Abstract
The prokaryotic and eukaryotic cells of the colon exist in a highly complex, but harmonious relationship. Disturbances in this remarkable symbiosis can result in the development of inflammatory bowel diseases (IBD). Although the etiology of IBD is not entirely understood, it is known that the chronic inflammation of Crohn’s disease, ulcerative colitis and chronic pouchitis are a result of an overly aggressive immune response to the commensal intestinal flora in genetically susceptible hosts. Recent studies have enhanced our ability to understand the interaction between the host and its intestinal microflora and the role the microflora plays in maintaining intestinal homeostasis. As we begin to understand the benefits conferred to the intestine by the microflora, the notion of modifying the composition of the bacterial load to improve human health has arisen. A significant body of research now exists investigating the role of probiotics and prebiotics in ameliorating chronic intestinal inflammation. This article will begin with an overview of the role of the commensal microflora in maintaining mucosal immune homeostasis, and how a dysregulated immune response to the intestinal microflora results in IBD. This will be followed by a summary of the use of probiotics and prebiotics in experimental and human IBD.
Keywords: Colitis, Crohn’s disease, Microflora, Immu-nity, Probiotics, Prebiotics
INTRODUCTION
At birth, the gastrointestinal tract is a sterile environment. Initial exposure of the gut to microbes occurs during the birthing process from the maternal fecal and vaginal flora. Within a few months after birth, a relatively stable microbial population is established[1]. This abundant, diverse and dynamic intestinal microflora normally lives in a complex, symbiotic relationship with the eukaryotic cells of the mucosa. About 100 trillion bacterial cells benefit from the constant nutrient flow, stable temperature and niches for various metabolic requirements provided by the intestinal environment. Likewise, the host benefits from the ability of the intestinal microflora to synthesize vitamin K, exert trophic effects on intestinal epithelial cells, salvage energy from unabsorbed food by producing short-chain fatty acids, inhibit the growth of pathogens, sustain intestinal barrier integrity and maintain mucosal immune homeostasis. Studies from germ-free animals reveal that the absence of resident intestinal microflora results in significant alterations in intestinal structure and function, including slender villi, shallow crypts, low leukocyte count[2,3], a decrease in the number and density of Peyer’s patches[4] and decreased stimulation of migrating motor complexes[5].
In their co-evolution with bacteria, vertebrates develop pattern-recognition receptors, which are activated by specific molecular patterns unique to bacteria, fungi and viruses that are absent in eukaryotes (lipopolysaccharides, peptidoglycan, ssRNA, muramyl dipeptide, flagellins, etc). These include the Toll-like receptors (TLRs) and nucleotide oligomerization domains (NODs). TLRs and NODs are critical for the initiation of innate immune defense responses. Activation of their signaling cascades usually results in the production of pro-inflammatory cytokines. TLR signaling also provides a link between innate and adaptive immunity, as TLR signaling results in the maturation of dendritic cells, which activate adaptive immune responses[6]. Although stimulation of these receptors in most parts of the immune system results in production of inflammatory cytokines, these ligands are not only tolerated by the gut mucosal immune system, but also essential for adaptation to intestinal bacteria and maintenance of homeostasis[7]. The tolerance to the intestinal microflora is not completely understood, but several aspects of commensal physiology have been defined which contribute to their inability to activate the immune system. Some commensal bacteria can modify TLR ligands, resulting in a hypoactive immune response. For example, the endotoxic portion of LPS is pentacylated in many Bacteroides species, and has minimal toxicity[8]. An important feature of commensal bacteria is their inability to penetrate the intestinal epithelial barrier. If some of these organisms do penetrate, they are usually rapidly phagocytosed by the innate mucosal immune system. Indeed, in a healthy host, the systemic immune system appears to be ignorant of the intestinal microflora[9]. Maintaining tolerance to these intestinal bacteria is a remarkable accomplishment achieved by the mucosal immune system, and disturbances in this bacterial-epithelial homeostasis result in considerable deleterious effects on the host.
Role of the commensal flora in IBD
Although many studies have investigated the possibility of a single infectious agent causing Crohn’s disease and ulcerative colitis, also called chronic inflammatory bowel disease (IBD), none has yet been discovered. The intestinal bacteria are now believed to be involved in the initiation and perpetuation of IBD. The prevailing theory explaining the development of IBD is that the adaptive immune system is hyper-responsive to the commensal intestinal microflora in genetically susceptible individuals[10]. This hypothesis is supported by several observations: most inflammation occurs in areas with the highest density of intestinal bacteria, broad spectrum antibiotics improve chronic intestinal inflammation, and surgical diversion of the fecal stream can prevent recurrence of Crohn’s disease. Most importantly, despite differences in the pathogenesis of chronic intestinal inflammation, a consistent feature of many animal models of IBD (such as IL-10 knockout mice and HLA-B27 transgenic rats) is the failure to develop chronic intestinal inflammation when these animals are raised in germ-free conditions[11,12]. Dysbiosis is also observed in IBD patients. Adherent and intramucosal bacteria, particularly Bacteroides spp, Escherichia coli (E. coli) and Enterobacterium spp are more abundant in patients with Crohn’s disease (CD) than in controls[13,14] (Figure 1). Bacterial overgrowth and dysbiosis are also associated with the development of chronic pouchitis, the inflammation of the ileal reservoir created after ileo-anal anastamosis following colectomy in ulcerative colitis (UC) patients[15]. In addition, several selected commensal bacterial species can induce and perpetuate colitis in genetically susceptible rodent models of chronic intestinal inflammation[16,17] (Figure 1).
Figure 1.
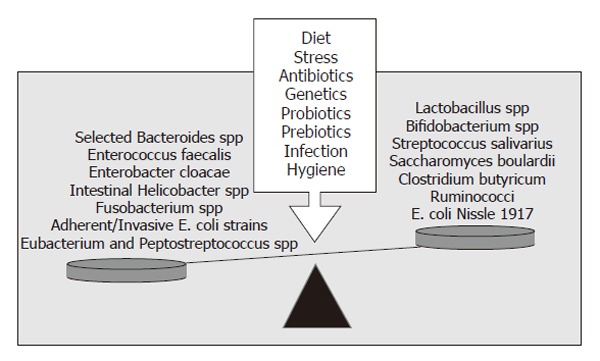
Microbial balance and dysbiosis. The pathogenic immune responses present in IBD are triggered by the presence of luminal bacteria. The balance of beneficial vs aggressive intestinal microbes is responsible for either mucosal homeostasis or chronic inflammation. A number of environmental and genetic factors influence the balance of beneficial vs aggressive microbes. Adapted from[63].
Other immune dysfunctions in IBD include aberrant secretion of pro-inflammatory cytokines and chemokines by intestinal epithelial cells and mucosal immune cells[18-20], altered TLR4 signaling[21], defective antigen presenting cell function[22] and lack of T-cell apoptosis[23]. It is now clear that ignorance of the systemic immune system to commensal intestinal microflora is lost in IBD patients, as shown by enhanced and persistent cell-mediated and humoral immune responses to these bacteria[24].
