Abstract
AIM: To evaluate contrast-enhanced ultrasonography (CEUS) using SonoVue® in the detection of liver metastases in patients with known extrahepatic primary tumors versus the combined gold standard comprising CT, MRI and clinical/histological data.
METHODS: It is an international multicenter study, and there were 12 centres and 125 patients (64 males, 61 females, aged 59 ± 11 years) involved, with 102 patients per protocol. Primary tumors were colorectal in 35 %, breast in 27 %, pancreatic in 17 % and others in 21 %. CEUS using SonoVue® was employed with a low-mechanical-index technique and contrast-specific software using Siemens Elegra, Philips HDI 5000 and Acuson Sequoia; continuous scanning for at least five minutes.
RESULTS: CEUS with SonoVue® increased significantly the number of focal liver lesions detected versus unenhanced sonography. In 31.4 % of the patients, more lesions were found after contrast enhancement. The total numbers of lesions detected were comparable with CEUS (55), triple-phase spiral CT (61) and MRI with a liver-specific contrast agent (53). Accuracy of detection of metastatic disease (i.e. at least one metastatic lesion) was significantly higher for CEUS (91.2 %) than for unenhanced sonography (81.4 %) and was similar to that of triple-phase spiral CT (89.2 %). In 53 patients whose CEUS examination was negative, a follow-up examination 3-6 mo later confirmed the absence of metastatic lesions in 50 patients (94.4 %).
CONCLUSION: CEUS is proved to be reliable in the detection of liver metastases in patients with known extrahepatic primary tumors and suspected liver lesions.
Keywords: CT, MRI, Metastasis, Detection, SonoVue®
INTRODUCTION
The continuous routine follow-up of cancer patients requires an easily available, reliable and cost-effective diagnostic method for the detection of liver metastases. Sonography is a widely used method for the detection of liver lesions, but is generally regarded as inferior to contrast-enhanced computed tomography (CT) and mag-netic resonance imaging (MRI). The detection of liver lesions with acoustic properties similar to those of the surrounding normal liver parenchyma has always been the significant limitation of grey scale (B-mode) imaging. To improve the detection of focal liver lesions, ultrasound imaging must also provide information on vascularity, exploiting the differences in blood flow between normal and pathological tissue. The concept of contrast-enhancing agents is not new, being derived from (bolus) dynamic computed tomography (CT) and magnetic resonance imaging (MRI).
Recent advances in contrast-enhanced techniques using high-mechanical-index imaging with Levovist® have improved the detection rate to a level similar to that attained using CT and MRI. This is clear from several, mostly single-centre, studies[1-5]. Owing to the high spatial resolution of sonography, CEUS may also detect subcentimetre metastases.
But there are still some important limitations since contrast-enhanced high-mechanical-index techniques must be performed intermittently and the examination technique is therefore difficult. It is expected that real-time scanning over the whole enhancement period of approximately three to five minutes using more stable contrast agents such as SonoVue® will facilitate routine use, making the examination easier and more reliable [6].
The purpose of this prospective international mul-ticenter study using a real-time technique was to assess the ability of low-mechanical-index contrast-enhanced ultrasound (CEUS) to detect liver metastases in the presence of a known primary tumor versus a combined gold standard.
MATERIALS AND METHODS
The rationale of this study was to evaluate the diagnostic value of dynamic contrast sonography for the assessment of liver metastases versus established reference methods (CT and MRI) in combination with all clinical data except ultrasound techniques as a combined gold standard.
Study design
The study was a prospective, multicenter, open-label, intraindividual comparison. The new diagnostic procedure, dynamic contrast-enhanced ultrasound (CEUS), was compared with conventional sonography (without assessment of vascularity using contrast enhancement) and contrast-enhanced triple-phase CT, the method most commonly used in routine diagnosis. Furthermore, contrast-enhanced MRI was performed and the results from other diagnostic tests (biopsy, clinical data, etc.) were collected where available, to define the final diagnosis using the combined gold standard.
Patients
Between August 2001 and June 2002, 131 patients were enrolled at 12 European centres (see list of contributing centres). Included were male and female patients with known extrahepatic primary tumors and an indication for diagnostic assessment of possible liver metastases. Exclusion criteria were age < 18 years, pregnant or lactating women, known allergies to ingredients of the contrast agent, unstable medical conditions impairing the diagnostic procedure or contraindications to such a procedure, insufficient sonographic window for liver examination and participation in another investigational study.
