 2000;1502:351–362. doi: 10.1016/s0925-4439(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 23.Mansour MA. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000;66:2583–2591. doi: 10.1016/s0024-3205(00)00592-0. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Rhee HM. Cardiovascular effects of mycelium extract of Ganoderma lucidum: inhibition of sympathetic outflow as a mechanism of its hypotensive action. Chem Pharm Bull (Tokyo) 1990;38:1359–1364. doi: 10.1248/cpb.38.1359. [DOI] [PubMed] [Google Scholar]
- 25.Lin JM, Lin CC, Chen MF, Ujiie T, Takada A. Radical scavenger and antihepatotoxic activity of Ganoderma formosanum, Ganoderma lucidum and Ganoderma neo-japonicum. J Ethnopharmacol. 1995;47:33–41. doi: 10.1016/0378-8741(95)01251-8. [DOI] [PubMed] [Google Scholar]
- 26.Lin ZB. Recent advances in Chinese Herbal Drugs-Actions and Uses. Ed. Beijing: Scientific Publishing House; 1999. pp. 133–140. [Google Scholar]
- 27.Kim YS, Eo SK, Oh KW, Lee C, Han SS. Antiherpetic activities of acidic protein bound polysacchride isolated from Ganoderma lucidum alone and in combinations with interferons. J Ethnopharmacol. 2000;72:451–458. doi: 10.1016/s0378-8741(00)00263-4. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Nishijima M. Studies on fungal polysaccharides. XXVII. Structural examination of a water-soluble, antitumor polysaccharide of Ganoderma lucidum. Chem Pharm Bull (Tokyo) 1981;29:3611–3616. doi: 10.1248/cpb.29.3611. [DOI] [PubMed] [Google Scholar]
- 29.Hikino H, Konno C, Mirin Y, Hayashi T. Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Med. 1985:339–340. [PubMed] [Google Scholar]
- 30.Liu J, Yang F, Ye LB, Yang XJ, Timani KA, Zheng Y, Wang YH. Possible mode of action of antiherpetic activities of a proteoglycan isolated from the mycelia of Ganoderma lucidum in vitro. J Ethnopharmacol. 2004;95:265–272. doi: 10.1016/j.jep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugar and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 32.Woolliams JA, Wiener G, Anderson PH, McMurray CH. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci. 1983;34:253–256. [PubMed] [Google Scholar]
- 33.Teppo AM, Maury CP. Radioimmunoassay of tumor necrosis factor in serum. Clin Chem. 1987;33:2024–2027. [PubMed] [Google Scholar]
- 34.Rechnagel RO, Glende EA. Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973;2:263–297. doi: 10.3109/10408447309082019. [DOI] [PubMed] [Google Scholar]
- 35.Poli G, Albano E, Dianzani MU. The role of lipid peroxidation in liver damage. Chem Phys Lipids. 1987;45:117–142. doi: 10.1016/0009-3084(87)90063-6. [DOI] [PubMed] [Google Scholar]
- 36.Koop DR, Chernosky A, Brass EP. Identification and induction of cytochrome P450 2E1 in rat Kupffer cells. J Pharmacol Exp Ther. 1991;258:1072–1076. [PubMed] [Google Scholar]
- 37.Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993;119:275–279. doi: 10.1006/taap.1993.1069. [DOI] [PubMed] [Google Scholar]
- 38.Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- 39.Center SA. Chronic liver disease: current concepts of disease mechanisms. J Small Anim Pract. 1999;40:106–114. doi: 10.1111/j.1748-5827.1999.tb03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Armstrong C, Leong W, Lees GJ. Comparative effects of metal chelating agents on the neuronal cytotoxicity induced by copper (Cu+2), iron (Fe+3) and zinc in the hippocampus. Brain Res. 2001;892:51–62. doi: 10.1016/s0006-8993(00)03195-4. [DOI] [PubMed] [Google Scholar]
- 41.Siegers CP, Steffen B, Younes M. Antidotal effects of deferrioxamine in experimental liver injury--role of lipid peroxidation. Pharmacol Res Commun. 1988;20:337–343. doi: 10.1016/s0031-6989(88)80070-5. [DOI] [PubMed] [Google Scholar]
- 42.Tsokos-Kuhn JO. Evidence in vivo for elevation of intracellular free Ca2+ in the liver after diquat, acetaminophen, and CCl4. Biochem Pharmacol. 1989;38:3061–3065. doi: 10.1016/0006-2952(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 43.Kim KC, Kim IG. Ganoderma lucidum extract protects DNA from strand breakage caused by hydroxyl radical and UV irradiation. Int J Mol Med. 1999;4:273–277. [PubMed] [Google Scholar]
- 44.Lee JM, Kwon H, Jeong H, Lee JW, Lee SY, Baek SJ, Surh YJ. Inhibition of lipid peroxidation and oxidative DNA damage by Ganoderma lucidum. Phytother Res. 2001;15:245–249. doi: 10.1002/ptr.830. [DOI] [PubMed] [Google Scholar]
- 45.Shi YL, James AE, Benzie IF, Buswell JA. Mushroom-derived preparations in the prevention of H2O2-induced oxidative damage to cellular DNA. Teratog Carcinog Mutagen. 2002;22:103–111. doi: 10.1002/tcm.10008. [DOI] [PubMed] [Google Scholar]
- 46.You YH, Lin ZB. Protective effects of Ganoderma lucidum polysaccharides peptide on injury of macrophages induced by reactive oxygen species. Acta Pharmacol Sin. 2002;23:787–791. [PubMed] [Google Scholar]
- 47.Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000;174:160–171. doi: 10.1034/j.1600-0528.2002.017411.x. [DOI] [PubMed] [Google Scholar]