At least 7 genetic loci conferring susceptibility to CD, ulcerative colitis (UC) or both, have been identified[25]. Interestingly, such susceptibility genes associated with CD involve polymorphisms of the NOD2 gene[26] (the pattern recognition receptor for muramyl dipeptide) which can result in hampered innate immune functions by impairment of TLR function[27], defective clearance of invasive bacteria by macrophages[28], and decreased production of defensins[29].
The recognition of the compelling association between intestinal microflora and the development of IBD has led to an abundance of studies investigating the therapeutic potential of altering luminal bacteria using probiotics and/or prebiotics.
Probiotics, prebiotics and synbiotics
Probiotics are defined as living organisms in food and dietary supplements which, upon ingestion, improve the health of the host beyond their inherent basic nutrition[30]. Probiotics are typically lactic acid bacteria selected from the gut flora, and are able to survive stomach acid and bile, maintain viability throughout extended periods of storage and are safe for human consumption. Other species have also shown some beneficial effects, such as E. coli Nissle 1917 and Saccharomyces boulardii (Figure 1). Probiotic bacteria have verifiable beneficial properties, including the ability to improve epithelial barrier function, modulate the mucosal immune system, and alter the intestinal flora.
Prebiotics are non-digestible dietary carbohydrates, e.g., lactosucrose, fructo- and galacto-oligosaccharides, inulin, psyllium, bran, germinated barley (Figure 2), which stimulate the growth and metabolism of endogenous enteric protective bacteria upon consumption. Beneficial effects of prebiotics are also associated with changes in colonic short-chain fatty acids (SCFA) due to fermentation by colonic bacteria[31]. Synbiotics are combinations of probiotics and prebiotics, and are also an emerging therapeutic modality. Restoration of normal microflora using probiotics, prebiotics or synbiotics has been investigated in numerous gastrointestinal and other disease states, including infectious diarrhea, H pylori infection, irritable bowel syndrome, colorectal cancer, lactase deficiency, pancreatitis, atopy, and IBD.
Figure 2.
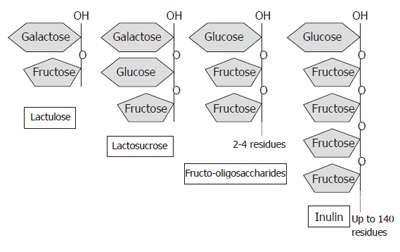
Basic structures of various prebiotic substances. Structurally, prebiotics are a mixture of polymers and oligomers comprising branching chains of fructose units.
Protective mechanisms of probiotics by ameliorating chronic intestinal inflammation
Probiotic bacteria have beneficial effects on the intestinal epithelia both directly and indirectly, including enhanced barrier function, modulation of the mucosal immune system, production of antimicrobials, and alteration of the intestinal microflora (Figure 3).
Figure 3.
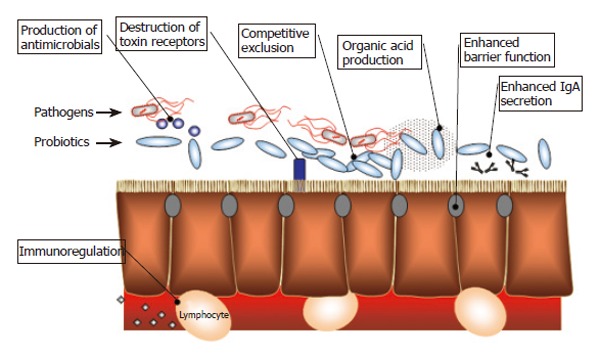
Mechanisms of probiotic activity.
Alteration of the mucosal immune system. The presence of probiotics has been shown to result in several modifications in the mucosal immune response, including augmented antibody production[32,33], increased phagocyte[34] and natural killer cell activity[35-38], modulation of the nuclear factor kappa-B (NFκB) pathway[39-41], and induction of T cell apoptosis[42]. Generally, probiotics increase the production of intestinal anti-inflammatory cytokines (such as IL-10 and TGF-β), while reducing the production of pro-inflammatory cytokines (e.g., TNF-α, interferon-γ, IL-8)[43-46]. Several probiotic bacteria, including B. breve, Streptococcus thermophilus, B. bifidum and Ruminococcus gnavus have been shown to secrete metabolites that reduce LPS-induced TNF-α secretion[47]. L. reuteri reduces TNF-α and Salmonella typhimurium induceds IL-8 secretion in vitro, by inhibiting nuclear translocation of NFκB and preventing the degradation of ΙκB[48]. Administration of the probiotic cocktail VSL#3 (consisting of L. acidophilus, L. bulgaricus, L. casei, L, plantarum, B. breve, B. infantis, B. longum, S. thermophilus) to IL-10 deficient mice results in colitis reduction and a concomitant reduction in mucosal secretion of TNF-α and interferon-γ[49]. E. coli Nissle 1917 is able to down-regulate the expansion of newly recruited T-cells into the mucosa and limit chronic intestinal inflammation[50]. In SAMP1/Yit mice, Lactobacillus casei strain Shirota inhibits IL-6 production in LPS-stimulated large intestinal lamina propria mononuclear cells and down-regulates nuclear translocation of NFκB[51]. Patients with a recent ileo-anal pouch anastamosis who responded to probiotic therapy have reduced mRNA levels of IL-1β, IL-8 and IFN-γ, and fewer polymorphonuclear cells compared with patients who receive placebo[52]. Probiotic treatment has also been shown to reduce IFN-γ and IL1-α expression and decrease inducible-nitric oxide synthase and gelatinase activities in pouch biopsy samples from patients with pouchitis[53]. In mucosal explants of ileal specimens from patients with Crohn’s disease, probiotics reduced TNF-α release and the number of CD4 cells[54]. In addition to live probiotics, components of probiotic bacteria can also exert effects on the mucosal immune system. For example, genomic DNA isolated from VSL#3 inhibits TNF-α-induced IL-8 secretion, mitogen-activated protein kinase activation and NFκB activation[41] in HT-29 cells.
Improved barrier function. Various probiotic bacteria can enhance intestinal epithelial barrier function. For example, oral administration of VSL#3 results in normalization of impaired colonic barrier function and restoration of intestinal epithelial integrity in IL-10 deficient mice and enhancement of epithelial resistance in T-84 cells[49]. Barrier function was enhanced not only by live bacteria, but also by a proteinaceous secreted product of VSL#3[49]. Several strains of lactobacilli are also capable of up-regulating intestinal MUC3 mRNA expression, thereby improving barrier function by increasing the mucus layer[55,56]. Lactobacillus GG (L. GG) improves barrier function by inhibiting apoptosis of intestinal epithelial cells[57]. S. thermophilus and L. acidophilus have been shown to enhance phosphorylation of actinin and occludin in the tight junction, thereby preventing the invasion of enteroinvasive E. coli into human intestinal epithelial cells[58].
