Abstract
AIM: Disabled-2 (DAB2) is a candidate tumor-suppressor gene identified in ovarian cancer that negatively influences mitogenic signal transduction of growth factors and blocks ras activity. In a recent study, we observed down-regulation of DAB2 transcripts in ESCCs using cDNA microarrays. In the present study, we aimed to determine the clinical significance of loss of DAB2 protein in esophageal tumorigenesis, hypothesizing that DAB2 promoter hypermethylation-mediated gene silencing may account for loss of the protein.
METHODS: DAB2 expression was analyzed by immunohistochemistry in 50 primary esophageal squamous cell carcinomas (ESCCs), 30 distinct hyperplasia, 15 dysplasia and 10 non-malignant esophageal tissues. To determine whether promoter hypermethylation contributes to loss of DAB2 expression in ESCCs, methylation status of DAB2 promoter was analyzed in DAB2 immuno-negative tumors using methylation-specific PCR.
RESULTS: Loss of DAB2 protein was observed in 5/30 (17%) hyperplasia, 10/15 (67%) dysplasia and 34/50 (68%) ESCCs. Significant loss of DAB2 protein was observed from esophageal normal mucosa to hyperplasia, dysplasia and invasive cancer (Ptrend < 0.001). Promoter hypermethylation of DAB2 was observed in 2 of 10 (20%) DAB2 immuno-negative ESCCs.
CONCLUSION: Loss of DAB2 protein expression occurs in early pre-neoplastic stages of development of esophageal cancer and is sustained down the tumorigenic pathway. Infrequent DAB2 promoter methylation in ESCCs suggests that epigenetic gene silencing is only one of the mechanisms causing loss of DAB2 expression in ESCCs.
Keywords: Disabled-2, DOC-2, Esophageal cancer, Promoter hypermethylation, Dysplasia
INTRODUCTION
Esophageal cancer is the most aggressive gastrointestinal malignancy ranking as the 6th most common cancer among males and 9th most common cancer among females globally[1]. Squamous cell carcinoma is the predominant histological subtype of esophageal cancer, characterized by high mortality rate and strong association with dietary habits and life style in India[2-4]. It is the 2nd most common cancer among males and 4th most common cancer among females in India[5]. Despite advances in multimodality therapy, due to late stage of diagnosis and poor efficacy of treatment, the prognosis for patients with ESCC still remains poor with an average 5-year survival of < 10% globally[6,7] and < 12% in India[8,9].
DOC-2/DAB2 (differentially expressed in ovarian carcinoma- 2/disabled-2) is a putative tumor suppressor gene that encodes a 96-ku mitogen-responsive phosphoprotein involved in signal transduction[10-15]. It inhibits mitogenic stimulation via the Ras pathway by binding to Grb2[13,14]. DAB2 has been shown to act as a negative regulator of c-Src in normal prostatic epithelium and cancer[16]. This interaction causes inactivation of Erk and Akt proteins critical for proliferation and survival of prostate cancer cells[16,17]. DAB2 is found in association with transforming growth factor-β (TGF-β) typeIand II receptors, while directly binding to the TGF-β signaling intermediates Smad2 and Smad3 through the PID domain[18]. DAB2 plays an important regulatory role in cellular differentiation and induction of differentiation in the absence of DAB2 expression commits the cell to apoptosis[19]. DAB2 functions as a negative regulator of canonical Wnt signaling by stabilizing the beta-catenin degradation complex[20]. Recently, treatment of mouse F9 embryonic carcinoma cells with glycosylceramide synthase inhibitors has been shown to result in depletion of gangliosides and delayed expression of DAB2, suggesting their involvement in F9 cell differentiation[21]. The aberrant expression of DAB2 has been reported in tumors, such as ovarian, prostate, choriocarcinoma, and breast[11,22-26]. Histologically, elevated levels of DAB2 are associated with an enriched basal cell compartment, a progenitor cell for glandular epithelium and may be involved in the homeostasis of rat prostate regeneration[23]. In addition, stable expression of DAB2 in cancer cell line has been shown to significantly reduce its in vitro growth rate, concomitant with an increase in cells in G1 and decrease in anchorage-independent growth on soft agar[11,22,23]. Therefore, DAB2 appears to be a potent negative regulator of cancer cell growth.
