Abstract
AIM: To identify the clinicopathological characteristics of lymph node-negative gastric carcinoma, and also to evaluate outcome indicators in the lymph node-negative patients.
METHODS: Of 2848 gastric carcinoma patients, 1524 (53.5%) were lymph node-negative. A statistical analysis was performed using the Cox model to estimate outcome indicators.
RESULTS: There was a significant difference in the recurrence rate between lymph node-negative and lymph node-positive patients (14.4% vs 41.0%, P < 0.001). The 5-year survival rate was significantly lower in lymph node-positive than in lymph node-negative patients (31.1% vs 77.4%, P < 0.001). Univariate analysis revealed that the following factors influenced the 5-year survival rate: patient age, tumor size, depth of invasion, tumor location, operative type, and tumor stage at initial diagnosis. The Cox proportional hazard regression model revealed that tumor size, serosal invasion, and curability were independent, statistically significant, prognostic indicators of lymph node-negative gastric carcinoma.
CONCLUSION: Lymph node-negative patients have a favorable outcome attributable to high curability, but the patients with relatively large tumors and serosal invasion have a poor prognosis. Curability is one of the most reliable predictors of long-term survival for lymph node-negative gastric carcinoma patients.
Keywords: Gastric carcinoma, Survival, Tumor size, Serosal invasion, Curability
INTRODUCTION
The presence or absence of lymph node metastasis is one of the most important prognostic indicators among several clinicopathological factors that influence the prognosis of patients with gastric carcinoma[1-4]. Several studies have been conducted to identify prognostic indicators in patients with lymph node-negative gastric carcinoma, but these studies have involved small numbers of patients[5-9]. In the present study, we compared lymph node-negative patients with lymph node-positive ones to identify the clinicopathological characteristics of lymph node-negative gastric carcinoma. We also evaluated outcome indicators for lymph node-negative carcinoma.
MATERIALS AND METHODS
Patients and specimens
From 1986 to 2000, 2848 patients with gastric carcinoma were treated in the Division of Gastroenterologic Surgery, Department of Surgery, Chonnam National University Medical School, Gwangju, Korea. Of these, 1524 (53.5%) were in the lymph node-negative group.
The clinicopathologic features of these patients with lymph node-negative gastric carcinoma were retrospectively reviewed. Information on the patient’s age, sex, tumor size, tumor location, macroscopic appearance, depth of invasion, hepatic metastasis, peritoneal dissemination, stage at the initial diagnosis, operative type, recurrence pattern, curability, and survival rate was obtained from the hospital records. The American Joint Committee on Cancer (AJCC) TNM Staging system was used for clinical and pathologic staging[10]. Histological evaluation was performed according to the Japanese General Rules for Gastric Cancer Study in Surgery and Pathology[11].
Statistical analysis
The survival rates of the patients were calculated using the Kaplan-Meier method and the relative prognostic importance of the parameters was investigated using the Cox proportional hazards model. The x2 was used to evaluate the statistical significance of differences and P values less than 0.05 were considered statistically significant.
RESULTS
Table 1 summarizes the clinical findings in 1524 (53.5%) patients with lymph node-negative gastric carcinoma and 1324 (46.5%) patients with lymph node-positive gastric carcinoma. There was no significant difference in the mean age between lymph node-negative and lymph node-positive patients (56.9 vs 57.1 years, respectively). Of the 1524 patients with lymph node-negative gastric carcinoma, 988 (64.8%) were male and 536 (35.2%) were female. There were 889 males (67.1%) and 435 females (32.9%) in the group of 1324 lymph node-positive patients. Although there were more males than females in each group, the gender ratio was the same in both groups. Dissection above the D2 lymph node was performed in most patients in each group (82.8% and 81.4% of the lymph node-positive and lymph node-negative patients, respectively). Subtotal gastrectomy was the procedure performed most frequently (80.4%) in patients with lymph node-negative gastric carcinoma. The curative resection rate was significantly higher (97.9%, 1492/1534) in lymph node-negative patients than in node-positive patients (77.3%; 1,024/1,324; P < 0.001), and the recurrence rate was significantly lower (14.4%) in lymph node-negative than in lymph node-positive patients (41.1%; P < 0.001). Peritoneal recurrence was the predominant type of recurrence in both groups.
