Abstract
AIM: To evaluate the mechanical and biochemical parameters of colonic anastomotic healing in hypercholesterolemic rats.
METHODS: Sixty rats were divided into two groups of 30 each according to their dietary regimens. The test group was fed with a high cholesterol-containing diet for two months while the control group had standard diet. These two groups were further divided into three subgroups consisting of ten rats each. After hypercholesterolemia was established, left colon resection and anastomosis were performed in both groups and samples from liver and abdominal aorta were taken to evaluate the systemic effects of hypercholesterolemia. Anastomotic wound healing, blow-out pressures and tissue hydroxyproline levels were evaluated.
RESULTS: The test group had a significant weight gain in two months. Microscopic examination of the abdominal aorta revealed no atherosclerotic change in none of the groups, but liver tissue specimens showed significant steatosis in the test group. Tissue hydroxyproline levels and anastomotic blow-out pressures were significantly lower in the test group than in the controls.
CONCLUSION: Hypercholesterolemia not only increases hydroxyproline levels and blow-out pressures but also worsens anastomotic wound healing.
Keywords: Hypercholesterolemia, Colonic anastomosis, Anastomotic wound healing
INTRODUCTION
Hypercholesterolemia is the major etiologic factor for atherosclerosis in Western countries[1]. However, the effects of hypercholesterolemia on anastomotic wound healing have not been studied.
Leakage in colonic anastomosis has a higher incidence of morbidity and mortality rate compared with that in small intestinal anastomosis. The overall leakage incidence of intestinal anastomosis is 2-35%[2]. Most of them are minimal and can be limited by the host defense mechanisms. Systemic and local factors play a role in anastomotic wound healing. Anemia, hypovolemia, low arterial PO2, neutropenia, low O2 saturation, malnutrition, vitamin deficiencies, zinc deficiency, jaundice, uremia and high dose corticosteroids are some of the systemic factors, while local factors include infection, intestinal contents, prophylactic antibiotics, technique of suturing and suture material, radiation and mesenteric vascular occlusion[2-4].
In experimental studies, microscopic healing in small intestine is better than that in the colon[5,6]. Intestinal anastomotic wound healing can be evaluated mechanically, biochemically and histopathologically. Mechanical evaluation depends on the blow-out pressure of the anastomotic line. This pressure can change with the inflation rate of the intestine or the in situ procedure[2,7-9]. Continuous suturing technique has no effect on this pressure[10].
Biochemically, collagen production rate and amount in the anastomosis line are the factors to be measured. Since the majority of the collagens are hydroxyproline, all measurements are made by detecting the hydroxyproline content[7,11,12]. Histopathologically, the only evidence is the infiltration of several kinds of inflammatory cells[2,7].
In this study, the mechanical and biochemical parameters of colonic anastomotic wound healing were evaluated in rats fed with a high cholesterol containing diet (2% cholesterol + 1% colic acid: Sigma Co., Manchester, UK) for two months[13]. Abdominal aortic and liver tissue samples were also obtained to evaluate the systemic effects of hypercholesterolemia.
MATERIALS AND METHODS
Experimental animals and grouping
Sixty rats of both sexes weighing 240-300 g used in this study were divided into two groups according to their dietary regimens. The test group consisted of 30 rats fed with a high cholesterol diet for two months while the control group had standard diet for the same period. The test group had a significant weight gain at the end of this period (Table 1).
Table 1.
Pre- and post-feeding body weights of hypercholesterolemic rats
| Pre-feeding weight (g) | Post-feeding weight (g) | |
| Rat 1 | 250 | 325 |
| Rat 2 | 255 | 330 |
| Rat 3 | 245 | 350 |
| Rat 4 | 240 | 310 |
| Rat 5 | 260 | 325 |
| Rat 6 | 250 | 335 |
| Rat 7 | 275 | 360 |
| Rat 8 | 280 | 355 |
| Rat 9 | 270 | 350 |
| Rat 10 | 240 | 340 |
| Mean | 256.5 | 338 |
These two groups were further divided into three subgroups, ten rats each group. Ten milliliter blood was taken from each rat for measurement of serum total cholesterol and triglyceride levels. In the test group, serum total cholesterol and triglyceride levels were significantly higher than in the control group (Figure 1). After hypercholesterolemia was established, left colon resection and anastomosis were performed in both groups and tissue samples were taken from liver and abdominal aorta.
