Abstract
AIM: To determine whether prior appendectomy modifies the phenotype and severity of Crohn’s disease.
METHODS: Appendectomy status and smoking habits were specified by direct interview in 2838 patients consecutively seen between 1995 and 2004. Occurrence of complications and therapeutic needs were reviewed retrospectively. Additionally, annual disease activity was assessed prospectively between 1995 and 2004 in patients who had not had ileocecal resection and of a matched control group.
RESULTS: Compared to 1770 non-appendectomized patients, appendectomized patients more than 5 years before Crohn’s disease diagnosis (n=716) were more often females, smokers, with ileal disease. Cox regression showed that prior appendectomy was positively related to the risk of intestinal stricture (adjusted hazard ratio, 1.24; 95% confidence interval, 1.13 to 1.36; P = 0.02) and inversely related to the risk of perianal fistulization (adjusted hazard ratio, 0.75; 95% confidence interval, 0.68 to 0.83; P = 0.002). No difference was observed between the two groups regarding the therapeutic needs, except for an increased risk of surgery in appendectomized patients, attributable to the increased prevalence of ileal disease. Between 1995 and 2004, Crohn’s disease was active during 50% of years in appendectomized patients (1 318 out of 2 637 patient-years) and 51% in non-appendectomized patients (1 454 out of 2 841 patient-years; NS).
CONCLUSION: Prior appendectomy is associated with a more proximal disease and has an increased risk of stricture and a lesser risk of anal fistulization. However, the severity of the disease is unaffected.
Keywords: Crohn’s disease, Appendectomy, Surgery, Smoking
INTRODUCTION
Two common environmental factors, cigarette smoking and appendectomy, have been found to play a role in inflammatory bowel diseases (IBD). Smoking has a role both in disease onset and disease course, and it is well established that it has opposite effects on the two diseases, beneficial in ulcerative colitis (UC) and deleterious in Crohn’s disease[1,2]. Previous appendectomy has a favourable effect on UC. Patients who have been appendectomized have a lesser risk of developing UC. Moreover, in the few appendectomized patients who develop UC, disease course is less severe, with a decreased need of colectomy compared to non-appendectomized patients[3,4]. Of note, the effects of smoking and appendectomy are additive[4]. In Crohn’s disease, the effect of previous appendectomy remains debated. Some series reported an increased risk of Crohn’s disease after appendectomy[5-7], and others did not[8-11]. These discrepancies may be partly linked to the inclusion or not of appendectomies performed close to the time of diagnosis. However, the largest study to date showed that the risk of Crohn’s disease is increased up to 20 years after appendectomy[7]. Data concerning the effect of appendectomy on the clinical course of Crohn’s disease are scarce and contradictory, one study reporting no effect[3] and another suggesting that previous appendectomy was associated with an increased risk of surgery[12]. In the study by Andersson et al[7], an increased risk of surgery for Crohn’s disease was observed only in patients with perforated appendicitis.
The aim of the present study was to analyse the phenotype and clinical course of Crohn’s disease in a large cohort of patients subjected to appendectomy compared with non-appendectomized patients.
MATERIALS AND METHODS
Patient population
From January 1995 to December 2004, all consecutive patients with Crohn’s disease who came to our unit were included in the study. The diagnostic criteria for Crohn’s disease were those of Lennard-Jones[13]
Appendectomy status and smoking habits
Appendectomy and smoking status were specified during a direct interview of the patients. The date of appendectomy was noted and patients were classified according to the time span between appendectomy and diagnosis. Patients were classified as smokers if they had smoked more than 7 cigarettes per week for at least six months during the six months preceding diagnosis of Crohn’s disease and/or thereafter[14].
Characteristics of Crohn’s disease
The characteristics of Crohn’s disease were completed according to the retrospective analysis of medical charts. The time of diagnosis was defined as the date of first detection of unequivocal inflammatory abnormalities of the intestine, as assessed from radiological, or endoscopic, or peroperative observations. The initial location of Crohn’s disease lesions was determined by colonoscopy and small bowel X-ray. After diagnosis, patients were followed clinically with 3 to 4 visits per year, and only investigated again in case of flare-up or development of new symptoms. Morphological investigations included proctosigmoidoscopy, colonoscopy, and small bowel X-ray. Upper gastrointestinal endoscopy was only performed in case of gastroesophageal symptoms. Subjects were explored for ano-perineal disease at each visit.
Location and behavior of Crohn’s disease were classified according to the Vienna classification[15]. First morphological demonstration of narrowing or penetrating complication was used to date the occurrence of the complication defining behavior[16].