Alteration of the intestinal flora. Probiotics suppress the growth and invasion of pathogens in several ways. They competitively exclude pathogenic bacteria by occupying the limited physical space in the mucus layer and on epithelial cells. They also engage pattern-recognition receptors and consume substrate otherwise available to other (pathogenic) microbes. In addition, probiotics render their microenvironment inauspicious for pathogens by secreting antimicrobial substances such as hydrogen peroxide, organic acids, and bacteriocins. For example, both in vitro and in vivo experiments demonstrate that B. infantis suppresses the growth of Bacteroides vulgatus[59]. VSL#3 has been shown to inhibit Salmonella dublin invasion into T-84 cells[49]. Patients with pouchitis treated with VSL#3 have been demonstrated to have increased bacterial diversity in the pouch, and decreased fungal diversity[60].
Probiotics may also alter the intestinal microflora by changing the fatty acid profile in the colon. VSL#3 probiotic strains are also capable of converting linoleic acid to conjugated linoleic acid, a fatty acid with anti-inflammatory and anti-carcinogenic properties[61].
Use of probiotics in inflammatory bowel disease treatment
Results from various animal studies and clinical trials using probiotics to treat intestinal inflammation have generated considerable excitement. Data are now emerging which suggest that probiotics are capable of preventing relapse of chronic intestinal inflammation. Some probiotics can even treat mild to moderately active IBD[62,63]. However, at present there is a relative lack of rigorously designed, randomized, placebo-controlled trials. Level 1 evidence is only available for the use of probiotics in post-operative chronic pouchitis while level 2 and 3 evidence supports the use of probiotics in treatment of CD and UC[62] (Table 1).
Table 1.
Clinical studies of probiotics in IBD
| Author | Design |
Group (Dose/d) (n) |
Results | |
| Probiotic | Comparator | |||
| Induction of remission of ulcerative colitis | ||||
| Kato 2004 [87] | DB, R, C | Bifidobacterium fermented milk (100 mL) (10) | Placebo (10) | Reduced UCDAI (P < 0.05) |
| Rembacken 1999 [85] | DB, R, C | E. coli Nissle 1917 (1 × 1011 cfu) (57) | Mesalamine (59) | As effective as mesalamine at attaining remission |
| Bibiloni 2005 [88] | Open-label | VSL#3 (3.6 × 109 cfu) (32) | None | 77% remission or response rate |
| Ishikawa 2003 [86] | R, C | Lactobacillus and Bifidobacterium-fermented milk (100 mL) (11) | Placebo (10) | Reduced exacerbation of symptoms (P < 0.01) |
| Borody 2003 [90] | Case reports | Fecal enema (6) | None | 100% remission |
| Maintenance of remission of ulcerative colitis | ||||
| Kruis 2004 [82] | DB, R, C | E. coli Nissle 1917(2.5-25 × 109 cfu) (162) | Mesalamine (165) | As effective as mesalamine at maintaining remission (P = 0.003) |
| Zocco 2006 [83] | Open-label | Lactobacillus GG (1.8 × 1010 cfu) (65) | Mesalamine (60) Mesalamine + LGG (62) | No difference in relapse rates at 12 mo. LGG more effective than mesalamine at prolonging relapse-free time (P < 0.05) |
| Shanahan 2006 [117] | DB, R, C | Lactobacillus salivarius or Bifidobacterium infantus (1 × 109 cfu) (52/group) | Placebo (53) | No improvement of time to relapse |
| Venturi 1999 [84] | Open-label | VSL#3 (1 × 1012 cfu) (20) | None | 75% maintained clinical and endoscopic remission |
| Induction of remission of Crohn’s disease | ||||
| Schultz 2004 [97] | DB, R, C | Lactobacillus GG (2 × 109 cfu) (5) | Placebo (6) | No difference in remission rates |
| McCarthy 2001 [118] | Open-label | Lactobacillus salivarius (1 × 1010 cfu) (25) | None | Reduced disease activity compared with baseline |
| Gupta 2000 [119] | Open-label | Lactobacillus GG (2 × 1010 cfu) (4) | None | Improvement in CDAI scores compared with baseline (P < 0.05) |
| Maintenance of remission of Crohn’s disease | ||||
| Prantera 2002 [95] | DB, R, C | Lactobacillus GG (1.2 × 1010 cfu) (23) | Placebo (22) | No significant difference in remission |
| Campieri 2000 [120] | R, C | VSL#3 (3 × 1011 cfu) (20) | Mesalamine (20) | Equivalent to mesalamine in preventing recurrence |
| Marteau 2006 [94] | DB, R, C | Lactobacillus johnsonii LA1(2 × 109 cfu) (48) | Placebo (50) | No difference in endoscopic recurrence |
| Malchow 1997 [93] | DB, R, C | E. coli Nissle 1917 (5 × 1010 cfu) (16) | Placebo (12) | No difference in remission rates |
| Bousvaros 2005 [96] | DB, R, C | Lactobacillus GG (2 × 1010 cfu) (39) | Placebo (36) | No difference in time to relapse |
| Guslandi 2000 [91] | R, C 6 mo | Saccharomyces boulardii (1 g/d) + mesalamine (2g) (16) | Mesalamine (16) | Significant prolongation of remission (P < 0.05) |
| Induction of remission of pouchitis | ||||
| Kuisma 2003 [80] | DB, R, C | Lactobacillus GG (1 × 1010 cfu) (10) | Placebo (10) | No difference in PDAI |
| Laake 2004 [121] | Open-label | Lactobacillus acidophilus and Bifidobacterium lactis-fermented milk (500 mL) (51) | None | Improved PDAI, no difference in histology |
| Gionchetti 2000 [78] | DB, R, C | VSL#3 (6 g) (20) | Placebo (20) | Increased remission time (P < 0.001) |
| Mimura 2004 [122] | DB, R, C | VSL#3 (6 g) (20 ) | Placebo (16) | Increased remission time (P < 0.0001) |
| Gionchetti 2003 [79] | DB, R, C | VSL#3 (1 × 1011) (20) | Placebo (20) | Increased remission time (P < 0.05) |
DB: Double-blind; R: Randomized; C: Controlled; UCDAI: Ulcerative colitis disease activity index; CDAI: Crohn’s disease activity index; LGG: Lactobacillus GG; PDAI: Pouchitis disease activity index.
