Abstract
AIM: To estimate the contribution of autoimmune thrombocytopenia to hepatitis C virus-related liver cirrhosis (type C cirrhosis), we evaluated the influence of splenectomy upon platelet-associated immunoglobulin G (PAIgG) levels and platelet numbers.
METHODS: PAIgG titers and immune markers were determined in 24 type C cirrhotic patients with an intact spleen, 17 type C cirrhotic patients submitted to splenectomy, and 21 non-C cirrhosis with an intact spleen.
RESULTS: Thrombocytopenia (PLT<15×104/μL) in type C cirrhosis was diagnosed in all patients with an intact spleen, 8 patients submitted to splenectomy, and in 19 non-C cirrhosis with intact spleen. Elevated titers of PAIgG at more than 25.0 ng/107cells were detected in all cirrhotic patients except for one splenectomized patient. PAIgG titers (ng/107cells) were significantly higher in the type C cirrhosis with an intact spleen (247.9 ± 197.0) compared with the splenectomized patients (125.6±87.8) or non-C cirrhosis (152.4 ± 127.4). PAIgG titers were negatively correlated with platelet counts in type C cirrhotic patients with an intact spleen. In comparison with the type C cirrhosis with an intact spleen, the splenectomized patients had a reduced CD4/CD8 ratio and serum neopterin levels. The spleen index (cm2) was negatively correlated with platelet counts in the non-C cirrhosis, but not in the type C cirrhosis.
CONCLUSION: Our data indicate that the autoimmune mechanism plays an important role in thrombocytosis complicated by HCV-positive cirrhosis. In addition, splenectomy may impair T cells function through, at least in part, a reduction of CD4/CD8 ratio, consequently suppressing PAIgG production.
Keywords: Platelet-associated immunoglobulin G, Autoimmune thrombocytopenia, Liver cirrhosis, Hepatitis C virus, Splenectomy, CD4/CD8 ratio
INTRODUCTION
Accompanying thrombocytopenia in patients with liver cirrhosis was initially attributed to an increase in platelet sequestration in the enlarged spleen secondary to portal hypertension[1]. Elevated platelet counts after procedures, such as transjugular intrahepatic portosystemic shunt[2,3], partial splenic embolization[4,5], splenectomy[6], and shunt operation[7,8], confirmed this theory. Nevertheless, controversial results on the relief of portal hypertension by surgical means have shown a reduction in spleen size with no concomitant persistent rise in platelet counts unless accompanied by liver transplantation[9]. Thus, the clinical significance of portal hypertension in the pathogenesis of thrombocytopenia in liver cirrhosis remains to be elucidated. Recently, not only impaired production of thrombopoietin, which regulates megakariocyte development[10,11], but also immune disturbance is thought to be responsible for thrombocytopenia associated with cirrhosis. In autoimmune-mediated thrombocytopenia, patients with chronic liver disease had a high incidence of platelet-associated immunoglobulin G (PAIgG)[12-16]. Pereira et al[12] detected autoantibodies that reacted specifically with platelet membrane glycoprotein in chronic liver disease, suggesting that an immune mechanism may participate in the induction or aggravation of thrombocytopenia.
Hepatitis C virus (HCV) has been identified as a major causative agent for parenterally transmitted non-A non-B hepatitis throughout the world[17]. Approximately 85% of HCV-infected individuals fail to clear the virus and develop persistent chronic hepatitis which leads to cirrhosis[18]. Chronic HCV infection is associated with an increased risk of liver cancer, and most cases of HCV-related hepatocellular carcinoma occur in the presence of cirrhosis. In addition, HCV infection is often associated with immune disturbance, including the development of autoantibodies and an increased frequency of immune-mediated disease[19]. We already hypothesized that chronic HCV infection may cause autoimmune thrombocytopenia because of prevalence of elevated levels of PAIgG and an inverse correlation between PAIgG titers and platelet counts[20]. On the other hand, Kosugi et al[21] reported that hepatitis C patients had a high incidence of PAIgM but relatively low levels of PAIgG, insisting that thrombocytopenia in hepatitis C patients was not due to anti-platelet autoantibodies. Substantial evidence exists that PAIgG is frequently identified in HCV-positive patients[20,22], but whether this immunoglobulin acts as a specific anti-platelet antibody remains unclear.