Figure 1.
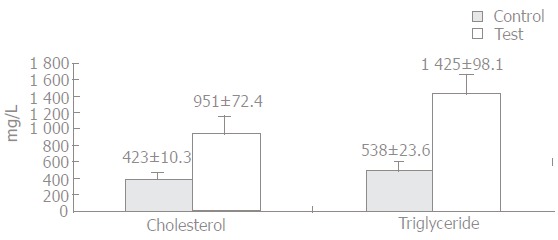
Mean cholesterol and triglyceride level in test and control groups.
Surgical technique
After an overnight fasting, intramuscular ketamin HCl (Ketalar, Parke Davies) was administered (50 mg/kg). One percent povidon-iodine was used for local cleansing. A midline laparotomy was performed and a segment of left colon (1 cm) was resected. Colo-colonic anastomosis was made using 7/0 polyproene (Prolene, Ethicon Inc., UK) with 8-10 stitches for a layer. There was no postoperative mortality.
In both groups, 10 rats underwent relaparotomy on the third day and 10 rats on the seventh day. Colon lumen was obliterated 10 cm distal to the anastomosis using 3/0 silk ligature. Colon was transected 10 cm proximal to the anastomotic line and an infusion set was inserted into the lumen. This set was connected to an infusion pump (Life Care 5000 Infusion System, Abbot, Illinois, USA) and isotonic sodium chloride was given at a rate of 2 mL/min into the lumen (Figure 2). The pressure at the moment of the first leakage observed from the suture line was recorded as the blow-out pressure. All measurements were done in situ. After the measurements, tissue samples were taken from the liver and abdominal aorta for histopathological examination. The experiment was terminated by creating a pneumothorax.
Figure 2.
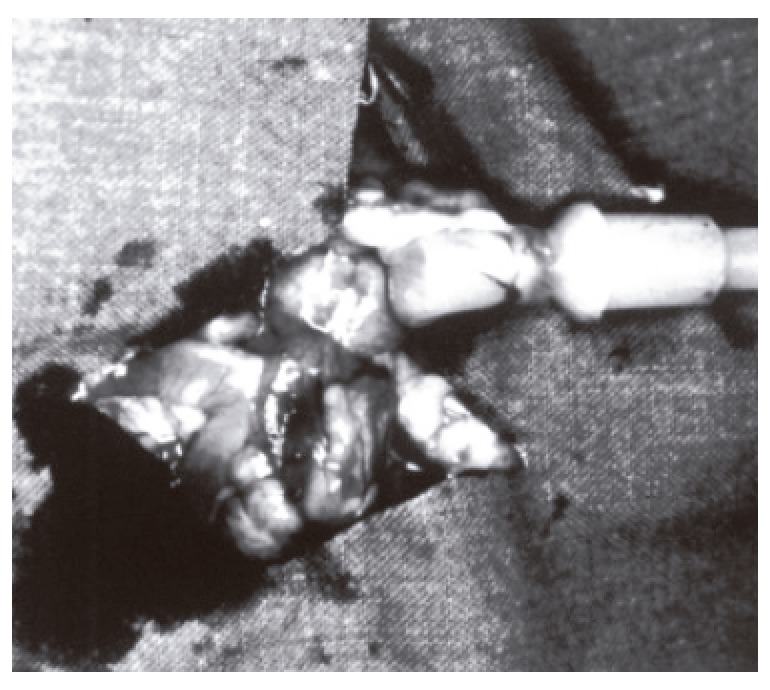
Insertion of an infusion pump into the colon.
In all groups, one centimeter segment of colon (including 0.5 cm proximal and 0.5 cm distal to the anastomotic line) was resected (Figure 3). The suture material was removed and the samples were kept in liquid nitrogen until the assay day.
Figure 3.
Anastomosis line.
Biochemical measurements were done as described previously[14]. The volume of hydroxyproline was defined as micromoles of hydroxyproline per gram of wet weight.
Statistical analysis
Mann Whitney U test was used for statistical analysis compare the blow-out pressures and hydroxyproline contents between groups. P < 0.05 was considered statistically significant.