Treatment of Crohn’s disease
Our treatment policy has been exposed elsewhere[16]. Flare-up episodes were treated with mesalamine (3-4 g daily) or prednisolone (1 mg/kg per day, progressively tapered after four weeks), according to the clinical severity. When steroid therapy failed, patients seen before 1999 were given a 3-wk course of enteral or parenteral nutrition; those seen after June 1999, when Infliximab became available in France, received Infliximab 5 mg/kg.
As maintenance treatment, we used aminosalicylates (sulphasalazine, olsalazine or mesalamine, 2-3 g daily) for asymptomatic or moderately active forms of the disease, and immunosuppressive drugs for severe forms (steroid-dependent or poorly responsive to steroids). Azathioprine 2 mg/kg per day was used as first line immunosuppressive drug. In case of repeated flare-ups or chronic active disease in a patient receiving azathioprine, its dosage was increased to 2.5-3 mg/kg per day. Intramuscular methotrexate (20-25 mg weekly) was used in patients unresponsive or intolerant to azathioprine. Its dosage was tapered progressively to 10-15 mg, and re-augmented in case of clinical relapse.
Although the overall strategy remained mostly unchanged, there was a clear tendency over time to initiate immunosuppressants earlier in the disease course. Surgery was reserved for stenotic and extra-parietal complications, or intractable forms after a well-conducted medical management.
Phenotype and severity of Crohn’s disease
Phenotyping Crohn’s disease took into account disease location and the occurrence of a stricturing or penetrating complication. Overall severity of the disease was assessed in two ways: first retrospectively, taking into account the importance of the medical therapy, i.e. need for glucocorticoid, nutritional support, immunosuppressive drugs, and Infliximab, and finally incidence of excisional surgery. Second, patients who had not had ileo-cecal resection prior to inclusion were followed-up prospectively from the date of inclusion to December 2004, and activity of the disease was assessed prospectively by analyzing the occurrence of a flare-up each year. A patient-year was considered as active if a flare-up or a complication occurred during the year, and inactive otherwise.
Statistics
Continuous data are expressed as mean (standard deviation), and differences between the groups were tested for significance by Student's t test. Discrete data are given as percentages, and comparisons were made with Pearson's Chi-square test.
The retrospective study analyzed the effect of prior appendectomy on the long-term course of Crohn’s disease. For this purpose, non-appendectomized patients were included in the control group until the time an ileo-cecal resection was eventually performed. To avoid biases related to the effect of ileo-cecal resection on the subsequent evolution of Crohn’s disease, appendectomized patients were also censored at the time of ileo-cecal resection. For actuarial analysis, the Kaplan-Meier model was used, with the date of diagnosis as starting point. The curves were compared by the Log-rank test. Multivariate analyses were performed with Cox proportional hazards regression to adjust for confounding. All baseline variables suspected to be possible predictors of complication or intestinal surgery [young age (< 20 years), old age (equal to or above 40 years), gender, ethnicity (Caucasian or not), socioeconomic status (high or low-moderate), diagnosis after 1987, familial history, extra-intestinal manifestations, smoking status, initial disease location (esophago-gastro-duodenal, jejunal, ileal, colonic, and anoperineal lesions)], were entered into the model. Diagnosis after 1987 was retained as a variable because the use of immunosuppressive therapy became frequent since that year. Results of analysis are presented as hazard ratios (HRs) with 95% confidence intervals.
The prospective analysis included consecutive Crohn’s disease patients seen between 1995 and 2004 who had had appendectomy but no ileo-cecal resection before 1995. Those patients were matched for sex, birth date (boxes of five years), date of diagnosis (boxes of five years), and Vienna classification disease location, with non-appendectomized patients without previous ileo-cecal resection. Patients of both groups were censored at the time of ileo-cecal resection. Calculations were performed using GB-stat statistical software.