The role of spleen in PAIgG production and modification of cell-mediated immunity was previously reported in patients with idiopathic thrombocytic purpura (ITP), in whom the prevalence of PAIgG was found to contribute to the development of autoimmune thrombocytopenia[23]. Chronic ITP patients characteristically had varied PAIgG levels post-splenectomy[24], but showed immediate normalization in splenectomy-responders[25]. In ITP patients, CD4+ and HLA-DR-restricted T cells to platelet membrane GPIIb-IIIa were found to involve in the production of anti-platelet autoantibodies[26]. These results suggest that splenectomy may modify PAIgG production and immune mediators in patients with chronic HCV infection. In the present study, we, in order to elucidate the role of autoimmune thrombocytopenia in the development HCV-positive liver cirrhosis, retrospectively evaluated the influence of splenectomy on PAIgG levels and platelet counts, as well as cellular and humoral immune mediators.
MATERIALS AND METHODS
Patients
In the present study, 41 patients with type C cirrhosis, who were referred to our hospital, were enrolled. They were found to be seropositive for HCV antibodies detected a second or third generation enzyme-linked immunosorbent assay (Ortho Diagnostic System Co., Ltd., Tokyo, Japan) and/or recombinant immunoblot assay (Chiron RIBA-2 and/or RIBA-3). Seventeen patients had undergone splenectomy in association with surgery for esophageal varices (the splenectomies). The mean duration of follow-up after splenectomy was 11.5 ± 4.7 years (range, 2 to 19 years). Twenty-four consecutive cirrhotic patients had an intact spleen (the non-splenectomies). In addition, 20 cirrhotic patients including 10 hepatitis B, 4 autoimmune hepatitis, 3 alcoholic and 3 idiopathic cirrhosis served as the non-C cirrhosis. All the non-splenectomies and non-C cirrhosis had esophageal varices complication, and eight of the non-splenectomies and five of the non-C cirrhosis received treatment for the varices by endoscopic sclerotherapy and/or band ligation.
Morphological diagnosis of cirrhosis was performed in all the splenectomies, 15 non-splenectomies and 12 non-C cirrhosis. The others were diagnosed clinically, and they had typical laboratory findings of liver cirrhosis and characteristic liver imaging on computed tomography or ultrasonography. Patients' characteristics are shown in Table 1. Clinical features were similar among the three groups. Informed consent was obtained from each patient according to the Helsinki Declaration.
Table 1.
Characteristics of the patients (n, mean±SD)
| Type C cirrhosis | Type C cirrhosis submitted to splenectomy | Non-C cirrhosis | |
| Number | 17 | 24 | 20 |
| Age (yr) | 62.6 ± 10.6 | 63.0 ± 7.1 | 61.8 ± 9.5 |
| Gender | |||
| Female | 10 | 15 | 10 |
| Male | 7 | 9 | 10 |
| Child’s classification | |||
| A | 11 | 13 | 9 |
| B | 4 | 6 | 7 |
| C | 2 | 4 | 4 |
| Association of HCC | 4 | 7 | 5 |
Non-C cirrhosis consisted of 10 hepatitis B, 4 autoimmune hepatitis, 3 alcoholic and 3 idiopathic cirrhosis. HCC: hepatocellular carcinoma.
Assays
Quantitative HCV-RNA was analyzed from serum samples by a combined reverse transcription PCR assay (Amplicor-HCV monitor). Peripheral blood counts and liver function parameters were measured by an autoanalyzer.