In a recent study, we observed down-regulation of DAB2 transcripts in ESCCs using cDNA microarrays (data not shown). To our knowledge, the clinical significance of down-regulation of DAB2 in ESCC remains to be determined. In the present study, we analyzed DAB2 protein expression in different stages of development of esophageal cancer viz., primary ESCC and paired non-malignant normal, hyperplasia and dysplasia. Loss of DAB2 protein was observed in high proportion of ESCCs and dysplasia. Therefore, we hypothesized epigenetic silencing of DAB2 gene in ESCCs. To test this hypothesis, the methylation status of the putative promoter (exon 1) of DAB2 was analyzed using methylation-specific PCR in ESCC tissues that showed loss of DAB2 protein.
MATERIALS AND METHODS
Tissue samples
The study was approved by Institutional Human Ethics Committee and informed consent was obtained from the patients prior to enrolment in the study. The tissue samples used in this study were collected from Department of Gastrointestinal Surgery, All India Institute of Medical Sciences, New Delhi, India. All the samples were histologically confirmed to be either ESCCs, esophageal hyperplasia, dysplasia or non-malignant tissues by the pathologist (SDG). The samples included 50 histologically confirmed ESCCs, 10 non-malignant esophageal mucosa, 30 hyperplasia and 15 dysplasia.
Immunohistochemistry
Immunohistochemical analysis of DAB2 protein was carried out in paraffin-embedded tissue sections (5 μm thickness). Briefly, the sections were deparaffinized in xylene, hydrated and incubated with 30 mL/L H2O2 in methanol for 5 min to inactivate the endogenous peroxidase. Slides were washed with Tris-buffered saline (TBS, 0.1 mol/L, pH7.4) and heated for 15 min at 100°C in 10 mmol/L sodium citrate buffer (pH 6.0). Thereafter, sections were incubated with anti-disabled-2 goat polyclonal antibody (C-20, dilution 1:50, Santa Cruz Biotechnology Inc., Santacruz, CA) at 4°C overnight in humidified chamber. Sections were incubated with biotinylated anti-mouse antiserum with horseradish peroxidase streptavidin conjugate (DAKO Labs, Glostrup, Denmark). After every incubation step, slides were washed with TBS thrice and color was developed using 3,3’-diaminobenzidine hydrochloride (DAB). Sections were counterstained with Mayer’s hematoxylin, and mounted with DPX mountant for evaluation. Normal ovary tissue sections were taken as positive control for DAB2 and in the negative control primary antibody was replaced by isotype-specific IgG (data not shown).
Bisulfide modification
Genomic DNA from tissues was isolated by phenol-chloroform method. Genomic DNA was treated with sodium bisulfite (Sigma-Aldrich, Bangalore, India) as previously described[27]. Briefly, 1 μg of DNA was denatured with 0.2 mol/L NaOH for 10 min at 37°C. Thirty microliters of 10 mmol/L hydroquinone (Sigma Aldrich) and 520 μL of 3 mol/L sodium bisulfite (pH 5.0) were added, followed by incubation at 50°C for 16 h. The modified DNA was purified using Wizard DNA purification columns (Promega, Madison, WI). The purified DNA was desulphonated with NaOH and precipitated with absolute ethanol in the presence of glycogen and ammonium acetate. DNA was resuspended in 20 μL of 1 mmol/L Tris (pH 8.0) and used for PCR amplification.
Methylation-specific PCR
Bisulfite-treated genomic DNA was amplified with either a methylation-specific or unmethylation-specific primer set for 35 cycles at 95°C for 5 min (hot started by adding Taq polymerase), followed by cycling with denaturation at 95°C for 30 s, primers annealing at 60°C for 30 s, and extension at 72°C for 1 min, as well as a final extension step at 72°C for 5 min. Methylation-specific primers span 6 CpG dinucleotides numbered 19-21 (forward) and 35-37 (reverse) of DAB2 exon1 (Gene accession No. AF218839). Similarly, unmethylation-specific primers span 8 CpG dinucleotides numbered 19-22 (forward) and 35-37 (reverse). The methylation-specific primers were designed using 5’-TATTTTTCGTCGGGAGTGGTCGC-3’ as the forward primer and 5’-ACTAACTATTACCTCCGTAAAACG-3’ as the reverse primer. The unmethylation-specific primers for site S1 were designed using 5’-GAATTATATTTTTTGTTGGGAGTGGTTGT-3’ as the forward primer and 5’-CCAACTAACTATTACCTCCATAAAACA -3’ as the reverse. These primer sequences were reported by Akiyama et al[28].
