Abstract
AIM: To investigate non-invasively the incidence of absorption of carbohydrates in diabetic patients during an oral glucose tolerance test (OGTT) and to determine whether malabsorption may be associated with insulin secretion and insulin resistance.
METHODS: A standard 75-g OGTT was performed in 82 diabetic patients. The patients received 75 g of anhydrous glucose in 225 mL of water after an overnight fasting and breath samples were collected at baseline and up to 120 min after ingestion. Breath hydrogen and methane concentrations were measured. Blood glucose and serum insulin concentrations were measured before ingestion and at 30, 60, 90, 120 min post-ingestion.
RESULTS: When carbohydrate malabsorption was defined as subjects with an increase of at least 10 ppm (parts per million) in hydrogen or methane excretion within a 2-h period, 28 (34%) had carbohydrate malabsorption. According to the result of increased breath test, 21 (75%) patients were classified as small bowel bacterial overgrowth and 7 (25%) as glucose malabsorption. Patients with carbohydrate malabsorption were older and had poor glycemic control as compared with those without carbohydrate malabsorption. The HOMA value, the sum of serum insulin during the test and the Δinsulin/Δglucose ratio were greater in patients with carbohydrate malabsorption.
CONCLUSION: Insulin resistance may be overestimated by using these markers if the patient has carbohydrate malabsorption, or that carbohydrate malabsorption may be present prior to the development of insulin resistance. Hence carbohydrate malabsorption should be taken into account for estimating insulin resistance and β-cell function.
Keywords: 75-g OGTT, Carbohydrate malabsorption, Bacterial overgrowth, Breath test, Insulin resistance
INTRODUCTION
The oral glucose tolerance test (OGTT) is a widely used procedure in the diagnoses of diabetes and intermediate stages of hyperglycemia. Plasma glucose and insulin responses during this test reflect the ability of pancreatic β-cells to secrete insulin and the sensitivity of tissues to insulin[1]. However, it was reported that 2%-20% of carbohydrates escape small intestinal absorption[2]. Based on this fact, digestion and absorption of carbohydrates may affect the results of OGTT. If the patient had carbohydrate malabsorption, plasma glucose and insulin responses during OGTT should be lower. Furthermore, an interesting observation was the outcome of OGTT yielding a remarkable intra-individual variability[3] and this was explained by the hypothesis that the velocity of the initial phase of glucose emptying from the stomach may depend on the grade of interdigestive antral motor activity at the time of glucose ingestion[4]. Although a many investigators have reported a close association between gastric emptying and insulin secretion, the prevalence of absorption of carbohydrates in diabetic patients has been unknown.
H2 breath tests have been used to evaluate intestinal transit, bacterial overgrowth, and disaccharidase deficiency[5-12]. Because bacteria represent the sole source of gut H2 and CH4, fasting breath H2 and CH4 gases have been used as markers of colonic fermentation[13,14]. As H2 production increases when a small amount of carbohydrate is supplied to colonic bacteria, the measurement of breath H2 concentration has been proposed as an indicator of carbohydrate malabsorption[6]. Similarly, breath CH4 excretion, which reflects an indirect measurement of the metabolism of the anaerobic colonic flora, has been measured[15,16]. Methanogenic bacteria utilize H2, carbon dioxide (CO2), and then synthesize CH4[17]. CH4 absorbed from the colon reaches the lung and excretes into the breath[18].
The aim of the present study was to investigate non-invasively the incidence of malabsorption of carbohydrates in diabetic patients during OGTT and to determine whether malabsorption may be associated with insulin secretion and insulin resistance.
MATERIALS AND METHODS
Patients
A standard 75-g OGTT was performed in 82 diet-controlled diabetic patients (42 women and 40 men; age range 30-84 years, average 62 years) without abdominal symptoms. Patients treated with alpha-glucosidase inhibitors were excluded from this study. None of the patients had a history of use of PPI, H2-receptor antagonist, antibiotics, steroids, or nonsteroidal anti-inflammatory drugs for a period of at least six month before the investigation. Patients who had a previous history of partial gastrectomy were also excluded from the study. The study was approved by our Local Ethical Committee.
Procedures
The patients received 75 g of anhydrous glucose in 225 mL of water in the sitting position after an overnight fasting. Breath samples were collected at baseline and at 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, and 120 min after ingestion. Breath H2 and CH4 concentrations were measured with breath analyzer TGA-2000 (TERAMECS, Kyoto) and expressed in parts per million (ppm). Linear accuracy response range was 2 to 150 ppm. An increase of at least 10 ppm within a 2-h period is indicative of bacterial overgrowth or glucose malabsorption[19]. Venous blood samples were obtained before ingestion and at 30, 60, 90, and 120 min after ingestion and blood glucose and serum insulin concentrations were measured.