Experimental colitis
More than 20 animal models of IBD are available[64] and have been widely used to study the efficacy and mechanisms of probiotics in ameliorating inflammation in order to provide support for human clinical trials. In IL-10 knockout mice, L. plantarum 299v[65], L. reuteri[46], L. salivarius subspecies salivarius 433118, B. infantis 35624[66], L. salivarius subspecies salivarius UCC118[67] and VSL#3[49] have all been shown to successfully attenuate intestinal inflammation. L. GG prevents recurrent colitis in HLA-B27 transgenic rats after antibiotic treatment, whereas L. plantarum has no effect[68]. Both VSL#3 and L. GG significantly ameliorate sulfhydryl-blocker iodoacetamide-induced colitis in rats, whereas they have no effect on dinitrobenzene sulfonic acid-induced colitis[69]. Improved inflammation in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis has also been demonstrated after oral administration of L. salivarius ssp. salivarius CECT5713 and L. plantarum NCIMB8826[70,71]. Dextran sulphate sodium (DSS)-induced colitis in mice is ameliorated by soluble bacterial antigens extracted from E. coli (strain Laves) or by Bifidobacterium strains breve, catenulatum, and longum[72,73]. Daily administration of live but not heat-killed auto-aggregating L. crispatus reduces the severity of DSS-colitis in mice[74]. L. reuteri significantly reduces the colonic inflammation caused by both acetic acid and methotrexate in rats[75,76]. Interestingly, DNA from VSL#3 has been reported to reduce colonic inflammation, thus improving intestinal barrier function in IL-10 KO mice and DSS-induced colitis[41,77].
Chronic pouchitis
Probiotics can maintain antibiotic-induced remission in patients with chronic pouchitis after colectomy for refractory UC. Gionchetti et al[78] have completed placebo-controlled trials using the probiotic cocktail VSL#3 in patients with chronic relapsing pouchitis, showing that the remission rate after 1 year is 90% in the group treated with VSL3# versus 60% in the placebo group. Another study by the same group also showed that VSL#3 is also capable of preventing the development of chronic pouchitis during the first year after pouch surgery for UC[79]. Ten percent of patients who took VSL#3 developed pouchitis, compared with 40% of the placebo group. A double-blinded, prospective, randomized placebo-controlled trial was carried out in 20 patients treated with L.GG versus placebo for 3-mo[80]. In contrast to the study with the probiotic cocktail VSL#3, no significant differences were observed in chronic pouchitis disease activity in L.GG-treated patients despite an increased total fecal lactobacilli:anaerobe ratio, illustrating the inefficacy of L. GG for this condition.
Ulcerative colitis
Numerous studies have investigated the use of probiotics for maintenance of remission of UC in humans. A small study investigating the use of non-pathogenic E. coli Nissle 1917 versus low-dose mesalamine showed that it can maintain remission of quiescent UC, with a relapse rate of 16%-67% in those treated with E.coli versus 11%-73% in the mesalamine group[81]. In a double-blind, randomized trial involving 327 patients, Kruis et al[82] compared the effectiveness of an oral preparation of E. coli Nissle 1917 with mesalamine for maintaining remission of UC, and found that at the end of the 12-mo study, there is no significant difference between the two study groups, with relapses occurring in 36.4% of the E. coli Nissle 1917 group and 33.9% of the mesalamine group (significant equivalence, P = 0.003). Recently, Zocco et al[83] investigated the efficacy of L. GG in maintaining remission of UC and found that there is no difference in relapse rate between the 3 groups after 6 and 12 mo. However, L. GG is more effective than standard mesalamine treatment in prolonging relapse-free time (P < 0.05). An open-label study of VSL#3 showed that 15/20 UC patients remain in remission after 1 year[84].
Several studies have addressed treatment of established UC with probiotic therapy. A study using E. coli Nissle 1917 has demonstrated its equivalence to mesalamine for inducing remission of UC[85]. Ishikawa et al[86] evaluated Bifidobacterium-fermented milk in the treatment of UC, and symptoms of exacerbation were observed in 3 of 11 patients in the treated group versus 9 of 10 patients in the untreated group after 1 year (P = 0.01). However, no difference was observed in endoscopic disease activity. A placebo-controlled trial with bifidobacteria-fermented milk for 12 wk in UC patients with active disease showed that endoscopic disease activity index and histological score are significantly reduced in the treatment group compared with those in placebo group[87]. Uncontrolled administration of daily VSL#3 for 3 mo induces remission in 19 of 30 patients (63%), with a response rate of 87%[88]. As in all of these reported studies, the increased luminal probiotic bacteria return to baseline levels within one month after stopping probiotic treatment, indicating only transient colonization by these probiotic bacteria. In another small study administration of a combination of 3 bifidobacterium species for 2 mo was superior (20% remission) to placebo (93% remission) in maintaining remission of UC induced by sulfasalazine and glucocorticoids[89]. This effect correlates with decreased mucosal TNF-α, IL-1β and increased mucosal IL-10 levels[89]. An interesting study by Borody et al[90] showed that altering the gut microflora in UC patients achieved dramatic outcomes by administration of a freshly prepares enema from a healthy donor to six patients with relapsing refractory UC after broad spectrum antibiotics. This results in a remarkable reversal of all symptoms after 4 mo and sustained remission after 1-3 year of treatment.
Crohn’s disease
Thus far, the use of probiotics for the prevention and treatment of CD is less substantiated than for the prevention and treatment of UC, although some studies certainly show promise. The effect of probiotics in maintaining remission of CD has been reported in an open-label study in patients receiving mesalamine alone versus mesalamine and S. boulardii[91]. At 6 mo, 37.5% of patients had a clinical relapse in the former group versus 6.3% of patients in the probiotic group. In an open-labeled study, McCarthy et al[92] reported that oral administration of L. salivarius UCC118 significantly reduces disease activity in patients with mild to moderate CD. In a randomized, placebo-controlled pilot study, patients with CD were treated with steroids and randomized to non-pathogenic E. coli Nissle 1917 as probiotic therapy or placebo[93]. After 1 year, there were fewer relapses in the probiotic group, but this was not statistically significant. Despite these promising studies, there are numerous reports on the inefficacy of some probiotics in CD. A randomized, placebo-controlled study of 98 patients showed that L. johnsonii LA1 is ineffective in preventing postoperative recurrence of CD[94]. A placebo-controlled trial with L. GG is also ineffective in preventing post-operative recurrence of CD in patients undergoing bowel resection[95]. Other studies have failed to detect benefits of L. GG in maintaining remission of CD in children[96] and adults[97,98]. Although L. GG has demonstrated its efficacy in treating rotaviral[99] and antibiotic-associated[100] diarrhea, results in IBD patients have been particularly underwhelming for this bacterial species, highlighting the species-specifity of colitis protection by probiotics.