Quantity of PAIgG was determined by a competitive micro enzyme-linked immunosorbent assay (ELISA) as described by Kawaguchi et al[27]. Briefly, platelets were separated from whole blood collected in EDTA, and washed with phosphate-buffered saline containing 11 g/L bovine serum albumin. The ELISA was performed in 96-well microplates coated with purified human IgG. A horseradish peroxidase-conjugated anti-human IgG antibody was incubated simultaneously with the samples, and visualized with o-phenylenediamine. PAIgG titers from 49 healthy individuals were within 25.0 ng/107 cells (mean, 16.4 ± 4.2 ng/107 cells)[20].
The proportion of CD4 and CD8 subsets of peripheral bloods was assayed by flow cytometry. The levels of neopterin and soluble interleukin-2 receptor (sIL2R) were measured using HPLC and Cell Free IL-2R EIA kit, respectively. Immunological markers and PAIgG were determined using fresh blood samples.
Spleen size in cirrhotic patients was evaluated as spleen index using ultrasonography as previously reported[28], in which spleen index correlated well with the volumes of resected spleens. Briefly, the spleen index was calculated as the product of the transverse diameter and its perpendicular diameter measured on the maximum cross-sectional image of the spleen, and expressed in cm2. Values obtained from healthy individuals were below 20 cm2, and the mean values of 28 healthy controls were 15 ± 7 cm2[28].
Statistical analysis
Values were expressed as mean±SD. Comparisons of the serum measurements among patients of the splenectomies, non-splenectomies and non-C cirrhosis were carried out using analysis of variance (ANOVA), and analyses of non-parametric data between two groups were carried out by Mann-Whitney U test. A correlation was calculated with the Spearman’s rank correlation coefficient.
RESULTS
Laboratory findings and spleen index
Thrombocytopenia (PLT<15×104/μL) was diagnosed in all the non-splenectomies (100%), eight of the splenectomies (47.1%), and 19 of the non-C cirrhosis (95.0%) (Table 2). Marked thrombocytopenia at less than 10×104/μL was obviously found in the non-splenectomies (79.2%) and the non-C cirrhosis (65.0%) as compared with the splenectomies (11.8%). Peripheral platelet and white blood cells were significantly higher in the splenectomies than that in the non-splenectomies and non-C cirrhosis (P < 0.01). Platelets and white blood cells were similar in the non-splenectomies and non-C cirrhosis. Red blood cell counts were not changed among the three groups. No difference was found among the three groups for liver function parameters except for total bilirubin levels and HCV-RNA titers.
Table 2.
Laboratory findings in the type C cirrhotic and non-C cirrhotic patients (n, mean±SD)
| Type C cirrhosis | Type C cirrhosis submitted to splenectomy | Non-C cirrhosis | |
| RBC (×104/μL) | 379 ± 61 | 374 ± 65 | 381 ± 63 |
| WBC (/μL) | 3 652 ± 1 080 | 5 855 ± 1 5951 | 3 832 ± 1 048 |
| Platelet (×104/μL) | 8.2 ± 2.9 | 16.1 ± 4.91 | 9.2 ± 3.4 |
| > 15.0 | 0 | 9 | 1 |
| 10.0-14.9 | 5 | 6 | 6 |
| < 9.9 | 19 | 2 | 13 |
| ALT (IU/L) | 58.2 ± 25.3 | 51.1 ± 35.2 | 40.6 ± 23.8 |
| Total bilirubin (mg/dL) | 1.4 ± 1.2 | 0.9 ± 0.31 | 2.3 ± 1.8 |
| Albumin (g/L) | 37 ± 4 | 37 ± 6 | 34 ± 8 |
| r-globulin (g/L) | 21 ± 0.5 | 22 ± 6 | 20 ± 9 |
| IgG (mg/ dL) | 2 454 ± 693 | no assay | 2 220 ± 877 |
| HCV RNA (kcopies/mL) | 523 ± 403 | 198 ± 2512 | |
| Splenic index (cm2) | |||
| -19 | 6cases | 5 cases | |
| 20-29 | 11cases | 9 cases | |
| 30 – 39 | 6 cases | 5 cases | |
| 40- | 1case | 3 cases |
ALT: alanine aminotransferase. 1: Significant difference compared with the type C cirrhosis with an intact spleen and non-C cirrhosis. 2: Significant difference compared with the type C cirrhosis with an intact spleen.