Table 1.
Clinical features of patients with node-negative and -positive gastric carcinoma
| Variables | Node-negative (n = 1524) (%) | Node-positive (n = 1324) (%) | P value |
| Age (mean, yr) | 56.9 ± 11.1 | 57.1 ± 11.6 | NS |
| Gender | NS | ||
| Male | 988 (64.8) | 889 (67.1) | |
| Female | 536 (35.2) | 435 (32.9) | |
| Extent of lymph node dissection | NS | ||
| <D2 | 262 (17.2) | 246 (18.6) | |
| ≥D2 | 1,262 (82.8) | 1,078 (81.4) | |
| Operative type | < 0.001 | ||
| Total gastrectomy | 251 (16.5) | 383 (28.9) | |
| Subtotal gastrectomy | 1,226 (80.4) | 902 (68.1) | |
| Proximal gastrectomy | 12 (0.8) | 3 (0.3) | |
| Others | 35 (2.3) | 36 (2.7) | |
| Curability | < 0.001 | ||
| Curative | 1,492 (97.9) | 1,024 (77.3) | |
| Non-curative | 32 (2.1) | 300 (22.7) | |
| Recurrence | < 0.001 | ||
| Locoregional | 29 (13.2) | 46 (8.5) | |
| Peritoneum | 163 (74.1) | 480 (88.4) | |
| Others | 28 (12.7) | 17 (3.1 ) |
NS, not significant.
The histopathological features are listed in Table 2. The mean tumor size in patients with lymph node-negative gastric carcinoma (2.9 cm) was significantly smaller than that in lymph node-positive patients (5.0 cm; P < 0.001). Most gastric carcinomas were located in the lower third of the stomach in both lymph node-negative (946 cases; 62.1%) and lymph node-positive patients (783 cases; 59.1%). There were no significant differences between the groups with respect to the locations of the carcinomas. An invasion depth limited to T2 was found more frequently in patients with lymph node-negative (75.3%) than in lymph node-positive patients (21.3%; P < 0.001). Hepatic metastases were found in 0.3% of the lymph node-negative patients and in 4.2% of the lymph node-positive patients. Peritoneal dissemination was present in 1.0% of the lymph node-negative patients and in 11.0% of the lymph node-positive patients. There was a significant difference between the groups in the number of cases with hepatic metastases and peritoneal dissemination. Poorly differentiated adenocarcinoma was found more frequently in patients with lymph node-positive gastric carcinoma than in lymph node-negative patients (48.5% vs 35.1%, P<0.001). Of the lymph node-negative patients, 1470 (96.4%) were classified as either stage I or II at the time of initial diagnosis.
Table 2.