RESULTS
Immunohistochemical analysis of DAB2
Immunohistochemical analysis was carried out to determine the expression of DAB2 protein in different stages of esophageal tumorigenesis. Table 1 summarizes the clinicopathological parameters of ESCC patients and expression status of DAB2 protein in the tumors. Strong cytoplasmic staining of DAB2 was observed in epithelial cells of all the 10 non-malignant (histologically normal) esophageal mucosa (Figure 1A). No detectable expression of DAB2 protein was observed in 5/30 (17%) hyperplasia (Figure 1B), 10/15 (67%) dysplasia (Figure 1C) and 34/50 (68%) ESCCs (Figure 1D). Only 32% of ESCCs showed weak to moderate DAB2 staining (Figure 1E). Significant loss of DAB2 protein expression was observed from esophageal normal mucosa to hyperplasia, dysplasia and SCC (Ptrend < 0.001). Significantly higher proportion of dysplastic tissues showed loss of DAB2 expression in comparison with hyperplasia (P = 0.002; OR = 9.998; 90% CI = 2.368-42.210).
Table 1.
Correlation of DAB2 protein expression with clinicopathological parameters of ESCC patients
| Parameters | Total cases |
DAB2 |
|
| n | Positive n (%) | Negative n (%) | |
| ESCC | 50 | 16 (32) | 34 (68) |
| Age | |||
| ≤ 40 yr | 14 | 4 (28) | 10 (72) |
| ≥ 40 yr | 36 | 12 (33) | 24 (67) |
| Gender | |||
| Male | 30 | 11 (37) | 19 (63) |
| Female | 20 | 5 (25) | 15 (75) |
| Tumor Stage | |||
| T2 | 3 | 1 | 2 |
| T3 | 36 | 11 (31) | 25 (69) |
| T4 | 11 | 4 (36) | 7 (64) |
| Hp Grading | |||
| WDSCC | 8 | 2 | 6 |
| MDSCC | 37 | 11 (30) | 26 (70) |
| PDSCC | 5 | 3 (60) | 2 (40) |
| Nodal stage | |||
| Node -ve | 23 | 8 (35) | 15 (65) |
| Node +ve | 27 | 8 (30) | 19 (70) |
| Normal | 10 | 10 | |
| Hyperplasia | 30 | 25 (83) | 5 |
| Dysplasia | 15 | 5 | 10 (67) |
Figure 1.
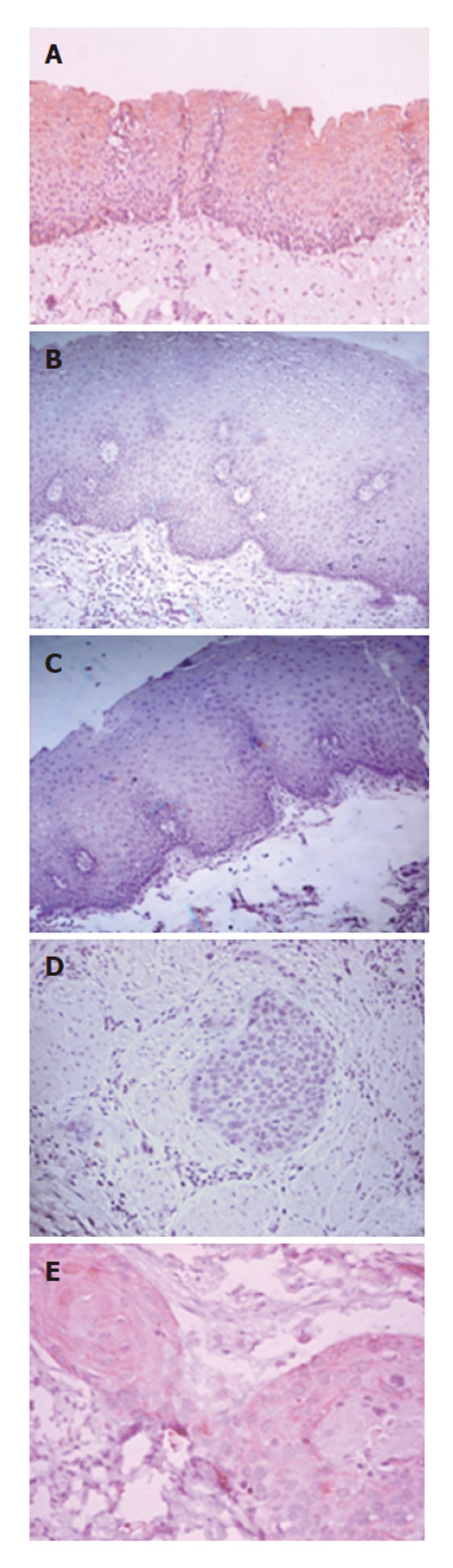
Immunohistochemical analysis of DAB2 in esophageal squamous cell carcinogenesis. A: Cytoplasmic expression of DAB2 in non-malignant normal esophageal mucosa; B: Hyperplastic esophageal mucosa showing no detectable expression of DAB2 protein; C: Dysplastic esophageal mucosa showing loss of DAB2 protein expression; D: ESCC section showing loss of DAB2 protein expression; and E: ESCC showing faint cytoplasmic DAB2 protein expression (A-D: Original magnification x 100; and E: Original magnification x 200).