Spleen index ranged widely from 9.6 to 42.0 cm2. The mean spleen index was above the upper limit of normal individuals (20 cm2) in both type C cirrhosis (24.6 ± 8.0 cm2) and non-C cirrhosis (23.5 ± 8.0 cm2).
Immunological markers
An elevation of PAIgG at more than 25.0 ng/107cells was observed in all cirrhotic patients except for one splenectomized patient. The mean PAIgG titers were significantly higher in the non-splenectomies as compared with the splenectomies (297.9 ± 197.0 vs 125.6 ± 87.8 ng/107cells, P < 0.05) and non-C cirrhosis (297.9 ± 197.0 vs 152.4 ± 127.4 ng/107cells, P < 0.05). There was no significant difference in PAIgG titers between the splenectomies and the non-C cirrhosis (Figure 1). In comparison with the type C cirrhotic patients, the splenectomies had reduced percentage of CD4 and similar percentage of CD8, resulting in a lower ratio of CD4/CD8 than those of the non-splenectomies. In addition, neopterin levels were significantly lower in the splenectomies as compared with the non-splenectomies, while sIL2R levels were similar between the two groups (Table 3).
Figure 1.
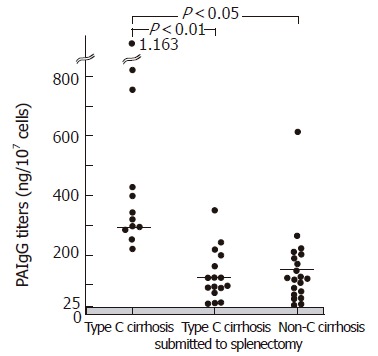
PAIgG titers in the type C cirrhosis with an intact spleen, type C cirrhosis submitted to splenomegaly, and non-C cirrhosis. The type C cirrhosis with an intact spleen had significant higher titers of PAIgG as compared with those of the splenectomized patients with type C cirrhosis and non-C cirrhosis.
Table 3.
Immunological markers in patients with type C cirrhosis (mean±SD)
| Type C cirrhosis | Type C cirrhosis submitted to splenectomy | |
| Neopterin (pmol/mL) | 8.3 ± 3.2 | 5.2 ± 2.5b |
| sIL2R (U/mL) | 850.7 ± 175.9 | 877.2 ± 458.6 |
| CD4 (%) | 43.1 ± 8.6 | 33.4 ± 7.6 |
| CD8 (%) | 27.6 ± 7.9 | 29.6 ± 9.6 |
| CD4/CD8 ratio | 1.8 ± 0.9 | 1.3 ± 0.4a |
sIL2R: Soluble interleukin-2 receptor. aP < 0.05 vs type C cirrhosis with an intact spleen; bP < 0.01 vs type C cirrhosis with an intact spleen.
Correlation between peripheral blood cells, immunological markers and spleen index
In the non-splenectomies, PAIgG titers were negatively correlated with platelet counts, and positively correlated with IgG and γ-globulin levels (Figure 2). In the non-C cirrhosis, PAIgG titers were neither correlated with platelet counts nor with IgG and γ-globulin levels. A significant negative correlation between the spleen index and platelet counts was found in the non-C cirrhosis, whereas not in the type C cirrhosis (Figure 3). In addition, the spleen index was negatively correlated with white blood cell counts in both type C and non-C cirrhosis. In the splenectomies, PAIgG titers were not correlated with platelet counts, γ-globulin levels, or the duration of follow-up after splenectomy.
Figure 2.