RESULTS
The rats in the test group gained significant weight (Table 1). The weight difference between the pre and post-feeding periods was statistically significant (P < 0.05).
After feeding with a high cholesterol containing diet for two months, a statistically significant difference serum cholesterol and triglyceride levels between the test and control groups was observed (P < 0.05, Figure 1). In the test group, serum total cholesterol level was 951 ± 72.4 mg/L and triglyceride level was 1425 ± 98.1 mg/L, while they were 423 ± 10.3 mg/L and 538 ± 23.6 mg/L in the control group, respectively.
On the third postoperative day, the mean blow-out pressure was 87.3 ± 11.4 mmHg in the test group and 150.5 ± 9.7 mmHg in the control group (Figure 4A). This difference was statistically significant (P < 0.05). The mean hydroxyproline level was 0.667 ± 0.09 in test group and 0.863 ± 0.05 in control group (P < 0.05) (Figure 4B).
Figure 4.
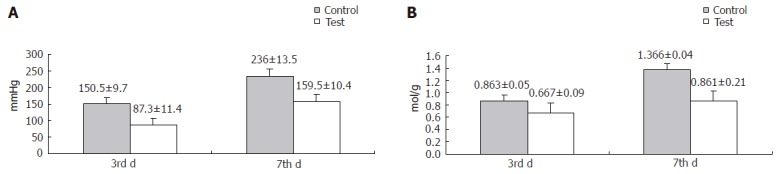
Mean blow-out pressure (A) and hydroxyproline level (B) in test and control groups.
On the seventh postoperative day, the blow-out pressure was 159.5 ± 10.4 mmHg in the test group and 236 ± 13.5 mmHg in the control group, while the hydroxyproline level was 0.861 ± 0.21 in the test group and 1.366 ± 0.06 in the control group, respectively. The difference in the parameters between the two groups was statistically significant (P < 0.05) (Figures 4A and 4B).
Liver tissue samples of the test group had a significant macroscopic steatosis compared to the control group (Figure 5A). In microscopic examination with hematoxilen-eosin staining, significant steatosis was also found in the test group (Figure 5B). No difference in microscopic results of the aortic specimens was found between the two groups.
Figure 5.
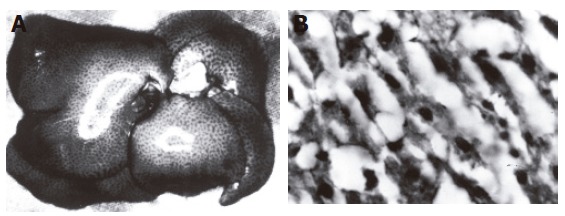
Macroscopic (A) and microscopic (B) steatosis in liver.
DISCUSSION
In this study, left colon anastomosis was performed in hypercholesterolemic rats and the mechanical and biochemical parameters of anastomotic wound healing were investigated. Anastomotic wound healing in the test group was worse than that in the control group in terms of hydroxypline levels and blow-out pressures. Significant steatosis was found in the liver specimens but no atherosclerotic changes in the aortic specimens. This finding is in accordance with several studies[15-17].
Factors influencing colonic healing have been widely studied[2,4]. Since blood flow is the major component of wound healing, hypercholesterolemia-induced atherosclerosis has a detrimental effect on intestinal anastomotic wound healing. However, the effects of hypercholesterolemia start long before the onset of atherosclerosis[16-20]. There are also reports on how hypercholesterolemia worsens vascular functions using different pathways of microendocrine system inside the endothelium[21-23]. Ross et al[24] have shown that monocytes accumulate on endothelium and damage it. Besides the macrovascular system, hypercholesterolemia also plays an important role in microvascular endothelial injury.
The cause of hypercholesterolemia-induced endothelial damage is predominantly determined by loss of endothelium-mediated relaxation. The mechanisms are as follows: decrease in the relaxing effect of NO/EDRF, ADP, thrombin, Ca++-ionophore A 23187 on endothelium[2,16,21,25,26]; increase in platelet aggregation and turnover as well as the sensitivity of platelets to aggregating substances due to the modification of effects of PGI2 and TXA2[27-29]; increase in the release of endothelium-derived vasoconstrictor substances such as serotonin and TXA2 through the cyclooxygenase pathway[22,27].