DAB2 exon1 methylation in ESCCs
To determine the possibility of promoter methylation-mediated gene silencing of DAB2, methylation status of putative promoter in exon1 of DAB2 gene was analyzed in 10 DAB2 immuno-negative ESCC tissues. Burkitt’s lymphoma cell line Raji was used as a positive control for hypermethylation of DAB2 gene, as reported by Akiyama et al[28]. As shown in Figure 2, 8 of 10 (80%) ESCCs showed signal (PCR amplicon) for the unmethylated DAB2 alleles, while 2 cases showed the presence of both unmethylated and methylated PCR amplicon, suggesting that DAB2 promoter hypermethylation may account for loss of DAB2 protein in these two tumors.
Figure 2.
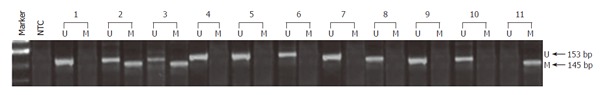
DAB2 exon-1 promoter hypermethylation. Methylation-specific PCR was done to determine the possibility of promoter methylation-mediated gene silencing of DAB2. Eight of 10 ESCCs showed signal (PCR amplicon) for the unmethylated DAB2 alleles (samples 1, 4-10), while 2 cases showed the presence of both unmethylated and methylated PCR amplicon (samples 2 and 3). Burkitt’s lymphoma cell line Raji was used as a positive control for hypermethylation of DAB2 gene (sample 11). In each sample, lane U represents the unmethylated product, while M represents the methylated product, lane NTC refers to the no template control.
DISCUSSION
In this study, we demonstrated, probably for the first time, significant loss of DAB2 protein in different stages of development and progression of ESCC, from normal to hyperplasia, dysplasia and invasive cancer (Ptrend < 0.001). Strong cytoplasmic expression of DAB2 protein was observed in all the 10 histologically non-malignant esophageal tissues. Loss of DAB2 protein was observed in preneoplastic stages, as early as in hyperplasia (in 5 of 30 hyperplastic tissues analyzed), suggesting that deregulation of DAB2 expression is likely to be an early event in esophageal tumorigenesis. Interestingly, loss of DAB2 protein was observed in significantly higher proportion of dysplastic tissues (10/15 cases) in comparison with hyperplasia (P = 0.002), indicating a critical impact of loss of this protein in evolution of dysplasia. Furthermore, loss of DAB2 in 34/50 (68%) of ESCCs observed in this study suggests that down-regulation of DAB2 protein is sustained down the tumorigenic pathway. To our knowledge, the clinical relevance of DAB2 in primary human esophageal tumors remains to be determined. Therefore, our study is important in demonstrating the clinical significance of aberrant DAB2 expression in primary ESCCs.