Histopathologic features of node-negative and -positive gastric carcinoma
| Variables | Node-negative (n = 1524) (%) | Node-positive (n = 1324) (%) | P value |
| Tumor size (mean, cm) | 2.9 ± 2.0 | 5.0 ± 2.7 | < 0.001 |
| Location | NS | ||
| Upper | 123 (8.1) | 141 (10.7) | |
| Middle | 443 (29.0) | 358 (27.0) | |
| Lower | 946 (62.1) | 783 (59.1) | |
| Whole | 12 (0.8) | 42 (3.2) | |
| Macroscopic appearance | NS | ||
| EGC | |||
| Elevated | 161 (20.0) | 27 (24.1) | |
| Depressed | 572 (71.2) | 69 (61.6) | |
| Flat | 71 (8.8) | 16 (14.3) | |
| Borrmann type | < 0.001 | ||
| 1 | 47 (6.5) | 54 (4.5) | |
| 2 | 183 (25.4) | 218 (18.0) | |
| 3 | 441 (61.3) | 812 (67.0) | |
| 4 | 49 (6.8) | 128 (10.5) | |
| Depth of invasion | < 0.001 | ||
| T1 | 804 (52.8) | 112 (8.5) | |
| T2 | 343 (22.5) | 170 (12.8) | |
| T3 | 338 (22.1) | 856 (64.7) | |
| T4 | 39 (2.6) | 186 (14.0) | |
| Hepatic metastasis | < 0.001 | ||
| H (-) | 1,519 (99.7) | 1,269 (95.8) | |
| H (+) | 5 (0.3) | 55 (4.2) | |
| Peritoneal dissemination | < 0.001 | ||
| P (-) | 1,509 (99.0) | 1,179 (89.0) | |
| P (+) | 15 (1.0) | 145 (11.0) | |
| Histologic type | <0.001 | ||
| Well-differentiated | 355 (23.3) | 165 (12.5) | |
| Moderately differentiated | 352 (23.1) | 339 (25.6) | |
| Poorly differentiated | 536 (35.1) | 642 (48.5) | |
| Signet ring cell | 189 (12.4) | 59 (4.5) | |
| Mucinous | 53 (3.5) | 95 (7.1) | |
| Others | 39 (2.6) | 24 (1.8) | |
| Stage | <0.001 | ||
| I | 1,145 (75.1) | 91 (6.9) | |
| II | 324 (21.3) | 124 (9.4) | |
| III | 30 (2.0) | 689 (52.0) | |
| IV | 25 (1.6) | 420 (31.7) |
NS, not significant
The overall survival rate of the lymph node-negative patients (77.4%) was higher than that of the lymph node-positive patients (31.1%; P < 0.001) (Figure 1). The 5-year survival rate of patients with early lymph node-negative gastric carcinoma was significantly higher than that of patients with early lymph node-positive gastric carcinoma (93.3% vs 84.3%, P = 0.0147). Patients with advanced lymph node-negative gastric carcinoma also had a significantly higher 5-year survival rate than that of patients with advanced lymph node-positive gastric carcinoma (66.9% vs 33.1%, P < 0.001). The clinicopathological variables tested by univariate analysis are shown in Table 3. The factors that influenced the 5-year survival rate were patient's age, tumor size, depth of invasion, tumor location, operative type, and tumor stage at initial diagnosis. Using the Cox proportional hazard regression model, tumor size, presence of serosal invasion, and curability emerged as independent, statistically significant, prognostic indicators(Table 4). The survival curves according to serosal invasion, tumor size, and curability for patients with lymph node-negative gastric carcinoma are presented in Figures 2, Figures 3, Figures 4.
Figure 1.
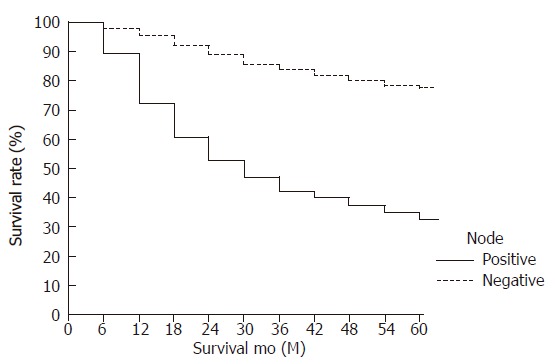
Survival curves of patients with lymph node-negative and node-positive gastric carcinoma (node-negative, 77.4% vs node-positive, 31.1%) (P < 0.001).
Table 3.