The above effects have been shown in vivo and in vitro in experimental models of rabbit aorta, monkey iliac artery, pig coronary artery and human coronary arteries[28]. Our study did not investigate these effects, but the effect of hypercholesterolemia on hydroxyproline level and blow-out pressure was emphasized.
Why hypercholesterolemia worsens anastomotic wound healing is still unknown. Luminal narrowing is the net result of atherosclerosis in both macro and microvascular systems. Consequently, ischemia and related injuries occur. Hypercholesterolemia is the major risk factor for atherosclerosis. In early phase of hypercholesterolemia, no morphological change takes place in arterial wall but vasospasm occurs due to neurohumoral mechanisms[30]. Atherosclerosis is an irreversible process but hypercholesterolemia is reversible and these effects are preventable.
In our study, anastomotic wound healing in the test group was worse than that in the control group. Since hypercholesterolemia predominantly affects the endothelium, this can partly be explained as a result of endothelial damage-induced vasospasm. But we did not measure the anastomotic blood flow or assess the endothelial injury. Although a relationship between hypercholesterolemia and wound healing was demonstrated, our results could not fully explain the mechanisms. Further studies are needed to reveal the relationship between hypercholesterolemia and anastomotic wound healing.
Footnotes
S- Editor Guo SY L- Editor Wang XL E- Editor Bai SH
References
- 1.Verbeuren TJ, Jordaens FH, Zonnekeyn LL, Van Hove CE, Coene MC, Herman AG. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986;58:552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- 2.Brasken P. Healing of experimental colon anastomosis. Eur J Surg. 1991;566(Suppl):1–51. [PubMed] [Google Scholar]
- 3.Foster ME, Laycock JR, Silver IA, Leaper DJ. Hypovolaemia and healing in colonic anastomoses. Br J Surg. 1985;72:831–834. doi: 10.1002/bjs.1800721019. [DOI] [PubMed] [Google Scholar]
- 4.Khoury GA, Waxman BP. Large bowel anastomoses. I. The healing process and sutured anastomoses. A review. Br J Surg. 1983;70:61–63. doi: 10.1002/bjs.1800700202. [DOI] [PubMed] [Google Scholar]
- 5.Hesp FL, Hendriks T, Lubbers EJ, de Boer HH. Wound healing in the intestinal wall. Effects of infection on experimental ileal and colonic anastomoses. Dis Colon Rectum. 1984;27:462–467. doi: 10.1007/BF02555541. [DOI] [PubMed] [Google Scholar]
- 6.Hesp WL, Hendriks T, Schillings PH, Lubbers EJ, de Boer HH. Histological features of wound repair: a comparison between experimental ileal and colonic anastomoses. Br J Exp Pathol. 1985;66:511–518. [PMC free article] [PubMed] [Google Scholar]
- 7.Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses. Parameters for repair. Dis Colon Rectum. 1990;33:891–901. doi: 10.1007/BF02051930. [DOI] [PubMed] [Google Scholar]
- 8.Jiborn H, Ahonen J, Zederfeldt B. Healing of experimental colonic anastomoses. The effect of suture technic on collagen concentration in the colonic wall. Am J Surg. 1978;135:333–340. doi: 10.1016/0002-9610(78)90062-4. [DOI] [PubMed] [Google Scholar]
- 9.Jiborn H, Ahonen J, Zederfeldt B. Healing of experimental colonic anastomoses. I. Bursting strength of the colon after left colon resection and anastomosis. Am J Surg. 1978;136:587–594. doi: 10.1016/0002-9610(78)90315-x. [DOI] [PubMed] [Google Scholar]
- 10.Jiborn H, Ahonen J, Zederfeldt B. Healing of experimental colonic anastomoses. II. Breaking strength of the colon after left colon resection and anastomosis. Am J Surg. 1978;136:595–599. doi: 10.1016/0002-9610(78)90316-1. [DOI] [PubMed] [Google Scholar]
- 11.Irvin TT. Collagen metabolism in infected colonic anastomoses. Surg Gynecol Obstet. 1976;143:220–224. [PubMed] [Google Scholar]