Signed informed consent was obtained from all study patients before enrollment. The study protocol was approved by the ethics committee of the Landesärztekammer Baden-Württemberg and the local ethics committee of each centre. The study complied with Good Clinical Practice and the Declaration of Helsinki.
Methods and procedures
Ultrasonography
Ultrasound examinations were performed using a high-end sonographic scanner [Siemens Elegra (Ensemble Contrast Imaging), Acuson Sequoia (Coherent Contrast Imaging), Philips ATL HDI 5000 (Pulse Inversion Harmonic Contrast Imaging] with contrast-enhanced imaging software as indicated in square brackets. The contrast examinations were performed with low insonation power (low-MI imaging, mean MI = 0.22), to avoid destruction of microbubbles. Optimized pre-settings were provided for each type of machine, adjusting the imaging parameters to the contrast agent used.
BR1 (SonoVue®, Bracco International) was used as ultrasound contrast agent. BR 1 contains microbubbles of sulphur hexafluoride gas surrounded by a flexible phospholipid shell, allowing contrast-specific imaging at low insonation power. Owing to the size of the SonoVue® microbubbles (mean 2.5 µm), this contrast agent remains (as a so-called blood pool agent) within the vascular system, unlike current X-ray and MRI contrast agents which spread into the interstitial fluid. Thus contrast wash-in and wash-out can be assessed continuously during the whole enhancement phase.
Using a 20-gauge needle, BR1 was injected as an intravenous bolus of 2.4 mL (1 mL/second) into the cubital vein, followed by a 3-10 mL saline bolus for flushing. Additional injections of 4.8 mL (up to 3 contrast injections) were given, if required, to allow optimization of the procedure. There was an interval of at least 6 min between each injection of SonoVue®. To allow contrast clearance of the previous contrast injection the bubbles were destroyed by using high insonation power.
Prior to injection of the contrast agent (native) and throughout contrast enhancement (arterial and portal-venous phase), the entire liver tissue was examined by conventional B-mode ultrasonography as recently described[2,7]. Unenhanced and contrast-enhanced examinations (including the native and arterial and portal-venous enhancement phases) were evaluated separately.
All examinations were documented on S-VHS videotapes and some examinations were additionally digitally stored on magnetic-optical discs (MOD).
Computed tomography (CT)
Standard triple-phase spiral CT examinations were per-formed and evaluated in the radiology departments or associated radiology units of each centre. The CT examinations include native, arterial and portal-venous phase scans, using bolus injections of 123 mL (mean, range 100-370 mL, in one patient 60 mL) iodinated contrast agent (300-350 mg/mL). In most cases a multislice scanner was used. Examinations were performed with a slice thickness ≤ 5 mm (in two patients 8 mm).
Magnetic resonance imaging (MRI)
Standard MRI examinations were performed and evaluated in the radiology departments or associated radiology units of each centre. The MRI examinations included native and liver-specific late-phase scans with T1 and T2 weighted images obtained by SE, TSE and/or GE sequences, using a liver-specific contrast agent (in 18 patients only dynamic phase with a Gd agent). Slice thickness was ≤ 6 mm (in 10 patients up to 10 mm).
Final reference diagnosis
The final reference diagnosis was defined by combining all available information from imaging (CT and MRI examinations) plus additional information from histology (17), surgery (8) and other clinical examinations (4). Thus the final reference diagnosis includes all information available at the end of the diagnostic evaluation, with the exception of the results from the ultrasound examination (being the test method).
Follow-up examination
Patients with negative findings at the initial examination (i.e. no metastatic lesions detected in the liver) were asked to come back for a follow-up examination 3-6 mo after the initial examination, either US, CT or MRI. This follow-up examination was used as an additional reference standard for patients with negative initial contrast-enhanced sono-graphy, to assess the predictive clinical value of contrast-enhanced sonography.
Safety and tolerability of the ultrasound contrast agent
All adverse events occurring during the examination and a 2 h post-examination observation period were collected and listed, irrespective of a causal relationship. Adverse events were assessed with regard to severity and causal relationship.