RESULTS
Appendectomy in relation to disease onset
Among 2926 patients with Crohn’s disease, 1068 patients (38% of those with appendectomy status known) had been appendectomized, including 87 after Crohn’s disease diagnosis. Compared to non-appendectomized patients, the 981 appendectomized patients before or at diagnosis were more often females (69 vs 58%, P < 0.001), Caucasians (90 vs 84%, P < 0.001), and active smokers (61 vs 48%, P < 0.001). They were also older at diagnosis (29.5 ± 12.1 vs 27.4 ± 12.7 years, P < 0.001), and the time span between first symptom attributable retrospectively to Crohn’s disease and Crohn’s disease diagnosis was longer (21.9 ± 48.9 vs16.5 ± 38.3 months in non-appendectomized patients, P = 0.003). Disease location differed significantly between the two groups, with a predominance of ileitis (L1 location) counterbalanced by a decrease of colitis (L2 location) in appendectomized patients (43 and 20%, vs 28 and 31%, respectively, in non-appendectomized patients, P < 0.001), without significant differences in the proportion of ileocolitis (L3) and upper digestive tract location (L4). Important differences were seen regarding gender distribution and disease location according to the time span between appendectomy and Crohn’s disease diagnosis (Figure 1). A significant female predominance was observed only in patients appendectomized more than one year prior to Crohn’s disease diagnosis. L1 was the main disease location whatever the interval between appendectomy and Crohn’s
Figure 1.
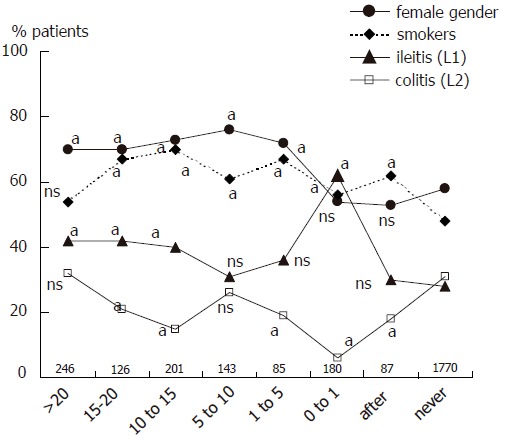
Characteristics of patients and Crohn’s disease (given as percentages) according to appendectomy status and the time span between appendectomy and diagnosis of Crohn’s disease. Numbers indicate the number of patients in each group. a and ns represent the P value of the comparison between the subgroup and the group of never appendectomized patients (aP < 0.05; ns: not significant).
disease diagnosis, however this increase was particularly marked in patients appendectomized at or within one year preceding the diagnosis. Compared to this latter subgroup, subgroups of appendectomized patients who had had appendectomy at various intervals prior to Crohn’s disease differed significantly by a higher proportion of females, a lower prevalence of L1 location and a higher prevalence of L2 location. Finally, there was a higher proportion of smokers in appendectomized patients whatever the date of appendectomy. To avoid inclusion of patients for whom appendectomy could have been performed for a missed diagnosis of Crohn’s disease, we excluded from further analysis, in addition to the 180 patients appendectomized within the year of diagnosis, the 85 patients in whom appendectomy had been performed within the five years before disease onset. Thus 716 patients were included in the retrospective analysis (Figure 2). Among those patients, appendectomy had been performed 5 to 76 years (median, 16 years) before diagnosis of Crohn’s disease, at a median age of 11 years (range 0-67).
Figure 2.
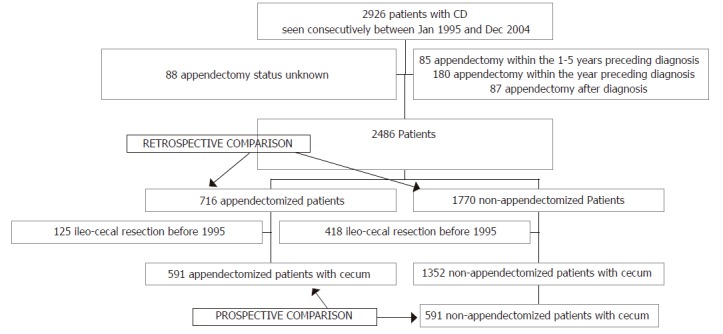
Flow chart of patients studied.