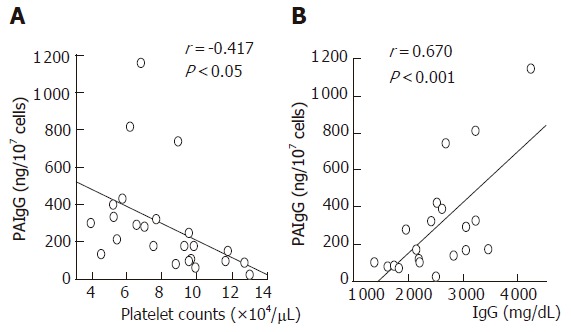
Relationships among PAIgG titters, platelet counts, and IgG levels in the type C cirrhosis with an intact spleen. PAIgG titers are negatively correlated with platelet counts, and positively correlated with IgG levels.
Figure 3.
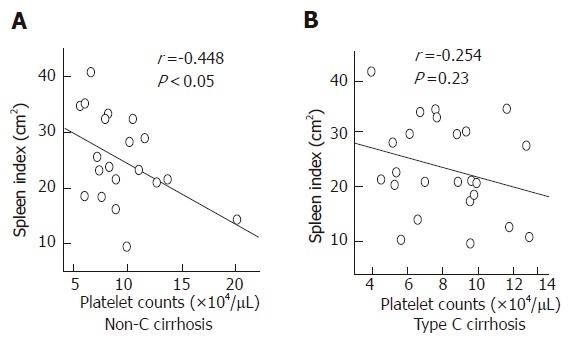
Relationship between the spleen index and platelet counts. A significant negative correlation is shown in the patients with non-C cirrhosis, but not in the type C cirrhosis with an intact spleen.
DISCUSSION
Our data demonstrated that the HCV-positive cirrhosis (type C cirrhosis) with an intact spleen had a significant rise of PAIgG as compared with the non-C cirrhosis, including hepatitis B infection, autoimmune hepatitis, alcohol abuse, and idiopathic cirrhosis. PAIgG titers were negatively correlated with platelet counts in the type C cirrhosis with an intact spleen. The type C cirrhotic patients submitted to splenectomy showed a significant elevation of platelet counts and reduction in PAIgG titers compared with those of the patients with an intact spleen. Similar PAIgG and platelet levels following splenectomy are commonly found in ITP patients, in whom the thrombocytopenia is responsible for the autoimmune mechanism mediated by a specific IgG bound to platelet membrane proteins[24,25]. There exist controversies regarding the clinical significance of PAIgG in pathogenesis of thrombocytopenia in the patients with liver disease[7,14,29,30]. Two studies on partial splenic artery embolization in patients with hypersplenism clearly confirmed an immunological mechanism mediated by PAIgG-induced thrombocytopenia accompanying liver cirrhosis[7,14]. These studies reported a significant rise in platelet numbers and a significant fall in PAIgG levels after partial splenic artery embolization. In the present study, the changes in PAIgG levels and platelet numbers among the type C cirrhotic patients with or without an intact spleen are practically similar to those previous results. These data support a key role of spleen in PAIgG production and that an autoimmune mechanism plays an important role in the development of thrombocytopenia associated with HCV-positive cirrhosis. On the contrary, a non-specific adsorption of elevated γ-globulin by platelets was suspiciously reported on chronic liver disease[27]. Our data found a negative correlation between PAIgG titers and platelet counts in the type C cirrhosis with an intact spleen, but not in the non-C cirrhosis. Presumably, the elevated PAIgG in the type C cirrhosis may act as anti-platelet autoantibodies. However, we found a positive correlation between PAIgG titers and IgG levels in the type C cirrhosis with an intact spleen. This result is in agreement with that of de Noronha et al[31] in the patients with various liver diseases. The methods used for quantitating PAIgG in their study as well as in our study appear to measure total PAIgG, which is not equivalent to platelet-specific autoantibodies. They suggested that increased platelet IgG was due to enhanced retention of plasma IgG in the platelets. In our study, despite similar γ-globulin levels, a relationship between PAIgG and γ-globulin was found neither in the splenectomized patients with type C cirrhosis nor in the non-C cirrhotic patients. Consequently, the theory of ‘enhanced retention of IgG in the platelets’ proposed by de Noronha et al [31] is deniable in the present study.