PREBIOTICS IN INFLAMMATORY BOWEL DISEASE
Experimental colitis
Studies using prebiotics have been performed mostly in animal models. Lactulose and inulin have been shown to attenuate inflammation in IL-10 knockout mice and DSS-induced colitis respectively[46,101]. The combination of inulin and oligofructose (mixture 1:1) is also effective in preventing the development of colitis in HLA-B27 transgenic rats[102]. This beneficial effect is observed in conjunction with an increase of intestinal bifidobacteria and lactobacilli. Another study in HLA-B27 transgenic rats showed that the effects of the synbiotic “SIM”, a combination of lactobacilli, bifidobacteria and the prebiotic inulin, are attributed to the inulin rather than the probiotics. The ingested probiotic bacteria are not detectable in the cecal content, yet the microflora profile of their cecal contents is altered[103]. In that study, inulin was also shown to specifically stimulate the growth of Bifidobacterium animalis. DSS-induced colitis rats fed with goat’s milk oligosaccharides maintain their body weight, have reduced colonic myeloperoxidase activity and clinical symptoms and increased MUC-3 expression compared with control rats[104]. Goat’s milk oligosaccharides also causes decreased anorexia, weight loss, bowel wall thickening and necrotic lesions in TNBS-induced colitis in rats, compared with untreated controls[105]. In another study of TNBS-induced colitis in rats, a 2-wk feeding of lactulose prior to the induction of colitis reduces myeloperoxidase activity, colonic TNFα and leukotriene B production, in conjunction with an increase of lactobacilli and bifidobacteria species in feces[106]. Lactulose has also demonstrated a dose-dependent beneficial effect on DSS-induced colitis in rats, including improvements of colonic ulceration areas, body weight changes, diarrhea, bloody stools and a reduction of myeloperoxidase activity and microscopic colitis[107].
However, not all studies using prebiotics have resulted in positive outcomes. Moreau et al[108] found that fructo-oligosaccharides are ineffective in improving DSS-induced colitis in rats. Holma et al[109] have reported a similar inefficacy of galacto-oligosaccharides in TNBS-colitis rats.
Several studies have investigated the use of an insoluble mixture of glutamine-rich protein and hemicellulose-rich dietary fiber termed germinated barley foodstuff (GBF). Fukuda et al[110] found that feeding GBF to rats with DSS-induced colitis resultes in significantly reduced colonic inflammation scores, and increased butyrate concentrations in cecal contents. Kanauchi et al[111] have observed similar results in DSS-colitis rats, and further determined that the dietary fiber component rather than the protein component of the GBF is responsible for the beneficial effects of GBF.
Ulcerative colitis
Table 2 lists the clinical trials using prebiotics to treat IBD. Although there is a paucity of human studies using prebiotics, the few emerging studies showed that there is potential for this treatment modality. A multi-centered open-label trial reported that oral administration of GBF to patients with mild to moderately active UC for 24 wk resultes in a significant decrease in clinical activity index, compared to controls[112]. An open-label study of 22 UC patients in remission showed that a daily oral intake of 20 g GBF resultes in a significantly improved clinical activity index and endoscopic score at 3, 6 and 12 mo, and a reduced relapse rate, compared with controls[113]. A recent randomized, double-blinded controlled trial by Furrie et al[114] examined the use of synbiotics in 18 patients with active UC, using a combination therapy of B. longum, inulin and oligofructose, and found that sigmoidoscopy inflammation scores are reduced in the synbiotic-treated population when compared to placebo. Intestinal levels of TNF-α and IL-1β are also reduced. Additionally, rectal biopsies have demonstrated reduced inflammation and greater epithelial regeneration in the synbiotic-treatment group.
Table 2.
Clinical studies of prebiotics in IBD
| Author | Design |
Group (Dose/d) (n) |
Results | |
| Probiotic | Comparator | |||
| Induction of remission of ulcerative colitis | ||||
| Kanauchi 2003 [112] | Open label | Conventional therapy + Germinated barley foodstuff (20-30 g) (21) | Conventional therapy | Improved UCDAI |
| Furrie 2005 [114] | DB, R, C | B. longum (2 × 1011) + inulin/oligofructose (6 g) (9) | Placebo (9) | Sigmoidoscopy scores reduced (P = 0.06) β-defensins, TNFα , IL-1α levels decreased (P < 0.05) |
| Maintenance of remission of ulcerative colitis | ||||
| Hanai 2004 [113] | Open label | Conventional therapy + Germinated barley foodstuff (20 g) (22) | Conventional therapy (37) | Improved UCDAI and endoscopic scores |
| Induction of remission of Crohn’s disease | ||||
| Lindsay 2006 [115] | Open label | Fructo-oligosaccharides (15 g) (10) | None | Increase in IL-10 expressing intestinal dendritic cells |
Crohn’s disease
A small, uncontrolled study of 15 active CD patients reported that 21 d of fructo-oligosaccharide (15 g) intake results in a significant decrease of disease activity, an increase of intestinal bifidobacteria and modifications of Toll-like receptors and IL-10 expression in mucosal dendritic cells[115].
CONCLUSION
The link between intestinal microflora and IBD is now well established, and altering the composition of the microflora using probiotics and prebiotics holds promise as a therapeutic strategy for ameliorating chronic intestinal inflammation. Future developments in this field must include rigorous double-blind, placebo-controlled trials, using probiotics and prebiotics along with a further understanding of their protective mechanisms. Due to their excellent safety profile and lack of serious side effects, there are few contraindications to the consumption of prebiotics, probiotics and synbiotics by IBD patients. Further understanding of the interactions between microbes and gastrointestinal tract will help identify which strains of bacteria and/or which prebiotics may be effective in the treatment of different types of chronic inflammatory disease.