Statistical analysis
Unenhanced and contrast-enhanced sonographies were compared by calculating the percentage difference with the two-sided 95% confidence interval. A difference of 10 % between methods was defined a priori as clinically significant. The assessment of contrast-enhanced sonography invariably included native (representing tissue) as well as contrast-enhanced sequences (representing vascularity), in parallel to the assessment of CT and MRI. This reflects clinical reality, where vascularity information is always assessed in combination with tissue information. Contrast sequences are performed as a supplement to native baseline sequences, not as an alternative.
For all methods sensitivity, specificity, accuracy, and negative and positive predictive values were calculated together with the respective 95% confidence intervals, using the combined final reference diagnosis as gold standard. Thus CT, MRI, histology, clinical data, etc, but not ultrasound were part of the gold standard. This could introduce a bias in favour of CT and MRI (for example, if sonography showed a small metastasis but all other CT + MRI did not, sonography was assessed as false positive), especially in cases where invasive confirmation was impossible for ethical reasons (6 lesions in 3 patients).
For the assessment of lesion numbers only patients having at most 8 lesions were considered, since in cases with a very high number of lesions the result is more indicative of the counting efforts and moreover there is no real clinical relevance. For the assessment of the presence of metastatic disease a patient was rated as positive if at least one lesion classifiable (on the basis of characteristic features mainly of the perfusion pattern, e.g. lack of portal-venous enhancement) as metastasis could be identified. Owing to the inclusion criteria, all patients had a current or previous primary nonhepatic tumor. The comparison of the methods included all patients for whom valid results from both methods were available, irrespective of the number of lesions. The assessment of follow-up data included all patients having negative metastatic disease at the initial examination (no metastases found with CEUS) and having follow-up data available.
As statistical tests the Wilcoxon signed rank test (com-parison of lesion numbers) and the McNemar test (assessment of metastatic disease) were used. For the comparison of CEUS vs CT the test was performed as a two-sided test (testing equivalence) and as a one-sided test (testing non-inferiority of CEUS vs CT).
RESULTS
Study population
Epidemiological data are summarized in Table 1. All 131 patients enrolled had a primary extrahepatic tumor (Table 2). 125 of the 131 patients received an adequate dose of the study medication (ultrasound contrast agent) and were considered eligible. 102 patients had no relevant protocol violations (per protocol population) and were used for the efficacy analysis. The reasons for exclusion from the primary efficacy analysis were (multiple instances possible): reference examination outside the stipulated time window of +/- 14 d (11 patients), reference examination missing or incomplete (8 patients), and inadequate visualization of the entire liver (5 patients).
Table 1.
Demographic data of the study population
| Parameter | Mean | Range | |
| Age (yr) | 59 ± 11 | 22 - 82 | |
| Weight (kg) | 76 ± 13 | 45 - 115 | |
| Height (cm) | 170 ± 8.9 | 152 - 192 | |
| Sex | 51.2 % male, 48.8 % female | ||
| Race | 99.2 % white, 0.08 % Asian |
Table 2.
Nature of primary tumor
| Parameter | Mean | Range |
| Primary tumor | n | % |
| Colorectal tumor | 44 | 35.2 |
| Breast tumor | 27 | 21.6 |
| Pancreas tumor | 17 | 13.6 |
| Bronchial tumor | 7 | 5.6 |
| Gastric tumor | 6 | 4.8 |
| Renal tumor | 3 | 2.4 |
| Endocrine gastrointestinal tumor | 2 | 1.6 |
| Melanoma | 2 | 1.6 |
| Others | 23 | 18.4 |
| In 6 patients, several primary tumors were present | ||
The examination of the liver was performed as part of the initial staging in 63 patients (50.4%), as follow-up examination in 52 patients (41.6%), for presurgical diagnosis in 11 patients (8.8 %) and for other purposes (abdominal pain, recurrent tumor staging, postsurgical assessment) in 3 patients (2.4 %) (multiple reasons possible, percentage related to n = 125).
62 patients had negative contrast-enhanced sonography, i.e. no metastatic lesions were found at the initial exam-ination. In 53 of these 62 patients, a follow-up examination was performed 3 - 6 mo after the initial examination and the absence of metastatic lesions was assessed additionally versus the information from follow-up.