Prognostic significance by univariate analysis of variables for patients with lymph node-negative gastric carcinoma
| Variables | 5-year survival (%) | P value |
| Age (yr) | < 0.01 | |
| < 65 | 79.1 | |
| ≥ 65 | 70.7 | |
| Gender | NS | |
| Male | 74.4 | |
| Female | 81.3 | |
| Tumor size (cm) | < 0.001 | |
| < 2.0 | 88.5 | |
| 2 - 4.9 | 79.2 | |
| ≥ 5 | 53.6 | |
| Depth of invasion | < 0.001 | |
| T1, T2 | 88.4 | |
| T3, T4 | 55.4 | |
| Location | < 0.001 | |
| Upper third | 49.9 | |
| Middle third | 67.9 | |
| Lower third | 62.3 | |
| Histologic type | NS | |
| Differentiated | 78.4 | |
| Undifferentiated | 75.7 | |
| Operative type | < 0.001 NS | |
| Total | 61.5 | |
| Subtotal | 80.9 | |
| Extent of lymph node dissection | ||
| < D2 | 67.6 | |
| ≥ D2 | 77.3 | |
| Stage | < 0.001 | |
| I | 88.4 | |
| II | 61.5 | |
| III | 17.9 | |
| IV | 22.3 |
NS, not significant
Table 4.
Multivariate analysis of significant prognostic factors for survival in lymph node negative gastric carcinoma patients using Cox proportional hazard regression model
| Variables | Risk ratio | 95% CI | P value |
| Age(yr) (< 65 vs ≥65) | 1.354 | 0.95-1.92 | NS |
| Gender (male vs female) | 0.923 | 0.66-1.28 | NS |
| Location (upper vs distal) | 0.731 | 0.49-1.09 | NS |
| Tumor size (mm) (< 50 vs ≥50) | 1.513 | 1.06-2.21 | < 0.05 |
| Histologic type (differentiated vs. undifferentiated) | 0.749 | 0.55-1.02 | NS |
| Serosal invasion (negative vs positive) | 3.409 | 2.42-4.80 | < 0.001 |
| Extent of lymph node dissection (< D2 vs ≥ D2) | 1.188 | 0.56-2.50 | NS |
| Curability (curative vs non-curative) | 3.84 | 2.11-7.00 | < 0.001 |
| Esophageal invasion (negative vs positive) | 1.007 | 0.34-2.97 | NS |
CI, confidence interval; NS, not significant.
Figure 2.
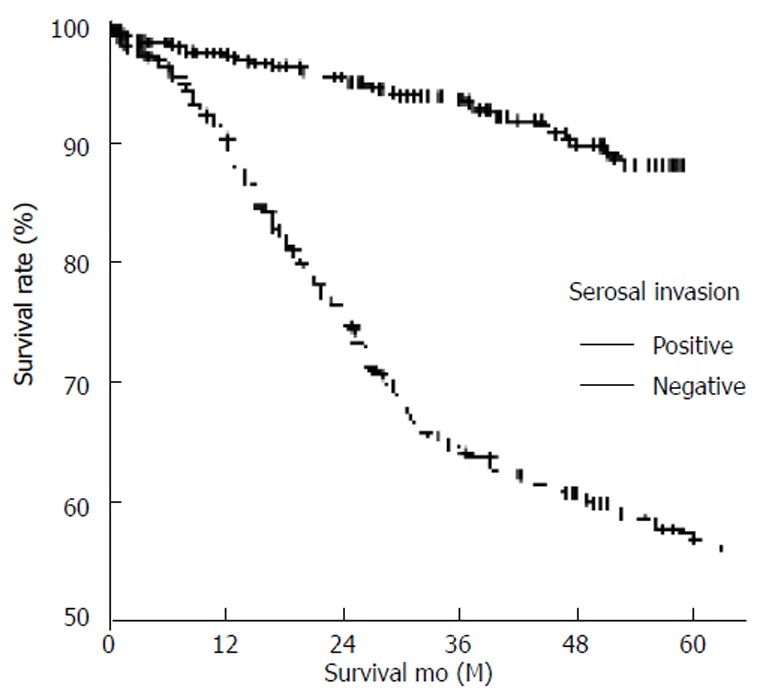
Survival curves of lymph node-negative gastric carcinoma according to serosal invasion (positive, 55.4% vs negative, 88.4%) (P < 0.001).