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
References
- 1.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 2.Sharma R, Schumacher U, Ronaasen V, Coates M. Rat intestinal mucosal responses to a microbial flora and different diets. Gut. 1995;36:209–214. doi: 10.1136/gut.36.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szentkuti L, Riedesel H, Enss ML, Gaertner K, Von Engelhardt W. Pre-epithelial mucus layer in the colon of conventional and germ-free rats. Histochem J. 1990;22:491–497. doi: 10.1007/BF01007234. [DOI] [PubMed] [Google Scholar]
- 4.Maeda Y, Noda S, Tanaka K, Sawamura S, Aiba Y, Ishikawa H, Hasegawa H, Kawabe N, Miyasaka M, Koga Y. The failure of oral tolerance induction is functionally coupled to the absence of T cells in Peyer’s patches under germfree conditions. Immunobiology. 2001;204:442–457. doi: 10.1078/0171-2985-00054. [DOI] [PubMed] [Google Scholar]
- 5.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 7.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 8.Williamson SI, Wannemuehler MJ, Jirillo E, Pritchard DG, Michalek SM, McGhee JR. LPS regulation of the immune response: separate mechanisms for murine B cell activation by lipid A (direct) and polysaccharide (macrophage-dependent) derived from Bacteroides LPS. J Immunol. 1984;133:2294–2300. [PubMed] [Google Scholar]
- 9.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 10.Bamias G, Nyce MR, De La Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143:895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 11.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, Weber J, Hoffmann U, Schreiber S, Dietel M, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 14.Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J. Alterations of the dominant faecal bacterial groups in patients with Crohn’s disease of the colon. Gut. 2003;52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruseler-van Embden JG, Schouten WR, van Lieshout LM. Pouchitis: result of microbial imbalance. Gut. 1994;35:658–664. doi: 10.1136/gut.35.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol. 2005;21:44–50. [PubMed] [Google Scholar]
- 17.Sydora B, Lupicki M, Martin SM, Backer J, Churchill TA, Fedorak RN, Dieleman LA. Enterobacter Cloacae Induces early onset cecal Inflammation in germ free interleukin-10 gene-deficient mice. Can J Gastroenterol. 2006;20:67A. [Google Scholar]
- 18.Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol. 2002;16:241–246. doi: 10.1155/2002/941087. [DOI] [PubMed] [Google Scholar]
- 19.Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818–826. doi: 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieleman LA, Hoentjen F, Qian BF, Sprengers D, Tjwa E, Torres MF, Torrice CD, Sartor RB, Tonkonogy SL. Reduced ratio of protective versus proinflammatory cytokine responses to commensal bacteria in HLA-B27 transgenic rats. Clin Exp Immunol. 2004;136:30–39. doi: 10.1111/j.1365-2249.2004.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 22.Qian BF, Tonkonogy SL, Hoentjen F, Dieleman LA, Sartor RB. Dysregulated luminal bacterial antigen-specific T-cell responses and antigen-presenting cell function in HLA-B27 transgenic rats with chronic colitis. Immunology. 2005;116:112–121. doi: 10.1111/j.1365-2567.2005.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boirivant M, Marini M, Di Felice G, Pronio AM, Montesani C, Tersigni R, Strober W. Lamina propria T cells in Crohn’s disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology. 1999;116:557–565. doi: 10.1016/s0016-5085(99)70177-0. [DOI] [PubMed] [Google Scholar]
- 24.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad T, Satsangi J, McGovern D, Bunce M, Jewell DP. Review article: the genetics of inflammatory bowel disease. Aliment Pharmacol Ther. 2001;15:731–748. doi: 10.1046/j.1365-2036.2001.00981.x. [DOI] [PubMed] [Google Scholar]
- 26.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 27.Netea MG, Kullberg BJ, de Jong DJ, Franke B, Sprong T, Naber TH, Drenth JP, Van der Meer JW. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn's disease. Eur J Immunol. 2004;34:2052–2059. doi: 10.1002/eji.200425229. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 29.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 31.Cherbut C. Inulin and oligofructose in the dietary fibre concept. Br J Nutr. 2002;87 Suppl 2:S159–S162. doi: 10.1079/BJNBJN2002532. [DOI] [PubMed] [Google Scholar]
- 32.Rinne M, Kalliomaki M, Arvilommi H, Salminen S, Isolauri E. Effect of probiotics and breastfeeding on the bifidobacterium and lactobacillus/enterococcus microbiota and humoral immune responses. J Pediatr. 2005;147:186–191. doi: 10.1016/j.jpeds.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 33.Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Shu Q, Gill HS. Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001 (DR20) against Escherichia coli O157: H7 infection in mice. FEMS Immunol Med Microbiol. 2002;34:59–64. doi: 10.1111/j.1574-695X.2002.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 35.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 36.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001;21:264–271. doi: 10.1023/a:1010979225018. [DOI] [PubMed] [Google Scholar]
- 37.Sheih YH, Chiang BL, Wang LH, Liao CK, Gill HS. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr. 2001;20:149–156. doi: 10.1080/07315724.2001.10719027. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa T, Asai Y, Tamai R, Makimura Y, Sakamoto H, Hashikawa S, Yasuda K. Natural killer cell activities of synbiotic Lactobacillus casei ssp. casei in conjunction with dextran. Clin Exp Immunol. 2006;143:103–109. doi: 10.1111/j.1365-2249.2005.02975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppée JY, Bourdet-Sicard R, Sansonetti PJ, Pédron T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- 41.Jijon H, Backer J, Diaz H, Yeung H, Thiel D, McKaigney C, De Simone C, Madsen K. DNA from probiotic bacteria modulates murine and human epithelial and immune function. Gastroenterology. 2004;126:1358–1373. doi: 10.1053/j.gastro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Di Marzio L, Russo FP, D'Alò S, Biordi L, Ulisse S, Amicosante G, De Simone C, Cifone MG. Apoptotic effects of selected strains of lactic acid bacteria on a human T leukemia cell line are associated with bacterial arginine deiminase and/or sphingomyelinase activities. Nutr Cancer. 2001;40:185–196. doi: 10.1207/S15327914NC402_16. [DOI] [PubMed] [Google Scholar]
- 43.Haller D, Bode C, Hammes WP, Pfeifer AM, Schiffrin EJ, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79–87. doi: 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maassen CB, van Holten-Neelen C, Balk F, den Bak-Glashouwer MJ, Leer RJ, Laman JD, Boersma WJ, Claassen E. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine. 2000;18:2613–2623. doi: 10.1016/s0264-410x(99)00378-3. [DOI] [PubMed] [Google Scholar]
- 45.Morita H, He F, Fuse T, Ouwehand AC, Hashimoto H, Hosoda M, Mizumachi K, Kurisaki J. Adhesion of lactic acid bacteria to caco-2 cells and their effect on cytokine secretion. Microbiol Immunol. 2002;46:293–297. doi: 10.1111/j.1348-0421.2002.tb02698.x. [DOI] [PubMed] [Google Scholar]