Retrospective analysis
The comparison of these 716 appendectomized patients with non-appendectomized patients is given in Table 1. The results were similar to the whole group of appendectomized patients, with a Crohn’s disease diagnosis later in life and a predominance of both women and active smokers. In addition, appendectomized patients were more often Caucasian, of low-to-moderate socioeconomic status, without family history, and had less extra-intestinal manifestations. The most frequent disease location according to Vienna classification was ileum only in appendectomized patients, whereas it was colon only in non-appendectomized patients. More detailed comparison of initial and cumulative disease location revealed that prior appendectomy was associated with a higher prevalence of ileal involvement, while distal colon, rectum, and anus were more frequently spared (Figure 3). Cumulative disease behavior was inflammatory (B1) in 46%, stricturing (B2) in 15%, and penetrating (B3) in 39% of appendectomized patients, vs 43% (B1), 11% (B2), and 46% (B3), respectively, in non-appendectomized patients. Because disease behavior is highly dependent on disease location and duration[16], the respective risks of stricture, intestinal perforation, and perianal perforation were calculated in each location group cumulatively (Table 2). These calculations demonstrated in appendectomized compared to non-appendectomized patients an increased risk of stricture in the L1 group, and a decreased risk of perianal perforating disease in the L2 group. Cox analysis in the whole population pooling the four location groups (2486 patients) confirmed that prior appendectomy was positively related to the risk of intestinal stricture (adjusted hazard ratio, 1.24; 95% confidence interval, 1.13 to 1.36; P = 0.02) and inversely related to the risk of perianal perforation (adjusted hazard ratio, 0.75; 95% confidence interval, 0.68 to 0.83; P = 0.002). The respective proportions of appendectomized and non-appendectomized patients who required oral steroids, enteral or parenteral nutrition, immunosuppressive therapy, infliximab, and surgery were not significantly different in the whole population nor in each location group (Table 3). In the whole population, the 10-yr cumulative need for first excisional surgery was significantly higher in appendectomized vs non-appendectomized patients (54 ± 2% vs. 48 ± 2%, respectively; P = 0.02). However, multivariate analysis selected as predictive factors of surgery young age (adjusted hazard ratio, 0.80; 95 % confidence interval, 0.74 to 0.86; P = 0.002), ileal involvement (adjusted hazard ratio, 1.73; 95% confidence interval, 1.60 to 1.87; P < 0.001) and colonic involvement (adjusted hazard ratio, 0.66; 95 % confidence interval, 0.62 to 0.71; P < 0.001) and did not confirm the specific role of appendectomy (P = 0.33). Within each location group, the 10-yr cumulative need for first excisional surgery did not differ significantly (L1 74 ± 4% vs 67 ± 3%; L2 27 ± 4% vs 38 ± 3; L3 50 ± 5% vs 39 ± 3%; L4 51 ± 8% vs 51 ± 4%).
Table 1.
Main characteristics of Crohn’s disease in non-appendectomized and appendectomized patients
| Non-appendectomized | Appendectomized | P | |
| Number of patients | 1770 | 716 | |
| Female gender | 1019 (58) | 515 (72) | 0.001 |
| Age at diagnosis (yr) | 27.5 ± 12.8 | 32.1 ± 13 | 0.001 |
| Duration of disease (yr)1 | 9.6 ± 8.6 | 9.1 ± 8.1 | NS |
| Caucasian ethnicity | 1484 (84) | 663 (93) | 0.001 |
| High socio-economic status | 535 (30) | 171 (24) | 0.001 |
| Disease onset after 1987 | 1374 (78) | 554 (77) | NS |
| Familial history | 272 (15) | 79 (11) | 0.004 |
| Current smokers | 856 (48) | 444 (62) | 0.001 |
| Disease location | |||
| Terminal ileum (L1) | 489 (28) | 277 (39) | |
| Colon (L2) | 548 (31) | 173 (24) | 0.001 |
| Ileocolon (L3) | 514 (29) | 187 (26) | |
| Upper gastrointestine (L4) | 219 (12) | 79 (11) | |
| Extra-intestinal manifestations | 1136 (64) | 422 (59) | 0.01 |
at last visit or when censored Number in parentheses is percentages.
Figure 3.
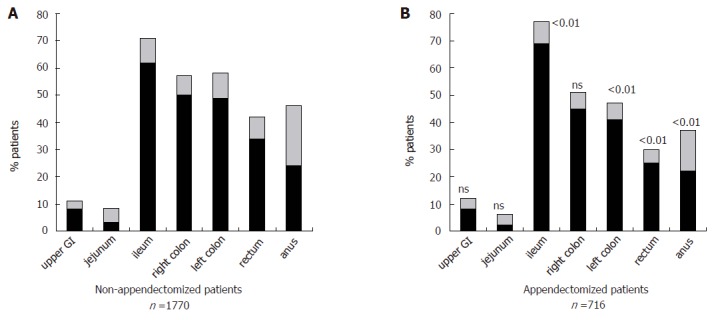
Initial and cumulative Crohn’s disease involvement of various segments of the digestive tract in non-appendectomized and appendectomized patients. The numbers above the columns indicate the P values comparing the frequencies of cumulative involvement of each segment between the two groups.
Table 2.