Number of metastatic lesions
The metastatic lesions could be identified most clearly in the portal-venous phase, as lesions lacking portal-venous enhancement surrounded by normal liver tissue (Figure 1 and Figure 2). Lack of portal-venous enhancement during CEUS occurred in 171 of 186 metastatic lesions (91.9 %). In 60 of 186 lesions (32.3 %) peripheral arterial enhancement could be detected (Figure 3). The number of lesions was assessable in 74 patients having fewer than 8 focal liver lesions. In 36 of these patients no metastatic lesion could be detected with any of the 3 imaging modalities (CEUS, CT, MRI). In the remaining 38 patients the number of metastatic lesions detected was 55 (CEUS), 61 (CT) and 53 (MRI). A comparison of the number of lesions per patient revealed that in 50 patients (67.6 %) the same number of metastases was found with CEUS and CT. In 16 patients (21.6 %) CT found more metastases, whereas in 8 patients (10.8 %) CEUS found more metastases. However, since the histological gold standard was not available for every lesion, the rates of true and false positive lesions cannot be reliably defined. The differences between the numbers of metastases detected were not statistically significant (Wilcoxon signed rank test: P = 0.28 and P = 0.95 for CEUS versus CT and CEUS versus MRT, respectively). Thus with all 3 imaging modalities a comparable number of metastatic lesions could be found.
Figure 1.
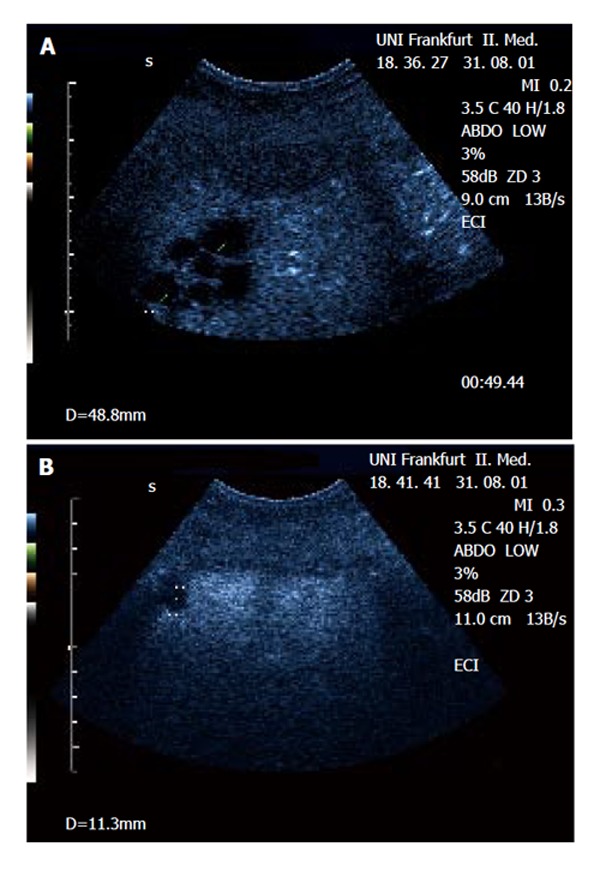
Demonstration of a focal (multicystic) lesion in a patient with cervix carcinoma using contrast-enhanced ultrasound (CEUS). A: The lesions can be delineated in the portal-venous phase as ‘black spots’ lacking portal-venous enhancement within normally enhanced liver tissue. B: An additional small lesion next to the diaphragm (not visible in native B-mode) was detected by CEUS but not with CT. Biopsy confirmed metastatic disease.
Figure 2.