Calculations
The homeostasis model assessment (HOMA), fasting serum insulin, the sum of serum insulin during the test, and the Δinsulin/Δglucose ratio were used as indexes of insulin resistance. The HOMA value was calculated as follows: HOMA = fasting plasma glucose (mg/dL) × fasting insulin (pmol/L)/405. The Δinsulin/Δglucose ratio was calculated as follows: (insulin at 30 min - insulin at baseline)/(glucose at 30 min - glucose at baseline).
Statistical analysis
All values were expressed as means ± SD. Comparisons of groups were made using the Mann-Whitney U test. A P value less than 0.05 was considered statistically significant.
RESULTS
Incidence of carbohydrate malabsorption
When carbohydrate malabsorption was defined as subjects with an increase of at least 10 ppm within a 2-h period, 34% (28/82) patients had carbohydrate malabsorption. As shown in Figures 1 and 2, small bowel bacterial overgrowth was defined as an increase of H2 and/or CH4 greater than 10 ppm above the baseline before the first 40 min, whereas carbohydrate malabsorption without small bowel bacterial overgrowth was defined as an increase of H2 and/or CH4 greater than 10 ppm above the baseline at 70 min and later. Of 28 patients with an increase of H2 and/or CH4, 21 (75%) patients were classified as small bowel bacterial overgrowth and 7 (25%) as carbohydrate malabsorption (Figure 3). All 28 patients had no abdominal symptom during OGTT.
Figure 1.
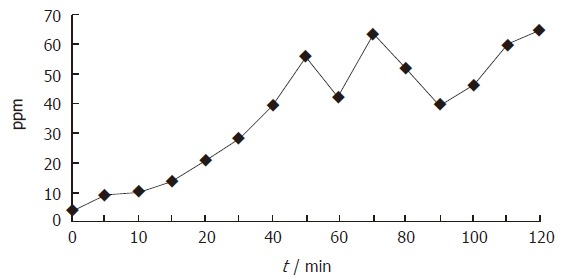
Changes in breath hydrogen concentration during OGTT in the patient with suspicious of small bowel bacterial overgrowth. An increase of H2 greater than 10 ppm above the baseline was found at 15 min.
Figure 2.
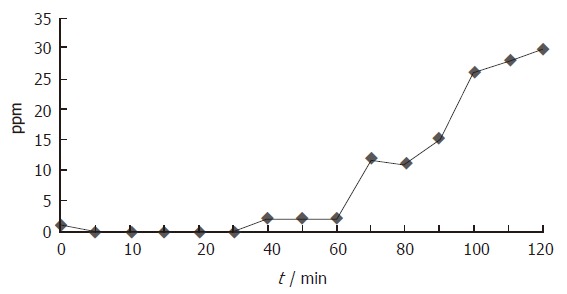
Changes in breath hydrogen concentration during OGTT in the patient with suspicious of carbohydrate malabsorption. Breath H2 concentration increased gradually from the beginning and reached 10 ppm over the baseline at 70 min and later.
Figure 3.
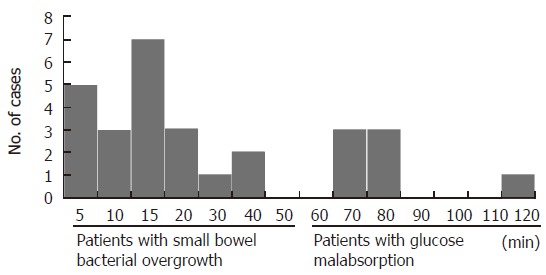
Distribution of time when breath hydrogen and/or methane levels increased more than 10 ppm over baseline.
As shown in Table 1, the values of HbA1c were 7.11% ± 1.70% and 6.43% ± 1.46% in patients with and without carbohydrate malabsorption (including small bowel bacterial overgrowth), respectively. Patients with carbohydrate malabsorption were older and had poor glycemic control when compared with those without carbohydrate malabsorption.
Table 1.
Characteristics of 82 patients with diabetes mellitus classified according to carbohydrate malabsorption status
| Carbohydrate malabsorption | P value | ||
| (+) | (-) | ||
| n | 28 | 54 | |
| Age | 63.3 ± 10.3 | 59.2 ± 9.7 | 0.34 |
| HbA1c | 7.1 ± 1.7 | 6.4 ± 1.5 | 0.17 |
| HOMA | 2.0 ± 2.1 | 1.9 ± 1.9 | 0.22 |
| ΣIR | 107.0 ± 95.3 | 117.0 ± 76.2 | 0.08 |
| ΔIR/ΔBG | 0.18 ± 0.27 | 0.14 ± 0.21 | 0.06 |
Carbohydrate malabsorption and insulin resistance
The HOMA values in the patients with carbohydrate malabsorption (2.0 ± 2.1) were greater than those without carbohydrate malabsorption (1.94 ± 1.86), but did not reach statistical significance (P = 0.22). Similarly, the sum of serum insulin during the test and the Δinsulin/Δglucose ratio were also greater in patients with carbohydrate mala-bsorption than those without carbohydrate malabsorption (Table 1), but the difference was not significant.