- 46.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 47.Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma D, Forsythe P, Bienenstock J. Live Lactobacillus rhamnosus [corrected] is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun. 2004;72:5308–5314. doi: 10.1128/IAI.72.9.5308-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 50.Sturm A, Rilling K, Baumgart DC, Gargas K, Abou-Ghazalé T, Raupach B, Eckert J, Schumann RR, Enders C, Sonnenborn U, et al. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. Infect Immun. 2005;73:1452–1465. doi: 10.1128/IAI.73.3.1452-1465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto S, Hara T, Hori T, Mitsuyama K, Nagaoka M, Tomiyasu N, Suzuki A, Sata M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin Exp Immunol. 2005;140:417–426. doi: 10.1111/j.1365-2249.2005.02790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lammers KM, Vergopoulos A, Babel N, Gionchetti P, Rizzello F, Morselli C, Caramelli E, Fiorentino M, d’Errico A, Volk HD, et al. Probiotic therapy in the prevention of pouchitis onset: decreased interleukin-1beta, interleukin-8, and interferon-gamma gene expression. Inflamm Bowel Dis. 2005;11:447–454. doi: 10.1097/01.mpa.0000160302.40931.7b. [DOI] [PubMed] [Google Scholar]
- 53.Ulisse S, Gionchetti P, D'Alò S, Russo FP, Pesce I, Ricci G, Rizzello F, Helwig U, Cifone MG, Campieri M, et al. Expression of cytokines, inducible nitric oxide synthase, and matrix metalloproteinases in pouchitis: effects of probiotic treatment. Am J Gastroenterol. 2001;96:2691–2699. doi: 10.1111/j.1572-0241.2001.04139.x. [DOI] [PubMed] [Google Scholar]
- 54.Borruel N, Carol M, Casellas F, Antolin M, de Lara F, Espin E, Naval J, Guarner F, Malagelada JR. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–664. doi: 10.1136/gut.51.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mack DR, Michail S, Wei S, McDougall L, Hollingsworth MA. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999;276:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 56.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiba T, Aiba Y, Ishikawa H, Ushiyama A, Takagi A, Mine T, Koga Y. The suppressive effect of bifidobacteria on Bacteroides vulgatus, a putative pathogenic microbe in inflammatory bowel disease. Microbiol Immunol. 2003;47:371–378. doi: 10.1111/j.1348-0421.2003.tb03368.x. [DOI] [PubMed] [Google Scholar]
- 60.Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, Gionchetti P, Blaut M, Campieri M, Fölsch UR, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55:833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ewaschuk JB, Walker JW, Diaz H, Madsen KL. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J Nutr. 2006;136:1483–1487. doi: 10.1093/jn/136.6.1483. [DOI] [PubMed] [Google Scholar]
- 62.Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:286–299. doi: 10.1097/00054725-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 64.Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2002;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, Sartor RB. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8:71–80. doi: 10.1097/00054725-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, Fitzgibbon J, O’Sullivan GC, Kiely B, Collins JK, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52:975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Mahony L, Feeney M, O’Halloran S, Murphy L, Kiely B, Fitzgibbon J, Lee G, O’Sullivan G, Shanahan F, Collins JK. Probiotic impact on microbial flora, inflammation and tumour development in IL-10 knockout mice. Aliment Pharmacol Ther. 2001;15:1219–1225. doi: 10.1046/j.1365-2036.2001.01027.x. [DOI] [PubMed] [Google Scholar]
- 68.Dieleman LA, Goerres MS, Arends A, Sprengers D, Torrice C, Hoentjen F, Grenther WB, Sartor RB. Lactobacillus GG prevents recurrence of colitis in HLA-B27 transgenic rats after antibiotic treatment. Gut. 2003;52:370–376. doi: 10.1136/gut.52.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shibolet O, Karmeli F, Eliakim R, Swennen E, Brigidi P, Gionchetti P, Campieri M, Morgenstern S, Rachmilewitz D. Variable response to probiotics in two models of experimental colitis in rats. Inflamm Bowel Dis. 2002;8:399–406. doi: 10.1097/00054725-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Diaz-Ropero MP, Olivares M, Xaus J, Zarzuelo A, Galvez J. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J Gastroenterol. 2005;11:5185–5192. doi: 10.3748/wjg.v11.i33.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pavan S, Desreumaux P, Mercenier A. Use of mouse models to evaluate the persistence, safety, and immune modulation capacities of lactic acid bacteria. Clin Diagn Lab Immunol. 2003;10:696–701. doi: 10.1128/CDLI.10.4.696-701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Konrad A, Mähler M, Flogerzi B, Kalousek MB, Lange J, Varga L, Seibold F. Amelioration of murine colitis by feeding a solution of lysed Escherichia coli. Scand J Gastroenterol. 2003;38:172–179. doi: 10.1080/00365520310000663. [DOI] [PubMed] [Google Scholar]
- 73.Setoyama H, Imaoka A, Ishikawa H, Umesaki Y. Prevention of gut inflammation by Bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with Bacteroides strains isolated from ulcerative colitis patients. Microbes Infect. 2003;5:115–122. doi: 10.1016/s1286-4579(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 74.Castagliuolo I, Galeazzi F, Ferrari S, Elli M, Brun P, Cavaggioni A, Tormen D, Sturniolo GC, Morelli L, Palù G. Beneficial effect of auto-aggregating Lactobacillus crispatus on experimentally induced colitis in mice. FEMS Immunol Med Microbiol. 2005;43:197–204. doi: 10.1016/j.femsim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Fabia R, Ar’Rajab A, Johansson ML, Willen R, Andersson R, Molin G, Bengmark S. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scand J Gastroenterol. 1993;28:155–162. doi: 10.3109/00365529309096063. [DOI] [PubMed] [Google Scholar]
- 76.Mao Y, Nobaek S, Kasravi B, Adawi D, Stenram U, Molin G, Jeppsson B. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology. 1996;111:334–344. doi: 10.1053/gast.1996.v111.pm8690198. [DOI] [PubMed] [Google Scholar]
- 77.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 79.Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 80.Kuisma J, Mentula S, Jarvinen H, Kahri A, Saxelin M, Farkkila M. Effect of Lactobacillus rhamnosus GG on ileal pouch inflammation and microbial flora. Aliment Pharmacol Ther. 2003;17:509–515. doi: 10.1046/j.1365-2036.2003.01465.x. [DOI] [PubMed] [Google Scholar]
- 81.Kruis W, Schutz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 1997;11:853–858. doi: 10.1046/j.1365-2036.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 82.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 84.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13:1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 85.Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 86.Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr. 2003;22:56–63. doi: 10.1080/07315724.2003.10719276. [DOI] [PubMed] [Google Scholar]
- 87.Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A, et al. Randomized placebo-controlled trial assessing the effect of bifidobacteria-fermented milk on active ulcerative colitis. Aliment Pharmacol Ther. 2004;20:1133–1141. doi: 10.1111/j.1365-2036.2004.02268.x. [DOI] [PubMed] [Google Scholar]
- 88.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539–1546. doi: 10.1111/j.1572-0241.2005.41794.x. [DOI] [PubMed] [Google Scholar]
- 89.Cui HH, Chen CL, Wang JD, Yang YJ, Cun Y, Wu JB, Liu YH, Dan HL, Jian YT, Chen XQ. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis. World J Gastroenterol. 2004;10:1521–1525. doi: 10.3748/wjg.v10.i10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn‘s disease. Dig Dis Sci. 2000;45:1462–1464. doi: 10.1023/a:1005588911207. [DOI] [PubMed] [Google Scholar]