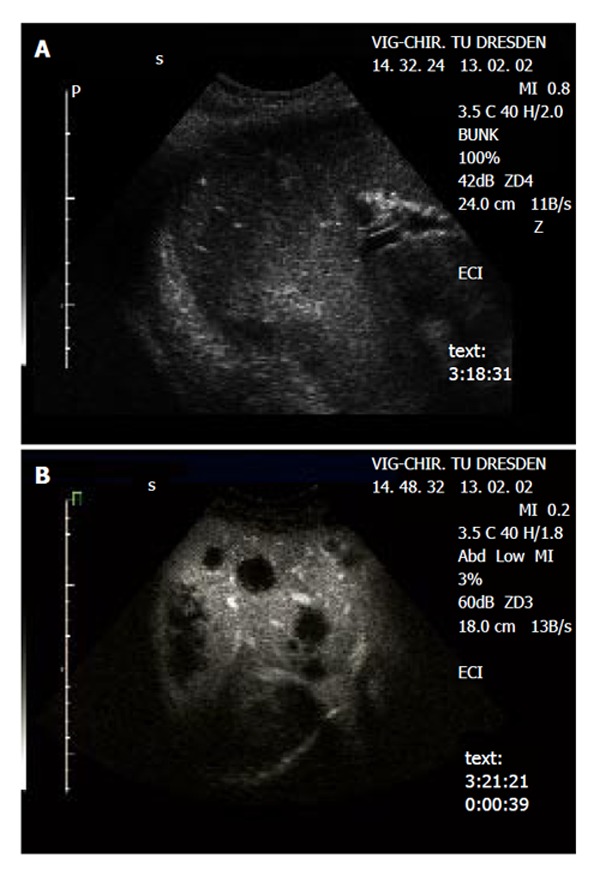
Detection of metastases in a patient with colorectal carcinoma. A: Native B-mode sonography revealed 3 metastases (segment 6/7) in agreement with CT, MRI revealed 4 metastases (segment 6/7 and 4). B: Contrast-enhanced sonography identified diffuse metastatic disease in both liver lobes. The metastatic lesions are clearly delineated in the portal-venous phase as ‘black spots’, due to the lack of portal-venous blood supply.
Figure 3.
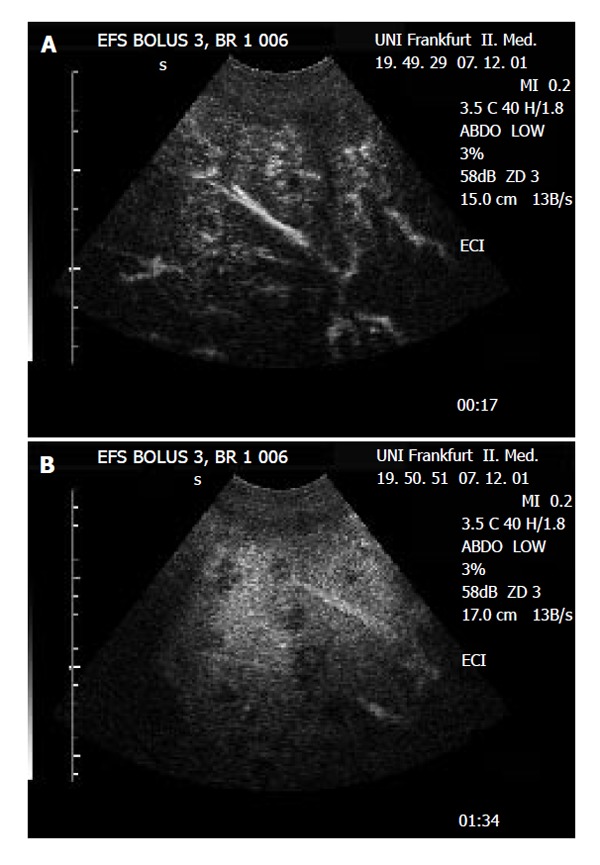
Detection of multiple metastatic lesions in the liver of a patient with sarcoma. A: In contrast-enhanced sonography, the lesions show a periperal enhancement in the arterial phase (“rim sign”), indicating the arterial blood supply to the peripheral proliferation zone of this metastatic lesions from a hypervascular tumor. B: During portal-venous phase, the lesions show lack of portal-venous enhancement (‘black spot’), indication the absence of portal-venous blood flow typical for malignant liver lesions.
Assessment of metastatic disease
Contrast-enhanced ultrasound (CEUS) versus conven-tional (unenhanced) ultrasound On a patient basis, the detection of metastatic disease (i.e. of liver metastases irrespective of the number) was assessed and compared with the combined gold standard. With the use of contrast enhancement, the number of correctly classified patients rose from 83 out of 102 (native US) to 93 out of 102 (CEUS), showing clear superiority of contrast-enhanced sonography over unenhanced sono-graphy (Table 3). The superiority of CEUS was statistically significant (McNemar test: P < 0.01).
Table 3.