The physical interaction of epithelial cells with the basement membrane ensures correct positioning and acts as a survival factor for epithelial cells. Cells that detach from the basement membrane often undergo apoptosis[29,30]. In tumors, this positional control is absent, resulting in disorganized cell proliferation[26]. Inactivation of a gene(s) controlling epithelial cell positioning may be a step in tumorigenicity. One of these genes is DAB2, which functions in cell positioning control and loss of DAB2 protein has been suggested to contribute to the basement membrane-independent, disorganized proliferation of tumor cells[26]. DAB2 expression in breast cancer cells resulted in sensitivity to suspension-induced cell death (anoikis)[31]. Loss of DAB2 expression and the loss of collagen IV and laminin-containing basement membrane are two critical events associated with morphologic dysplastic changes of the ovarian surface epithelium as a step in tumorigenicity[32,33].
Basement membrane (BM) can regulate differentiation, proliferation and polarity of esophageal epithelium and its integrity is important for carcinogenesis. Studies aimed to explore the effect of the BM changes induced by chronic inflammation on esophageal carcinogenesis have suggested that BM changes with aberrant proliferation of esophageal epithelia[34]. The most salient finding of our study is the significant loss of DAB2 protein expression in pre-neoplastic lesions, such as dysplasia in comparison with non-malignant esophageal epithelium and hyperplasia (P = 0.002). Based on the studies in ovarian cancer and transitional cell carcinoma and our observations in esophageal dysplasia and ESCC, we hypothesize that down-regulation of DAB2 protein in dysplasia may be an important step in loss of epithelial cell positioning, aberrant proliferation and tumorigenicity. Therefore, loss of DAB2 protein may serve as a candidate molecular marker for pre-neoplastic lesions.
Akiyama et al[28] showed epigenetic silencing of GATA-4 and GATA-5 but not GATA-6 transcription factor genes and their potential downstream anti-tumor target genes in colorectal and gastric cancer. GATA6 and histone deacetylase inhibitor synergistically induce DAB2 gene expression in transitional cell carcinoma (TCC) cell lines[35]. Histone acetylation status associated with the 5’ upstream regulatory sequence of DAB2 gene is one of the key determinants of its activity. GATA6 can specifically induce DAB2 promoter activity. Increased histone acetylation and the presence of GATA6 have a synergistic effect on DAB2 promoter activity which results in elevation of DAB2 protein expression. Although the underlying mechanism leading to high GATA6 and/or acetyl H3 levels in TCC cell lines is still unclear, it is likely that enzymes responsible for epigenetic regulation, such as histone modification or DNA methylation, could play a role. Therefore, we analyzed the methylation status of DAB2 gene in esophageal tumors that showed loss of DAB2 protein. Methylation-specific PCR showed methylation of DAB2 promoter in 2 of the 10 DAB2 immuno-negative ESCCs analyzed. These findings suggest that epigenetic silencing of DAB2 is infrequent in ESCCs and accounts for down-regulation of the protein in only a subset of esophageal tumors. Thus, there is a need to investigate other mechanisms that may be responsible for loss of DAB2 protein in ESCCs harboring the unmethylated DAB2 promoters. A parallel study on DAB2 protein expression and promoter methylation in our laboratory showed similar discordance between loss of protein expression and epigenetic silencing of the gene in breast cancer. In silico analysis suggested that post-transcriptional, micro RNA-mediated targeting of DAB2 mRNA may be another mechanism for gene silencing (unpublished data of Bagadi SAR et al). It will be worthwhile to determine if micro RNA is involved in targeting of DAB2 mRNA accounting for loss of DAB2 protein in ESCCs as well.
In conclusion, our data suggest that loss of DAB2 protein occurs in early pre-neoplastic stages and is sustained down the tumorigenic pathway of esophageal squamous cell carcinogenesis, underscoring its potential as a candidate molecular marker for pre-neoplastic lesions. Furthermore, DAB2 exon-1 promoter hypermethylation is an infrequent event in ESCCs.