- 12.Jiborn H, Ahonen J, Zederfeldt B. Healing of experimental colonic anastomoses. III. Collagen metabolism in the colon after left colon resection. Am J Surg. 1980;139:398–405. doi: 10.1016/0002-9610(80)90302-5. [DOI] [PubMed] [Google Scholar]
- 13.Musanti R, Chiari A, Ghiselli G. Peritoneal macrophage cholesteryl ester content as a function of plasma cholesterol in rats. Arterioscler Thromb. 1991;11:1111–1119. doi: 10.1161/01.atv.11.4.1111. [DOI] [PubMed] [Google Scholar]
- 14.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 15.Chinellato A, Ragazzi E, Petrelli L, Paro M, Mironov A, Aliev G. Effect of cholesterol-supplemented diet in heritable hyperlipidemic Yoshida rats: functional and morphological characterization of thoracic aorta. Atherosclerosis. 1994;106:51–63. doi: 10.1016/0021-9150(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 16.Schuschke DA, Saari JT, Ackermann DM, Miller FN. Progressive microcirculatory changes caused by hypercholesterolemia in rats. Am J Physiol. 1990;258:H1464–H1469. doi: 10.1152/ajpheart.1990.258.5.H1464. [DOI] [PubMed] [Google Scholar]
- 17.Schuschke DA, Joshua IG, Miller FN. Comparison of early microcirculatory and aortic changes in hypercholesterolemic rats. Arterioscler Thromb. 1991;11:154–160. doi: 10.1161/01.atv.11.1.154. [DOI] [PubMed] [Google Scholar]
- 18.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 19.Cohen RA, Zitnay KM, Haudenschild CC, Cunningham LD. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ Res. 1988;63:903–910. doi: 10.1161/01.res.63.5.903. [DOI] [PubMed] [Google Scholar]
- 20.Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992;85:1927–1938. doi: 10.1161/01.cir.85.5.1927. [DOI] [PubMed] [Google Scholar]
- 21.Gilligan DM, Guetta V, Panza JA, García CE, Quyyumi AA, Cannon RO 3rd. Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation. 1994;90:35–41. doi: 10.1161/01.cir.90.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Lüscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 23.Osborne JA, Lento PH, Siegfried MR, Stahl GL, Fusman B, Lefer AM. Cardiovascular effects of acute hypercholesterolemia in rabbits. Reversal with lovastatin treatment. J Clin Invest. 1989;83:465–473. doi: 10.1172/JCI113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross R, Faggiotto A, Bowen-Pope D, Raines E. The role of endothelial injury and platelet and macrophage interactions in atherosclerosis. Circulation. 1984;70:III77–III82. [PubMed] [Google Scholar]
- 25.Schwartz CJ, Kelley JL, Nerem RM, Sprague EA, Rozek MM, Valente AJ, Edwards EH, Prasad AR, Kerbacher JJ, Logan SA. Pathophysiology of the atherogenic process. Am J Cardiol. 1989;64:23G–30G. doi: 10.1016/0002-9149(89)90952-1. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Nerem RM. The pathogenesis of atherosclerosis: an overview. Clin Cardiol. 1991;14:I1–16. doi: 10.1002/clc.4960141302. [DOI] [PubMed] [Google Scholar]
- 27.Lopez JA, Brown BP, Armstrong ML, Piegors DJ, Heistad DD. Response of the mesenteric circulation to serotonin in normal and atherosclerotic monkeys: implications for the pathogenesis of non-occlusive intestinal ischaemia. Cardiovasc Res. 1989;23:117–124. doi: 10.1093/cvr/23.2.117. [DOI] [PubMed] [Google Scholar]
- 28.Shimokawa H, Vanhoutte PM. Impaired endothelium-dependent relaxation to aggregating platelets and related vasoactive substances in porcine coronary arteries in hypercholesterolemia and atherosclerosis. Circ Res. 1989;64:900–914. doi: 10.1161/01.res.64.5.900. [DOI] [PubMed] [Google Scholar]
- 29.Zeiher AM, Drexler H, Wollschläger H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 30.Nickenig G. Central role of the AT(1)-receptor in atherosclerosis. J Hum Hypertens. 2002;16 Suppl 3:S26–S33. doi: 10.1038/sj.jhh.1001436. [DOI] [PubMed] [Google Scholar]