Patients with correct diagnosis (existence of metastatic disease) with unenhanced sonography and contrast-enhanced sonography (CEUS)
| CEUS | ||||
| Correct diagnosis | ||||
| Yes | No | |||
| Unenhanced | Yes | 82 | 1 | 83 |
| sonography | No | 11 | 8 | 19 |
| correct diagnosis | ||||
| 93 | 9 | 102 | ||
Contrast-enhanced ultrasound (CEUS) versus CT The correctly diagnosed number of patients with metas-tatic disease was 91 out of 102 patients in the case of triple-phase CT, compared with the 93 out of 102 patients who were correctly diagnosed using CEUS (Table 4). Thus con-trast-enhanced ultrasound and spiral CT showed comparable accuracy for the detection of metastatic liver disease.
Table 4.
Patients with correct diagnosis (existence of metastatic disease) with contrast-enhanced sonography (CEUS) and triple-phase spiral CT
| CEUS | ||||
| Correct diagnosis | ||||
| Yes | No | |||
| CT | Yes | 83 | 8 | 91 |
| correct diagnosis | No | 10 | 1 | 11 |
| 93 | 9 | 102 | ||
The slight difference in favour of contrast-enhanced ultra-sound was not statistically significant. The McNemar test revealed a two-sided 95 % confidence interval [-6.2 %; +10.1%],not clearly exceeding the stipulated 10 % equivalence range.In comparison to the final reference diagnosis the sensitivi-ties were 84.6 %, 88.5 % and 92.3 % and the respective specificities were 78.0%, 94.0 % and 89.2% (unenhanced sonography, contrast-enhanced sonography and CT). Thus the accuracy for the detection of metastatic liver disease rose from 81.4 % to 91.2 % on use of contrast-enhancement for sonography, compared with 89.2 % for spiral CT. Of the three methods, contrast-enhanced ultrasound showed the best specificity and accuracy for the detection/exclusion of metastatic liver disease.
Contrast-enhanced ultrasound (CEUS) versus follow-up examination Of the 62 patients with a negative diagnosis (metastatic disease) on contrast-enhanced sonography, 53 had a follow-up examination 3-6 months after the initial examination. In 47 patients (88.7 %) no additional lesions could be found at the follow-up examination. In 3 patients (5.7 %) new lesions were found, which turned out not to be metastatic. Only 3 patients (5.7 %) showed new metastatic lesions at the follow-up examination which were not diagnosed at the initial CEUS examination. One of these patients also showed no lesion on initial CT and MRI, so that this metastasis initially could not be detected by any of the 3 imaging modalities. In the other 2 patients, CEUS initially detected 5 lesions and 1 lesion respectively, but these lesions were not cl-assified as metastatic and so, based on CEUS alone, the initial diagnosis was false negative. In the first patient (with 5 lesions), CT identified 1 of these lesions as metastasis with peripheral portal-venous enhancement and MRI with SPIO particles identified 2 lesions as metastases. At follow-up, 3 lesions proved to be metastatic lesions. In the other patient (with 1 lesion), all methods (CT, MRI and CEUS) initially classified the lesion as nonmetastatic, and only at follow-up were 3 metastatic lesions identified. However, even versus the 3-6 mo follow-up, CEUS showed 94.4% correct assessment for nonexistence of metastatic liver disease, demonstrating the suitability of contrast-enhanced ultrasound for follow-up examinations of patients with primary extrahepatic tumors.
Safety and tolerability of the ultrasound contrast agent
The patients received 1-3 bolus injections of 2.4 or 4.8 mL SonoVue®, with a total dose of 2.4 mL (11.2 %), 4.8 mL (12.8 %), 7.2 mL (41.6 %), 9.6 mL (5.6 %) or 12.0 mL (28.8%) per patient. Only one adverse event - dry mouth of mild intensity - was reported. Thus the overall adverse event rate per patient in this study was 0.8%.
DISCUSSION
Brightness(B)-mode ultrasonography is highly sensitive and specific in characterizing cysts and calcifications, leading to a definitive diagnosis, but shows several limitations in patients with primary and secondary liver tumors. In addition, some focal lesions have the same echogenicity of normal liver parenchyma leading to false negative findings. To improve the detection of focal liver lesions, ultrasound imaging must also provide information on vascularity, exploiting the differences in blood flow between normal and pathological tissue.