Footnotes
S- Editor Liu Y L- Editor Kumar M E- Editor Liu WF
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Malkan G, Mohandas KM. Epidemiology of digestive cancers in India. I. General principles and esophageal cancer. Indian J Gastroenterol. 1997;16:98–102. [PubMed] [Google Scholar]
- 3.Nayar D, Kapil U, Joshi YK, Sundaram KR, Srivastava SP, Shukla NK, Tandon RK. Nutritional risk factors in esophageal cancer. J Assoc Physicians India. 2000;48:781–787. [PubMed] [Google Scholar]
- 4.Phukan RK, Chetia CK, Ali MS, Mahanta J. Role of dietary habits in the development of esophageal cancer in Assam, the north-eastern region of India. Nutr Cancer. 2001;39:204–209. doi: 10.1207/S15327914nc392_7. [DOI] [PubMed] [Google Scholar]
- 5.Gajalakshmi V, Swaminathan R, Shanta V. An Independent Survey to Assess Completeness of Registration: Population Based Cancer Registry, Chennai, India. Asian Pac J Cancer Prev. 2001;2:179–183. [PubMed] [Google Scholar]
- 6.Montesano R, Hollstein M, Hainaut P. Genetic alterations in esophageal cancer and their relevance to etiology and pathogenesis: a review. Int J Cancer. 1996;69:225–235. doi: 10.1002/(SICI)1097-0215(19960621)69:3<225::AID-IJC13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 8.Gupta NM, Jindal R, Prakash O, Gupta R, Bhasin DK. Comparison of the clinical profile and outcome for squamous cell carcinoma and adenocarcinoma of the distal esophagus and cardia in India. Surg Today. 2001;31:400–404. doi: 10.1007/s005950170129. [DOI] [PubMed] [Google Scholar]
- 9.Yeole BB, Kumar AV. Population-based survival from cancers having a poor prognosis in Mumbai (Bombay), India. Asian Pac J Cancer Prev. 2004;5:175–182. [PubMed] [Google Scholar]
- 10.Xu XX, Yang W, Jackowski S, Rock CO. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem. 1995;270:14184–14191. doi: 10.1074/jbc.270.23.14184. [DOI] [PubMed] [Google Scholar]
- 11.Mok SC, Chan WY, Wong KK, Cheung KK, Lau CC, Ng SW, Baldini A, Colitti CV, Rock CO, Berkowitz RS. DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene. 1998;16:2381–2387. doi: 10.1038/sj.onc.1201769. [DOI] [PubMed] [Google Scholar]
- 12.Xu XX, Yi T, Tang B, Lambeth JD. Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene. 1998;16:1561–1569. doi: 10.1038/sj.onc.1201678. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CP, Ely BD, Pong RC, Wang Z, Zhou J, Hsieh JT. The role of DOC-2/DAB2 protein phosphorylation in the inhibition of AP-1 activity. An underlying mechanism of its tumor-suppressive function in prostate cancer. J Biol Chem. 1999;274:31981–31986. doi: 10.1074/jbc.274.45.31981. [DOI] [PubMed] [Google Scholar]
- 14.Zhou J, Hsieh JT. The inhibitory role of DOC-2/DAB2 in growth factor receptor-mediated signal cascade. DOC-2/DAB2-mediated inhibition of ERK phosphorylation via binding to Grb2. J Biol Chem. 2001;276:27793–27798. doi: 10.1074/jbc.M102803200. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Tseng CP, Pong RC, Chen H, McConnell JD, Navone N, Hsieh JT. The mechanism of growth-inhibitory effect of DOC-2/DAB2 in prostate cancer. Characterization of a novel GTPase-activating protein associated with N-terminal domain of DOC-2/DAB2. J Biol Chem. 2002;277:12622–12631. doi: 10.1074/jbc.M110568200. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Scholes J, Hsieh JT. Characterization of a novel negative regulator (DOC-2/DAB2) of c-Src in normal prostatic epithelium and cancer. J Biol Chem. 2003;278:6936–6941. doi: 10.1074/jbc.M210628200. [DOI] [PubMed] [Google Scholar]
- 17.Zhoul J, Hernandez G, Tu SW, Huang CL, Tseng CP, Hsieh JT. The role of DOC-2/DAB2 in modulating androgen receptor-mediated cell growth via the nongenomic c-Src-mediated pathway in normal prostatic epithelium and cancer. Cancer Res. 2005;65:9906–9913. doi: 10.1158/0008-5472.CAN-05-1481. [DOI] [PubMed] [Google Scholar]