DISCUSSION
Impaired intestinal and gastric motility are frequent findings in diabetic patients[19,20]. However, there is a wide range of symptoms in gastrointestinal motility disorders, and the degree of motility disorders correlate poorly with severity of symptoms. Intestinal peristalsis as well as gastric acid secretion are the most important factors protecting against the small bowel bacterial overgrowth[21]. Although delayed gastrointestinal transit potentially causes bacterial overgrowth, in contrast, a rapid transit may also cause diarrhea due to an increase in intraluminal contents that reach the cecum. It is, therefore, possible that both rapid and delayed gastrointestinal transit cause diarrhea because of bacterial overgrowth or carbohydrate malabsorption. However, patients with bacterial overgrowth may also be asymptomatic[22]. Although gastrointestinal symptoms are present in 50%-70% of diabetic patients, the association between symptoms and bacterial overgrowth in diabetic patients has been unknown.
In the present study, 34% (28/82) patients without abdominal symptoms, including diarrhea, had carbohydrate malabsorption, including 7 patients classified as small bowel bacterial overgrowth. It has been unclear how much of small bowel bacterial overgrowth is opposed to carbohydrate malabsorption. The results of the present study suggest that carbohydrate malabsorption occurs more often in diabetic patients than small bowel bacterial overgrowth.
There is increasing evidence that postprandial hyperglycemia has a major role in the pathogenesis of diabetic macrovascular complications[23,24]. It is widely recognized that postprandial glycemia is potentially dependent on a number of factors, including the rate of carbohydrate entry into the small intestine, small intestinal digestion and absorption, insulin secretion, peripheral insulin sensitivity, and hepatic and muscle glucose metabolism[25]. In addition, postprandial secretion of insulin is prompted as much by the incretin hormones as by entry of glucose into the blood, and the release of incretins is dependent on rates of nutrient entry into the small intestine[26,27]. Our observations confirmed that one third of patients with diet-controlled type 2 diabetes and without abdominal symptoms had carbohydrates malabsorption, which might contribute to postprandial hyperglycemia[25]. Some previous studies also described approximately 35% of the variance in initial postprandial blood glucose concentrations after a 75-g oral glucose load[28,29]. Since patients with carbohydrate malabsorption tended to be older and to have poor glycemic control as compared with those without carbohydrate malabsorption, it is possible that patients with long-standing or poor-controlled diabetes may more often have carbohydrate malabsorption. Conversely, the result and its interpretation of 75-g OGTT might be influenced by carbohydrate malabsorption.
A standard 75-g OGTT has also been used to assess insulin resistance and insulin release. For example, fasting plasma insulin concentrations have been used as an index of insulin resistance, and the 30-min ratio of changes in plasma insulin and glucose has been used as an index of β-cell function[30,31]. Insulin resistance, evaluated by the HOMA value, the sum of serum insulin during the test, and the Δinsulin/Δglucose ratio were greater in patients with carbohydrate malabsorption than those without carbohydrate malabsorption. However, these differences were not significant. This suggests that insulin resistance might be overestimated by using these markers if the patient has carbohydrate malabsorption, or that carbohydrate malabsorption might be present prior to the development of insulin resistance. In conclusion, it is more desirable that carbohydrate malabsorption should be taken into account for estimating insulin resistance and β-cell function.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Ma WH
References
- 1.Reaven GM, Brand RJ, Chen YD, Mathur AK, Goldfine I. Insulin resistance and insulin secretion are determinants of oral glucose tolerance in normal individuals. Diabetes. 1993;42:1324–1332. doi: 10.2337/diab.42.9.1324. [DOI] [PubMed] [Google Scholar]
- 2.Stephen AM, Haddad AC, Phillips SF. Passage of carbohydrate into the colon. Direct measurements in humans. Gastroenterology. 1983;85:589–595. [PubMed] [Google Scholar]
- 3.MCDONALD GW, FISHER GF, BURNHAM C. REPRODUCIBILITY OF THE ORAL GLUCOSE TOLERANCE TEST. Diabetes. 1965;14:473–480. doi: 10.2337/diab.14.8.473. [DOI] [PubMed] [Google Scholar]
- 4.Thompson DG, Wingate DL, Thomas M, Harrison D. Gastric emptying as a determinant of the oral glucose tolerance test. Gastroenterology. 1982;82:51–55. [PubMed] [Google Scholar]