Figure 3.
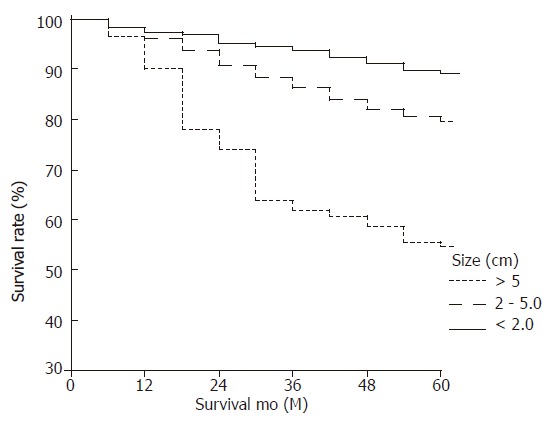
Survival curves of lymph node-negative gastric carcinoma according to tumor size (< 2cm, 88.5% vs 2-5 cm, 79.2% vs ≥5 cm, 53.6%) (P < 0.001).
Figure 4.
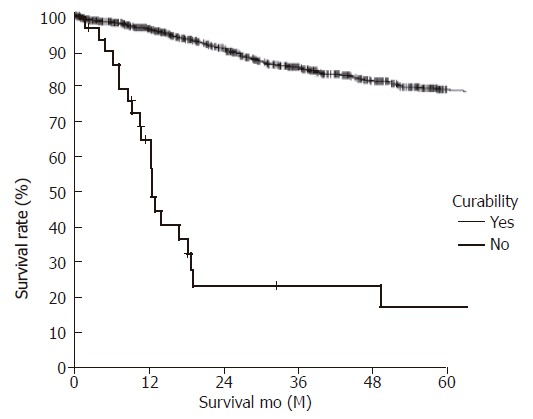
Survival curves of lymph node-negative gastric carcinoma according to curability (curative, 78.9% vs non-curative, 17.4%) (P < 0.001).
DISCUSSION
In Korea, gastric carcinoma is the leading cause of death as a result of a malignant neoplasm. Although most patients with lymph node-negative gastric carcinoma have a better prognosis than lymph node-positive ones, some lymph node-negative patients have recurrence and poor survival. The identification of factors associated with poor survival in patients with lymph node-negative gastric carcinoma is important. In this study, we compared node-negative and node-positive patients in order to identify the clinicopathological characteristics of lymph node-negative gastric carcinoma. We also evaluated outcome indicators in lymph node-negative patients.
In addition to lymph node invasion, the depth of wall invasion emerged as one of the most important prognostic indicators of lymph node-negative gastric carcinoma[12]. Adachi et al[13] reported that the depth of wall invasion provided useful prognostic information in patients with gastric carcinoma. In the present study, serosal invasion also emerged as a statistically significant independent prognostic indicator using the Cox proportional hazard regression model.
Whether age is a prognostic indicator for lymph node-negative gastric carcinoma is controversial[3,14]. Moriguch et al[15] reported that age at operation was a significant prognostic indicator in patients with early gastric carcinoma. Similarly, Adachi et al[13] reported that patient's age was the second most important prognostic indicator in patients with lymph node-negative gastric carcinoma. Furthermore, some investigators have reported that survival rates were lower in elderly patients with gastric carcinoma[16,17]. Contrary to the aforementioned reports, our study of patients with lymph node-negative gastric carcinoma revealed that age did not affect the survival rate.
Whether tumor size independently correlates with the prognosis for gastric carcinoma is also controversial. In the present study, there was a significant difference in tumor size between lymph node-negative and lymph node-positive patients (2.9cm vs 5.0cm), and node-negative patients with large tumors had poor survival. Some investigators have stressed that tumor size is an independent prognostic indicator for gastric carcinoma, whereas others believe that tumor size does not independently influence survival. Adachi et al[13] reported that tumor size served as a simple prognostic indicator for gastric carcinoma. By contrast, Yokota et al[18] reported that the presence of lymph node metastasis, depth of invasion, and tumor location were more important prognostic indicators than tumor size. Maruyama[2] reported findings that supported the conclusions of Yokota et al.