It was recently shown that CEUS using contrast-specific nonlinear high-mechanical-index imaging techni-ques improves the detection rate of liver metastases in comparison with B-mode ultrasound, achieving a detection rate similar to that reported for computed tomography and magnetic resonance imaging techni-ques[1,2,4,5,8-9] In these recently published detection studies mainly using Levovist® in the portal venous and liver-specific late phase in patients with known malignancies, additional lesions could be found in 30-55 % of patients. Additionally, it was shown that examination techniques employing Levovist® allow differentiation of histologically proven benign and malignant liver lesions. In 79 patients with histologically proven malignant liver lesions and in 95 patients with benign liver lesions it was shown that hypoechoic contrast enhancement in the portal venous or late phase as a predictive sign of malignancy had 100 % sensitivity in patients mostly without underlying liver disease. Homogeneous Levovist® enhancement in the portal venous and late phase had 93 % specificity as an indicator of benign disease[7]. It should be noted that there were no false negative findings in patients without underlying parenchymal liver disease. Furthermore, a lower interobserver variability was found in contrast-enhanced sonography than in baseline ultrasonography[10]. But there are still some important limitations since contrast-enhanced high-mechanical-index techniques must be performed intermittently and the examination technique is therefore difficult.
The present study employed a new-generation contrast medium (SonoVue®) allowing real-time imaging, providing similar information to the more difficult intermittent imaging technique using Levovist®. This method prov-ed useful in routine application and is easy to learn. Co-ntrast-enhanced sonography using low-mechanical-index techniques with SonoVue® has also proved useful intraoperatively[11] and in conjunction with 3D techniques[12] and additionally may differentiate between adenoma and focal nodular hyperplasia[13]. It was shown that these techniques gave a statistically significant improvement in the accuracy of detection of metastatic disease versus unenhanced sonography. In comparison with baseline ultrasound the number of metastatic lesions increased with a sensitivity comparable to contrast-enhanced triple-phase CT. In two thirds of patients the same numbers of metastatic lesions were found with contrast-enhanced sonography and CT. In the remaining patients, sometimes CT and sometimes sonography found more lesions, with no significant superiority of one method. Since contrast-enhanced sonography was not part of the gold standard this may introduce a possible bias in the calculation of accuracy. There were two patients in whom CEUS found a lesion but CT and MRI did not. One patient (female, 54 years, with breast cancer) had a 29 mm lesion and the other (female, 67 years, with pancreatic cancer) had an 11 mm lesion, which were both non-enhancing in the portal-venous phase. However, owing to the definition of the gold standard both were rated as false positive for CEUS, since no biopsy was obtained for clarification. This illustrates the limitation of such studies comparing imaging methods, since there is no absolute non-invasive gold standard. Additionally, contrast-enhanced sonography has the best specificity and accuracy for detection or exclusion of metastatic disease when compared with unenhanced sonography and triple-phase spiral CT. In 11 % of the patients the existence of lesions could be ruled out, indicating the high specificity of contrast-enhanced sono-graphy.
Monitoring of adverse events was mandatory and performed in all patients and demonstrated no relevant reaction, leading to an excellent tolerability of the ultra-sound contrast agent.
In conclusion, contrast-enhanced ultrasound in the portal venous and late phase following injection of Sono-Vue® considerably improves the detection of liver tumors compared with conventional B-mode sonography and is therefore a suitable method for the follow-up of patients with primary extrahepatic cancer.
ACKNOWLEDGMENTS
The following persons participated as investigators of the SonoVue® study group in the study, collecting and evaluating the data (numbers of patients enrolled): Germany - C.F. Dietrich, G. Schuessler and A. Ignee, Johann Wolfgang Goethe University Hospital Frankfurt (12), R. Fessl and J. Demharter, Central Hospital Augsburg (12), W. Kratzer and K. Hirschbühl, University Hospital Ulm (20), K. Hauenstein, University Hospital Rostock (9), D. Hahn, A. Trusen and M. Beissert, University Hospital Würzburg (8), W. Blank, K. Wild, U. Schwaiger and B. Braun, Hospital am Steinenberg, Reutlingen (5), A. Bunk, University Hospital Dresden (12), U. Vossas, Marien Hospital, Düsseldorf (12), D. Strobel , University Hospital Erlangen (14), W. Koch, Leopoldina Hospital, Schweinfurt (8), Netherlands - M. Oudkerk, E.J. van der Jagt, C.A. Jansen and H. Alkefaji, University Hospital Groningen (7), Belgium - E. Danse, P. Trefois and E. Avalos, University Hospital Brussels (12).