- 5.Hoekstra JH. Fructose breath hydrogen tests in infants with chronic non-specific diarrhoea. Eur J Pediatr. 1995;154:362–364. doi: 10.1007/s004310050304. [DOI] [PubMed] [Google Scholar]
- 6.Levitt MD, Donaldson RM. Use of respiratory hydrogen (H2) excretion to detect carbohydrate malabsorption. J Lab Clin Med. 1970;75:937–945. [PubMed] [Google Scholar]
- 7.Bond JH, Levitt MD. Use of pulmonary hydrogen (H 2 ) measurements to quantitate carbohydrate absorption. Study of partially gastrectomized patients. J Clin Invest. 1972;51:1219–1225. doi: 10.1172/JCI106916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maffei HV, Metz GL, Jenkins DJ. Hydrogen breath test: Adaptation of a simple technique to infants and children. Lancet. 1976;1:1110–1111. doi: 10.1016/s0140-6736(76)90069-6. [DOI] [PubMed] [Google Scholar]
- 9.Bond JH Jr, Levitt MD, Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975;85:546–555. [PubMed] [Google Scholar]
- 10.La Brooy SJ, Male PJ, Beavis AK, Misiewicz JJ. Assessment of the reproducibility of the lactulose H2 breath test as a measure of mouth to caecum transit time. Gut. 1983;24:893–896. doi: 10.1136/gut.24.10.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Götze H, Ptok A. Orocaecal transit time in patients with Crohn disease. Eur J Pediatr. 1993;152:193–196. doi: 10.1007/BF01956142. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes JM, Middleton P, Jewell DP. The lactulose hydrogen breath test as a diagnostic test for small-bowel bacterial overgrowth. Scand J Gastroenterol. 1979;14:333–336. doi: 10.3109/00365527909179892. [DOI] [PubMed] [Google Scholar]
- 13.Le Marchand L, Wilkens LR, Harwood P, Cooney RV. Use of breath hydrogen and methane as markers of colonic fermentation in epidemiologic studies: circadian patterns of excretion. Environ Health Perspect. 1992;98:199–202. doi: 10.1289/ehp.9298199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perman JA, Modler S, Barr RG, Rosenthal P. Fasting breath hydrogen concentration: normal values and clinical application. Gastroenterology. 1984;87:1358–1363. [PubMed] [Google Scholar]
- 15.McKay LF, Eastwood MA, Brydon WG. Methane excretion in man--a study of breath, flatus, and faeces. Gut. 1985;26:69–74. doi: 10.1136/gut.26.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjørneklett A, Jenssen E. Relationships between hydrogen (H2) and methane (CH4) production in man. Scand J Gastroenterol. 1982;17:985–992. [PubMed] [Google Scholar]
- 17.Stadtman TC. Methane fermentation. Annu Rev Microbiol. 1967;21:121–142. doi: 10.1146/annurev.mi.21.100167.001005. [DOI] [PubMed] [Google Scholar]
- 18.Bond JH Jr, Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971;133:572–588. doi: 10.1084/jem.133.3.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahamsson H. Gastrointestinal motility disorders in patients with diabetes mellitus. J Intern Med. 1995;237:403–409. doi: 10.1111/j.1365-2796.1995.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 20.Iber FL, Parveen S, Vandrunen M, Sood KB, Reza F, Serlovsky R, Reddy S. Relation of symptoms to impaired stomach, small bowel, and colon motility in long-standing diabetes. Dig Dis Sci. 1993;38:45–50. doi: 10.1007/BF01296772. [DOI] [PubMed] [Google Scholar]
- 21.Kirsch M. Bacterial overgrowth. Am J Gastroenterol. 1990;85:231–237. [PubMed] [Google Scholar]
- 22.Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am. 1996;25:361–378. doi: 10.1016/s0889-8529(05)70328-5. [DOI] [PubMed] [Google Scholar]
- 23.Del Prato S. In search of normoglycaemia in diabetes: controlling postprandial glucose. Int J Obes Relat Metab Disord. 2002;26 Suppl 3:S9–S17. doi: 10.1038/sj.ijo.0802172. [DOI] [PubMed] [Google Scholar]
- 24.Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care. 2001;24:1397–1402. doi: 10.2337/diacare.24.8.1397. [DOI] [PubMed] [Google Scholar]
- 25.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001;24:371–381. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann C, Göke R, Richter G, Fehmann HC, Arnold R, Göke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 27.Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, Göke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993;36:857–862. doi: 10.1007/BF00400362. [DOI] [PubMed] [Google Scholar]
- 29.Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643–1648. [PubMed] [Google Scholar]
- 30.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20:1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 31.Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138–1141. doi: 10.2337/diacare.19.10.1138. [DOI] [PubMed] [Google Scholar]