- 18.Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prunier C, Howe PH. Disabled-2 (Dab2) is required for transforming growth factor beta-induced epithelial to mesenchymal transition (EMT) J Biol Chem. 2005;280:17540–17548. doi: 10.1074/jbc.M500974200. [DOI] [PubMed] [Google Scholar]
- 20.Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH. Regulation of the Wnt signaling pathway by disabled-2 (Dab2) EMBO J. 2003;22:3084–3094. doi: 10.1093/emboj/cdg286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato T, Zakaria AM, Uemura S, Ishii A, Ohno-Iwashita Y, Igarashi Y, Inokuchi J. Role for up-regulated ganglioside biosynthesis and association of Src family kinases with microdomains in retinoic acid-induced differentiation of F9 embryonal carcinoma cells. Glycobiology. 2005;15:687–699. doi: 10.1093/glycob/cwi055. [DOI] [PubMed] [Google Scholar]
- 22.Fulop V, Colitti CV, Genest D, Berkowitz RS, Yiu GK, Ng SW, Szepesi J, Mok SC. DOC-2/hDab2, a candidate tumor suppressor gene involved in the development of gestational trophoblastic diseases. Oncogene. 1998;17:419–424. doi: 10.1038/sj.onc.1201955. [DOI] [PubMed] [Google Scholar]
- 23.Tseng CP, Ely BD, Li Y, Pong RC, Hsieh JT. Regulation of rat DOC-2 gene during castration-induced rat ventral prostate degeneration and its growth inhibitory function in human prostatic carcinoma cells. Endocrinology. 1998;139:3542–3553. doi: 10.1210/endo.139.8.6159. [DOI] [PubMed] [Google Scholar]
- 24.Schwahn DJ, Medina D. p96, a MAPK-related protein, is consistently downregulated during mouse mammary carcinogenesis. Oncogene. 1998;17:1173–1178. doi: 10.1038/sj.onc.1202038. [DOI] [PubMed] [Google Scholar]
- 25.Fazili Z, Sun W, Mittelstaedt S, Cohen C, Xu XX. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene. 1999;18:3104–3113. doi: 10.1038/sj.onc.1202649. [DOI] [PubMed] [Google Scholar]
- 26.Sheng Z, Sun W, Smith E, Cohen C, Sheng Z, Xu XX. Restoration of positioning control following Disabled-2 expression in ovarian and breast tumor cells. Oncogene. 2000;19:4847–4854. doi: 10.1038/sj.onc.1203853. [DOI] [PubMed] [Google Scholar]
- 27.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akiyama Y, Watkins N, Suzuki H, Jair KW, van Engeland M, Esteller M, Sakai H, Ren CY, Yuasa Y, Herman JG, et al. GATA-4 and GATA-5 transcription factor genes and potential downstream antitumor target genes are epigenetically silenced in colorectal and gastric cancer. Mol Cell Biol. 2003;23:8429–8439. doi: 10.1128/MCB.23.23.8429-8439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 30.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SC, Makino K, Xia W, Kim JS, Im SA, Peng H, Mok SC, Singletary SE, Hung MC. DOC-2/hDab-2 inhibits ILK activity and induces anoikis in breast cancer cells through an Akt-independent pathway. Oncogene. 2001;20:6960–6964. doi: 10.1038/sj.onc.1204873. [DOI] [PubMed] [Google Scholar]
- 32.Capo-Chichi CD, Smith ER, Yang DH, Roland IH, Vanderveer L, Cohen C, Hamilton TC, Godwin AK, Xu XX. Dynamic alterations of the extracellular environment of ovarian surface epithelial cells in premalignant transformation, tumorigenicity, and metastasis. Cancer. 2002;95:1802–1815. doi: 10.1002/cncr.10870. [DOI] [PubMed] [Google Scholar]
- 33.Roland IH, Yang WL, Yang DH, Daly MB, Ozols RF, Hamilton TC, Lynch HT, Godwin AK, Xu XX. Loss of surface and cyst epithelial basement membranes and preneoplastic morphologic changes in prophylactic oophorectomies. Cancer. 2003;98:2607–2623. doi: 10.1002/cncr.11847. [DOI] [PubMed] [Google Scholar]
- 34.Zhang GH, Su M, Tian DP. [Effect of chronic inflammation-induced basement membrane changes on esophageal carcinogenesis] Ai Zheng. 2005;24:1071–1075. [PubMed] [Google Scholar]
- 35.Zhou J, Hernandez G, Tu SW, Scholes J, Chen H, Tseng CP, Hsieh JT. Synergistic induction of DOC-2/DAB2 gene expression in transitional cell carcinoma in the presence of GATA6 and histone deacetylase inhibitor. Cancer Res. 2005;65:6089–6096. doi: 10.1158/0008-5472.CAN-04-3672. [DOI] [PubMed] [Google Scholar]