- 92.McCarthy J, O’Mahony L, Dunne C, Kelly P, Feeney M, Kiely B, O’Sullivan G, Collins JK, Shanahan F. An open trial of a novel probiotic as an alternative to steroids in mild/moderately active Crohn’s disease. Gut. 2001;49:A2447. [Google Scholar]
- 93.Malchow HA. Crohn's disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn's disease. J Clin Gastroenterol. 1997;25:653–658. doi: 10.1097/00004836-199712000-00021. [DOI] [PubMed] [Google Scholar]
- 94.Marteau P, Lémann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, Cadiot G, Soulé JC, Bourreille A, Metman E, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn's disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55:842–847. doi: 10.1136/gut.2005.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405–409. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, Goldin B, Hartigan L, Kugathasan S, Levy J, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis. 2005;11:833–839. doi: 10.1097/01.mib.0000175905.00212.2c. [DOI] [PubMed] [Google Scholar]
- 97.Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC. Lactobacillus GG in inducing and maintaining remission of Crohn's disease. BMC Gastroenterol. 2004;4:5. doi: 10.1186/1471-230X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prantera C, Scribano ML. Probiotics and Crohn’s disease. Dig Liver Dis. 2002;34 Suppl 2:S66–S67. doi: 10.1016/s1590-8658(02)80168-2. [DOI] [PubMed] [Google Scholar]
- 99.Szymański H, Pejcz J, Jawień M, Chmielarczyk A, Strus M, Heczko PB. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains--a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 2006;23:247–253. doi: 10.1111/j.1365-2036.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 100.Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564–568. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- 101.Videla S, Vilaseca J, Antolín M, García-Lafuente A, Guarner F, Crespo E, Casalots J, Salas A, Malagelada JR. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96:1486–1493. doi: 10.1111/j.1572-0241.2001.03802.x. [DOI] [PubMed] [Google Scholar]
- 102.Hoentjen F, Welling GW, Harmsen HJ, Zhang X, Snart J, Tannock GW, Lien K, Churchill TA, Lupicki M, Dieleman LA. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11:977–985. doi: 10.1097/01.mib.0000183421.02316.d5. [DOI] [PubMed] [Google Scholar]
- 103.Schultz M, Munro K, Tannock GW, Melchner I, Göttl C, Schwietz H, Schölmerich J, Rath HC. Effects of feeding a probiotic preparation (SIM) containing inulin on the severity of colitis and on the composition of the intestinal microflora in HLA-B27 transgenic rats. Clin Diagn Lab Immunol. 2004;11:581–587. doi: 10.1128/CDLI.11.3.581-587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lara-Villoslada F, Debras E, Nieto A, Concha A, Gálvez J, López-Huertas E, Boza J, Obled C, Xaus J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin Nutr. 2006;25:477–488. doi: 10.1016/j.clnu.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 105.Daddaoua A, Puerta V, Requena P, Martínez-Férez A, Guadix E, de Medina FS, Zarzuelo A, Suárez MD, Boza JJ, Martínez-Augustin O. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2006;136:672–676. doi: 10.1093/jn/136.3.672. [DOI] [PubMed] [Google Scholar]
- 106.Camuesco D, Peran L, Comalada M, Nieto A, Di Stasi LC, Rodriguez-Cabezas ME, Concha A, Zarzuelo A, Galvez J. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel Dis. 2005;11:265–271. doi: 10.1097/01.mib.0000160808.30988.d9. [DOI] [PubMed] [Google Scholar]
- 107.Rumi G, Tsubouchi R, Okayama M, Kato S, Mozsik G, Takeuchi K. Protective effect of lactulose on dextran sulfate sodium-induced colonic inflammation in rats. Dig Dis Sci. 2004;49:1466–1472. doi: 10.1023/b:ddas.0000042248.48819.ad. [DOI] [PubMed] [Google Scholar]
- 108.Moreau NM, Martin LJ, Toquet CS, Laboisse CL, Nguyen PG, Siliart BS, Dumon HJ, Champ MM. Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. Br J Nutr. 2003;90:75–85. doi: 10.1079/bjn2003867. [DOI] [PubMed] [Google Scholar]
- 109.Holma R, Juvonen P, Asmawi MZ, Vapaatalo H, Korpela R. Galacto-oligosaccharides stimulate the growth of bifidobacteria but fail to attenuate inflammation in experimental colitis in rats. Scand J Gastroenterol. 2002;37:1042–1047. doi: 10.1080/003655202320378239. [DOI] [PubMed] [Google Scholar]
- 110.Fukuda M, Kanauchi O, Araki Y, Andoh A, Mitsuyama K, Takagi K, Toyonaga A, Sata M, Fujiyama Y, Fukuoka M, et al. Prebiotic treatment of experimental colitis with germinated barley foodstuff: a comparison with probiotic or antibiotic treatment. Int J Mol Med. 2002;9:65–70. [PubMed] [Google Scholar]
- 111.Kanauchi O, Iwanaga T, Andoh A, Araki Y, Nakamura T, Mitsuyama K, Suzuki A, Hibi T, Bamba T. Dietary fiber fraction of germinated barley foodstuff attenuated mucosal damage and diarrhea, and accelerated the repair of the colonic mucosa in an experimental colitis. J Gastroenterol Hepatol. 2001;16:160–168. doi: 10.1046/j.1440-1746.2001.02427.x. [DOI] [PubMed] [Google Scholar]
- 112.Kanauchi O, Mitsuyama K, Homma T, Takahama K, Fujiyama Y, Andoh A, Araki Y, Suga T, Hibi T, Naganuma M, et al. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med. 2003;12:701–704. [PubMed] [Google Scholar]
- 113.Hanai H, Kanauchi O, Mitsuyama K, Andoh A, Takeuchi K, Takayuki I, Araki Y, Fujiyama Y, Toyonaga A, Sata M, et al. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13:643–647. [PubMed] [Google Scholar]
- 114.Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O'neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54:242–249. doi: 10.1136/gut.2004.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lindsay JO, Whelan K, Stagg AJ, Gobin P, Al-Hassi HO, Rayment N, Kamm MA, Knight SC, Forbes A. Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Gut. 2006;55:348–355. doi: 10.1136/gut.2005.074971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol. 2003;15:697–698. doi: 10.1097/00042737-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 117.Shanahan F, Guraner F, von Wright A, Vilpponene-Salmela T, O’Donoghue D, Kiely B. A one year, randomised, double-blind, placebo controlled trial of a lactobacillus or a bidifobacterium probiotic for maintenance of steroid-induced remission of ulcerative colitis. Gastroenterology. 2006;130(Suppl2):A44. [Google Scholar]
- 118.McCarthy J, O’Mahony L, Dunne C. An open trial of a novel probiotic as an alternative to steroids in mild/moderately active Crohn’s disease. Gut. 2001;49(Suppl III):A2447. [Google Scholar]
- 119.Gupta P, Andrew H, Kirschner BS, Guandalini S. Is lactobacillus GG helpful in children with Crohn’s disease Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr. 2000;31:453–457. doi: 10.1097/00005176-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 120.Campieri M, Rizzello F, Venturi A. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gastroenterology. 2000;118:A781. [Google Scholar]
- 121.Laake KO, Line PD, Grzyb K, Aamodt G, Aabakken L, Røset A, Hvinden AB, Bakka A, Eide J, Bjørneklett A, et al. Assessment of mucosal inflammation and blood flow in response to four weeks' intervention with probiotics in patients operated with a J-configurated ileal-pouch-anal-anastomosis (IPAA) Scand J Gastroenterol. 2004;39:1228–1235. doi: 10.1080/00365520410009320. [DOI] [PubMed] [Google Scholar]
- 122.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