Surgical resection is the only potentially curative modality for localized gastric carcinoma. Adachi et al[13] stated that it was reasonable to conclude that the extent of lymph node dissection did not influence the survival of patients without lymph node metastasis. They stressed that the macroscopic diagnosis of lymph node involvement was unreliable and recommended D2 lymph node dissection for curative treatment of node-negative gastric carcinoma. Harrison et al[19] also recommended extended lymph node dissection for the improvement of survival of patients with lymph node-negative gastric carcinoma. We concurred with the aforementioned recommendations, and performed dissection above the D2 lymph node in most patients with lymph node-negative gastric carcinoma. In accordance with most reports, curative resection offered the only chance of long-term survival. In our series, this approach allowed us to achieve a high rate of curative resection (97.0%) and favorable outcomes in the lymph node-negative patients.
In this study, 5-year survival rates were different between patients with lymph node-negative and lymph node-positive gastric carcinoma. The 5-year survival rate was significantly lower for lymph node-positive patients than for lymph node-negative ones (35.8% vs 79.2%). Bruno et al[8] and Harrison et al[19] reported overall survival rates of 72% and 79%, respectively, for patients with lymph node-negative gastric carcinoma. Many factors influence the 5-year survival rate. Yokota et al[20] reported that tumor size, vascular microinvasion, and the cancer-stromal relationship were the most reliable predictors of 5-year survival for patients with lymph node-negative gastric carcinoma, while Adachi et al[21] reported that depth of wall invasion and patient's age were the most important prognostic indicators. Bruno et al[8] found that serosal invasion, residual disease, and poor differentiation were independent prognostic indicators of gastric carcinoma. Our univariate analysis revealed that age, tumor size, depth of invasion, tumor location, operative type, and tumor stage at initial diagnosis were prognostic indicators of lymph node-negative gastric carcinoma. In addition, in the present study, the Cox proportional hazard regression model revealed that tumor size, presence of serosal invasion, and curability were prognostic indicators of lymph node-negative gastric carcinoma.
In conclusion, we found that lymph node-negative gastric carcinoma is associated with a favorable outcome. We also found that tumor size, serosal invasion, and curative resection are the most reliable predictors of long-term survival for patients with lymph node-negative gastric carcinoma.
Footnotes
S- Editor Wang J L- Editor Zhang JZ E- Editor Liu WF
References
- 1.Bozzetti F, Bonfanti G, Morabito A, Bufalino R, Menotti V, Andreola S, Doci R, Gennari L. A multifactorial approach for the prognosis of patients with carcinoma of the stomach after curative resection. Surg Gynecol Obstet. 1986;162:229–234. [PubMed] [Google Scholar]
- 2.Maruyama K. The most important prognostic factors for gastric cancer patients. Scand J Gastroenterol. 1987;22:63–68. [Google Scholar]
- 3.Adachi Y, Ogawa Y, Sasaki Y, Yukaya H, Mori M, Sugimachi K. A clinicopathologic study of gastric carcinoma with reference to age of patients. J Clin Gastroenterol. 1994;18:287–290. doi: 10.1097/00004836-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba H, Maehara Y, Takeuchi H, Inutsuka S, Okuyama T, Adachi Y, Akazawa K, Sugimachi K. Effect of lymph node dissection on the prognosis in patients with node-negative early gastric cancer. Surgery. 1995;117:165–169. doi: 10.1016/s0039-6060(05)80080-7. [DOI] [PubMed] [Google Scholar]