Semple et al[32] indicated that CD4+ T-helper cells from patients with ITP are stimulated by normal platelet antigen(s) to secrete IL-2 and may modulate the enhanced anti-platelet autoantibody response. The underlying mechanisms for the production of anti-platelet autoantibodies require CD4+ and HLA-DR-restricted T cells to GPIIb-IIIa in ITP patients[26]. GPIb+ cells isolated from spleens of patients with chronic ITP strongly expressed HLA-DR, and splenic T cells had a high level of in vitro platelet-stimulated IL-2 secretion as compared with the controls[33]. Thus, the spleen is such an important site for antibody production in patients with chronic ITP that the levels of PAIgG rapidly normalize in response to a splenectomy[24,34]. Our data showed that compared with the type C cirrhosis with an intact spleen, the type C cirrhosis submitted to splenectomy had lower CD4/CD8 ratio because of a reduced percentage of CD4+ T cells. Neopterin is secreted by macrophages when stimulated for helper functions in the proliferation and activation of T cells. This immune marker was significantly decreased in the type C cirrhosis submitted to splenectomy compared to the type C cirrhotic patients with an intact spleen, supporting impaired T cell function following splenectomy. Taken together, previous and present results indicate that splenectomy may impair T cell function in HCV-positive liver cirrhosis through, at least in part, a reduction in CD4/CD8 ratio, which thereby suppresses PAIgG production. The data from the present study provide some indications that the spleen induces helper T-cell proliferation, but a further research is necessary to clarify the role of spleen in PAIgG production and T helper lymphocyte cytokine production.
Hypersplenism due to portal hypertension is believed as a major cause of thrombocytopenia complicated with liver cirrhosis; however, studies on the relief of portal hypertension have yielded the controversial results that observable reduction in spleen size is not associated with a concomitant persistent rise in platelet counts, unless accompanied by liver transplantation[8,9]. Thus, the role played by immune disturbance as well as thrombopoietin is an attractive possibility in the pathogenesis of thrombocytopenia concomitant with liver cirrhosis. Noguchi et al[5] observed the improved numbers and survival time of platelets and reduction in the raised PAIgG levels by partial splenic artery embolization in cirrhotic patients with hypersplenism. Owing to a negative correlation between platelet numbers and splenic volume before and after the procedure, they concluded that partial splenic artery embolization may not only reduce the increased platelet pool in the spleen, but also improve the autoimmune thrombocytopenia induced by cirrhosis. In the present study, the elevated platelets and reduced PAIgG levels in the splenectomized patients support their results. In addition, the present study showed that spleen size was correlated with platelets counts in the non-C cirrhotic patients. The data suggested that hypersplenism due to portal hypertension is assumed to be a major cause of thrombocytopenia observed in the non-C cirrhotic patients. If no significant relationship is found between the spleen index and platelet counts in HCV-positive cirrhotic patients, autoimmune disturbance may be the most likely principal mechanism for thrombocytopenia rather than sequestration and/or destruction of platelets secondary to hypersplenism.
In the present retrospective study, we confirmed that the autoimmune disturbance induces thrombocytopenia associated with liver cirrhosis which is related to HCV-infection. Prospective pre- and post-splenectomy studies should elucidate the role of spleen in PAIgG production, platelet sequestration and destruction, as well as cellular and humoral immunity.
ACKNOWLEDGMENTS
Authors thank Dr. Ishii, Dr. Kakizaki, Dr. Kabeya, Dr. Katakai and Dr. Takezawa for collecting blood samples.