The study was sponsored by Bracco Altana Pharma, Konstanz, Germany. Monitoring, data management, data analysis and statistical evaluation were done by an independent contract research organization (Medidata). Discussion of study protocol and study data and the decision to submit the paper for publication took place at investigators’ meetings. One author (CG) contributing to this discussion is an employee of the sponsor.
C. Greis designed and coordinated the study and contributed to the manuscript. C. Dietrich, E. Danse, and W. Kratzer contributed to the manuscript. We thank A. Möller, D. Fritsche and U. Doniat from Medidata, Konstanz, Germany for study monitoring, source data verification, data management, statistical analysis and support for the interpretation of the study results.
Footnotes
S- Editor Guo SY L- Editor Zhang JZ E- Editor Wu M
References
- 1.Harvey CJ, Blomley MJ, Eckersley RJ, Heckemann RA, Butler-Barnes J, Cosgrove DO. Pulse-inversion mode imaging of liver specific microbubbles: improved detection of subcentimetre metastases. Lancet. 2000;355:807–808. doi: 10.1016/S0140-6736(99)04545-6. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, Claudon M, Calliada F, Correas JM, LaFortune M, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361–370. doi: 10.1148/radiol.2272011833. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht T, Hoffmann CW, Schmitz SA, Schettler S, Overberg A, Germer CT, Wolf KJ. Phase-inversion sonography during the liver-specific late phase of contrast enhancement: improved detection of liver metastases. AJR Am J Roentgenol. 2001;176:1191–1198. doi: 10.2214/ajr.176.5.1761191. [DOI] [PubMed] [Google Scholar]
- 4.Dalla Palma L, Bertolotto M, Quaia E, Locatelli M. Detection of liver metastases with pulse inversion harmonic imaging: preliminary results. Eur Radiol. 1999;9 Suppl 3:S382–S387. doi: 10.1007/pl00014079. [DOI] [PubMed] [Google Scholar]
- 5.Esteban JM, Molla MA, Tomas C, Maldonado L. Improved detection of liver metastases with contrast-enhanced wideband harmonic imaging: comparison with CT findings. Eur J Ultrasound. 2002;15:119–126. doi: 10.1016/s0929-8266(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 6.Hohmann J, Albrecht T, Oldenburg A, Skrok J, Wolf KJ. Liver metastases in cancer: detection with contrast-enhanced ultrasonography. Abdom Imaging. 2004;29:669–681. doi: 10.1007/s00261-004-0175-6. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CF, Ignee A, Trojan J, Fellbaum C, Schuessler G. Improved characterisation of histologically proven liver tumors by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut. 2004;53:401–405. doi: 10.1136/gut.2003.026260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernatik T, Becker D, Neureiter D, Hänsler J, Frieser M, Schaber S, Hahn EG, Strobel D. [Detection of liver metastases--comparison of contrast--enhanced ultrasound using first versus second generation contrast agents] Ultraschall Med. 2003;24:175–179. doi: 10.1055/s-2003-40060. [DOI] [PubMed] [Google Scholar]
- 9.Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27–34. doi: 10.1002/hep.20728. [DOI] [PubMed] [Google Scholar]
- 10.von Herbay A, Vogt C, Häussinger D. Late-phase pulse-inversion sonography using the contrast agent levovist: differentiation between benign and malignant focal lesions of the liver. AJR Am J Roentgenol. 2002;179:1273–1279. doi: 10.2214/ajr.179.5.1791273. [DOI] [PubMed] [Google Scholar]
- 11.Moug SJ, Horgan PG, Leen E. Contrast-enhanced ultrasonography during liver surgery (Br J Surg 2004; 91: 1165-1167) Br J Surg. 2004;91:1527. doi: 10.1002/bjs.4852. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich CF. [3D real time contrast enhanced ultrasonography, a new technique] Rofo. 2002;174:160–163. doi: 10.1055/s-2002-20102. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich CF, Schuessler G, Trojan J, Fellbaum C, Ignee A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78:704–707. doi: 10.1259/bjr/88181612. [DOI] [PubMed] [Google Scholar]