- 6.Adachi Y, Mori M, Maehara Y, Kitano S, Sugimachi K. Prognostic factors of node-negative gastric carcinoma: univariate and multivariate analyses. J Am Coll Surg. 1997;184:373–377. [PubMed] [Google Scholar]
- 7.Maehara Y, Tomoda M, Tomisaki S, Ohmori M, Baba H, Akazawa K, Sugimachi K. Surgical treatment and outcome for node-negative gastric cancer. Surgery. 1997;121:633–639. doi: 10.1016/s0039-6060(97)90051-9. [DOI] [PubMed] [Google Scholar]
- 8.Bruno L, Nesi G, Montinaro F, Carassale G, Boddi V, Bechi P, Cortesini C. Clinicopathologic characteristics and outcome indicators in node-negative gastric cancer. J Surg Oncol. 2000;74:30–32. doi: 10.1002/1096-9098(200005)74:1<30::aid-jso7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Hyung WJ, Lee JH, Choi SH, Min JS, Noh SH. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562–567. doi: 10.1007/BF02573892. [DOI] [PubMed] [Google Scholar]
- 10.AJCC cancer staging manual. 6th ed. Springer - Verlag. 2002 [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Sugimachi K. Clinicopathologic studies of gastric carcinoma. Semin Surg Oncol. 1990;6:19–27. doi: 10.1002/ssu.2980060106. [DOI] [PubMed] [Google Scholar]
- 13.Adachi Y, Oshiro T, Mori M, Maehara Y, Sugimachi K. Tumor size as a simple prognostic indicator for gastric carcinoma. Ann Surg Oncol. 1997;4:137–140. doi: 10.1007/BF02303796. [DOI] [PubMed] [Google Scholar]
- 14.Mitsudomi T, Matsusaka T, Wakasugi K, Takenaka M, Kume K, Fujinaga Y, Teraoka H, Iwashita A. A clinicopathological study of gastric cancer with special reference to age of the patients: an analysis of 1,630 cases. World J Surg. 1989;13:225–30; discussion 230-1. doi: 10.1007/BF01658407. [DOI] [PubMed] [Google Scholar]
- 15.Moriguchi S, Odaka T, Hayashi Y, Nose Y, Maehara Y, Korenaga D, Sugimachi K. Death due to recurrence following curative resection of early gastric cancer depends on age of the patient. Br J Cancer. 1991;64:555–558. doi: 10.1038/bjc.1991.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houry S, Amenabar J, Rezvani A, Huguier M. Should patients over 80 years old be operated on for colorectal or gastric cancer. Hepatogastroenterology. 1994;41:521–525. [PubMed] [Google Scholar]
- 17.Takeda J, Tanaka T, Koufuji K, Kodama I, Tsuji Y, Kakegawa T. Gastric cancer surgery in patients aged at least 80 years old. Hepatogastroenterology. 1994;41:516–520. [PubMed] [Google Scholar]
- 18.Yokota T, Ishiyama S, Saito T, Teshima S, Yamada Y, Iwamoto K, Takahashi M, Murata K, Yamauchi H. Is tumor size a prognostic indicator for gastric carcinoma. Anticancer Res. 2002;22:3673–3677. [PubMed] [Google Scholar]
- 19.Harrison LE, Karpeh MS, Brennan MF. Extended lymphadenectomy is associated with a survival benefit for node-negative gastric cancer. J Gastrointest Surg. 1998;2:126–131. doi: 10.1016/s1091-255x(98)80002-4. [DOI] [PubMed] [Google Scholar]
- 20.Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Takahashi M, Kikuchi S, Yamauchi H. Significant prognostic factors in patients with node-negative gastric cancer. Int Surg. 1999;84:331–336. [PubMed] [Google Scholar]
- 21.Adachi Y, Suematsu T, Shiraishi N, Tanimura H, Morimoto A, Kitano S. Perigastric lymph node status as a prognostic indicator in patients with gastric cancer. Br J Surg. 1998;85:1281–1284. doi: 10.1046/j.1365-2168.1998.00833.x. [DOI] [PubMed] [Google Scholar]