Footnotes
S- Editor Wang J L- Editor Kumar M E- Editor Bi L
References
- 1.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45:645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanyal AJ. The use and misuse of transjugular intrahepatic portasystemic shunts. Curr Gastroenterol Rep. 2000;2:61–71. doi: 10.1007/s11894-000-0053-5. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez OA, Lopera GA, Patel V, Encarnacion CE, Palmaz JC, Lee M. Improvement of thrombocytopenia due to hypersplenism after transjugular intrahepatic portosystemic shunt placement in cirrhotic patients. Am J Gastroenterol. 1996;91:134–137. [PubMed] [Google Scholar]
- 4.Sangro B, Bilbao I, Herrero I, Corella C, Longo J, Beloqui O, Ruiz J, Zozaya JM, Quiroga J, Prieto J. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology. 1993;18:309–314. [PubMed] [Google Scholar]
- 5.Noguchi H, Hirai K, Aoki Y, Sakata K, Tanikawa K. Changes in platelet kinetics after a partial splenic arterial embolization in cirrhotic patients with hypersplenism. Hepatology. 1995;22:1682–1688. [PubMed] [Google Scholar]
- 6.Shah R, Mahour GH, Ford EG, Stanley P. Partial splenic embolization. An effective alternative to splenectomy for hypersplenism. Am Surg. 1990;56:774–777. [PubMed] [Google Scholar]
- 7.Koyanagi N, Iso Y, Higashi H, Kitano S, Sugimachi K. Increased platelet count as a screening test for distal splenorenal shunt patency. Am J Surg. 1988;156:29–33. doi: 10.1016/s0002-9610(88)80164-8. [DOI] [PubMed] [Google Scholar]
- 8.Mutchnick MG, Lerner E, Conn HO. Effect of portacaval anastomosis on hypersplenism. Dig Dis Sci. 1980;25:929–938. doi: 10.1007/BF01308044. [DOI] [PubMed] [Google Scholar]
- 9.Yanaga K, Tzakis AG, Shimada M, Campbell WE, Marsh JW, Stieber AC, Makowka L, Todo S, Gordon RD, Iwatsuki S. Reversal of hypersplenism following orthotopic liver transplantation. Ann Surg. 1989;210:180–183. doi: 10.1097/00000658-198908000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rios R, Sangro B, Herrero I, Quiroga J, Prieto J. The role of thrombopoietin in the thrombocytopenia of patients with liver cirrhosis. Am J Gastroenterol. 2005;100:1311–1316. doi: 10.1111/j.1572-0241.2005.41543.x. [DOI] [PubMed] [Google Scholar]
- 11.Aref S, Mabed M, Selim T, Goda T, Khafagy N. Thrombopoietin (TPO) levels in hepatic patients with thrombocytopenia. Hematology. 2004;9:351–356. doi: 10.1080/10245330400010620. [DOI] [PubMed] [Google Scholar]
- 12.Pereira J, Accatino L, Alfaro J, Brahm J, Hidalgo P, Mezzano D. Platelet autoantibodies in patients with chronic liver disease. Am J Hematol. 1995;50:173–178. doi: 10.1002/ajh.2830500305. [DOI] [PubMed] [Google Scholar]
- 13.Skootsky SA, Rosove MH, Langley MB. Immune thrombocytopenia and response to splenectomy in chronic liver disease. Arch Intern Med. 1986;146:555–557. [PubMed] [Google Scholar]
- 14.Kajiwara E, Akagi K, Azuma K, Onoyama K, Fujishima M. Evidence for an immunological pathogenesis of thrombocytopenia in chronic liver disease. Am J Gastroenterol. 1995;90:962–966. [PubMed] [Google Scholar]
- 15.Samuel H, Nardi M, Karpatkin M, Hart D, Belmont M, Karpatkin S. Differentiation of autoimmune thrombocytopenia from thrombocytopenia associated with immune complex disease: systemic lupus erythematosus, hepatitis-cirrhosis, and HIV-1 infection by platelet and serum immunological measurements. Br J Haematol. 1999;105:1086–1091. doi: 10.1046/j.1365-2141.1999.01469.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanjo A, Satoi J, Ohnishi A, Maruno J, Fukata M, Suzuki N. Role of elevated platelet-associated immunoglobulin G and hypersplenism in thrombocytopenia of chronic liver diseases. J Gastroenterol Hepatol. 2003;18:638–644. doi: 10.1046/j.1440-1746.2003.03026.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, Miyamura T, Dienstag JL, Alter MJ, Stevens CE. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 18.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 19.Gregorio GV, Choudhuri K, Ma Y, Pensati P, Iorio R, Grant P, Garson J, Bogdanos DP, Vegnente A, Mieli-Vergani G, et al. Mimicry between the hepatitis C virus polyprotein and antigenic targets of nuclear and smooth muscle antibodies in chronic hepatitis C virus infection. Clin Exp Immunol. 2003;133:404–413. doi: 10.1046/j.1365-2249.2003.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol. 1996;24:135–140. doi: 10.1016/s0168-8278(96)80021-3. [DOI] [PubMed] [Google Scholar]
- 21.Kosugi S, Imai Y, Kurata Y, Tomiyama Y, Shiraga M, Honda S, Nishikawa M, Yonezawa T, Kanakura Y, Matsuzawa Y. Platelet-associated IgM elevated in patients with chronic hepatitis C contains no anti-platelet autoantibodies. Liver. 1997;17:230–237. doi: 10.1111/j.1600-0676.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 22.Hernández F, Blanquer A, Linares M, López A, Tarín F, Cerveró A. Autoimmune thrombocytopenia associated with hepatitis C virus infection. Acta Haematol. 1998;99:217–220. doi: 10.1159/000040842. [DOI] [PubMed] [Google Scholar]
- 23.McMillan R, Tani P, Mason D. The demonstration of antibody binding to platelet-associated antigens in patients with immune thrombocytopenic purpura. Blood. 1980;56:993–995. [PubMed] [Google Scholar]
- 24.Luiken GA, McMillan R, Lightsey AL, Gordon P, Zevely S, Schulman I, Gribble TJ, Longmire RL. Platelet-associated IgG in immune thrombocytopenic purpura. Blood. 1977;50:317–325. [PubMed] [Google Scholar]
- 25.Ware R, Kinney TR, Friedman HS, Falletta JM, Rosse WF. Prognostic implications for direct platelet-associated IgG in childhood idiopathic thrombocytopenic purpura. Am J Pediatr Hematol Oncol. 1986;8:32–37. doi: 10.1097/00043426-198608010-00007. [DOI] [PubMed] [Google Scholar]
- 26.Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102:1393–1402. doi: 10.1172/JCI4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi R, Haruna S, Hikiji K, Higashi Y, Tsukada Y. Elevation of platelet-associated IgG in aplastic anemia. J Clin Lab Anal. 1992;6:130–135. doi: 10.1002/jcla.1860060306. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi H, Higuchi N, Shimamura R, Hirata Y, Kudo J, Niho Y. Sonographic assessment and grading of spleen size. J Clin Ultrasound. 1991;19:21–25. doi: 10.1002/jcu.1870190106. [DOI] [PubMed] [Google Scholar]
- 29.McGrath KM, Stuart JJ, Richards F 2nd. Correlation between serum IgG, platelet membrane IgG, and platelet function in hypergammaglobulinaemic states. Br J Haematol. 1979;42:585–591. doi: 10.1111/j.1365-2141.1979.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 30.Mueller-Eckhardt C, Kayser W, Mersch-Baumert K, Mueller-Eckhardt G, Breidenbach M, Kugel HG, Graubner M. The clinical significance of platelet-associated IgG: a study on 298 patients with various disorders. Br J Haematol. 1980;46:123–131. doi: 10.1111/j.1365-2141.1980.tb05942.x. [DOI] [PubMed] [Google Scholar]
- 31.de Noronha R, Taylor BA, Wild G, Triger DR, Greaves M. Inter-relationships between platelet count, platelet IgG, serum IgG, immune complexes and severity of liver disease. Clin Lab Haematol. 1991;13:127–135. doi: 10.1111/j.1365-2257.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 32.Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78:2619–2625. [PubMed] [Google Scholar]
- 33.Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, Freedman J. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- 34.Cines DB, Schreiber AD. Immune thrombocytopenia. Use of a Coombs antiglobulin test to detect IgG and C3 on platelets. N Engl J Med. 1979;300:106–111. doi: 10.1056/NEJM197901183000302. [DOI] [PubMed] [Google Scholar]