5-yr and 10-yr cumulative risks of intestinal and perianal complications in non-appendectomized and appendectomized patients according to disease location
|
Non-appendectomized |
Appendectomized |
||||||||
| Location | L1 | L2 | L3 | L4 | L1 | L2 | L3 | L4 | |
| Number of patients | 493 | 546 | 513 | 218 | 279 | 172 | 186 | 79 | |
| Intestinal stricture | 5-yr | 23 ± 2 | 2 ± 1 | 9 ± 1 | 22 ± 3 | 30 ± 3 b | 3 ± 2 | 12 ± 3 | 29 ± 6 |
| 10-yr | 34 ± 3 | 7 ± 1 | 17 ± 2 | 42 ± 5 | 46 ± 4 b | 6 ± 2 | 24 ± 4 | 42 ± 7 | |
| Intestinal perforation | 5-yr | 30 ± 2 | 7 ± 1 | 12 ± 2 | 12 ± 2 | 27 ± 3 | 4 ± 2 a | 13 ± 3 | 10 ± 4 |
| 10-yr | 42 ± 3 | 12 ± 2 | 25 ± 2 | 23 ± 4 | 37 ± 4 | 4 ± 2 a | 23 ± 4 | 29 ± 7 | |
| Perianal fistulization | 5-yr | 14 ± 2 | 32 ± 2 | 31 ± 2 | 23 ± 3 | 11 ± 2 | 19 ± 3 b | 26 ± 4 | 15 ± 4 |
| 10-yr | 18 ± 2 | 49 ± 3 | 41 ± 3 | 29 ± 4 | 17 ± 3 | 33 ± 5 b | 40 ± 5 | 21 ± 6 | |
P < 0.001,
P < 0.05 vs appendectomized non-appendectomized patients (log rank). Other curves were not significantly different.
Table 3.
Cumulative therapeutic needs in non-appendectomized and appendectomized patients according to disease location
| Non-appendectomized | Appendectomized | |||||||
| Location | L1 | L2 | L3 | L4 | L1 | L2 | L3 | L4 |
| Number of patients | 493 | 546 | 513 | 218 | 279 | 172 | 186 | 79 |
| Oral or IV steroids | 396 (80) | 469 (86) | 462 (90) | 195 (89) | 228 (82) | 150 (87) | 173 (93) | 71 (90) |
| Enteral or parenteral nutrition | 53 (11) | 1 | 94 (18) | 48 (22) | 23 (8) | 0 | 35 (19) | 13 (16) |
| Azathioprine or methotrexate | 163 (33) | 305 (56) | 290 (57) | 132 (61) | 100 (36) | 83 (48) | 101 (54) | 48 (61) |
| Infliximab | 9 (2) | 73 (13) | 48 (9) | 19 (9) | 4 (1) | 11 (6) | 14 (8) | 5 (6) |
| Intestinal resection | 283 (57) | 173 (32) | 176 (34) | 79 (36) | 165 (59) | 42 (24) | 73 (39) | 30 (38) |
Numbers in parentheses are percentages. Comparison between non-appendectomized and appendectomized patients revealed no significant difference
Prospective study
The prospective study included 591 appendectomized patients and 591 non-appendectomized matched controls, both selected on the basis of not having had ileo-cecal resection at inclusion into follow-up. The two groups were well matched (Table 4). One hundred and forty-five appendectomized (25%) and 143 non-appendectomized patients (24%), respectively, had an ileo-cecal resection after inclusion. After censoring, the median length of follow-up for the two cohorts was 4 years and encompassed a total of 5478 patient-years. The rate of years with active disease was 50% in appendectomized patients (1 318 out of 2 637 patient-years) vs 51% in non-appendectomized patients (1 455 out of 2 841 patient-years, NS). By contrast, in the same series of patients, non- or ex-smoking was associated with a decreased activity rate (1 521 out of 3 210 patient-years, 47%), compared to current smoking (1 252 out of 2 268, 55%; P < 0.001). Figure 4 gives the percentage of patient-years with active disease and hospitalization respectively, according to both smoking and appendectomy status. Previous appendectomy had no effect on year-by-year disease activity while smoking was significantly deleterious.
Table 4.
Comparison between two groups of patients included in prospective follow-up study
| Non-appendectomized | Appendectomized | |
| Number of patients | 591 | 591 |
| Number of females | 427 | 427 |
| Mean age at diagnosis (yr) | 31.8 ± 13.8 | 32.3 ± 13.4 |
| Mean age at inclusion (yr) | 34.0 ± 13.9 | 34.8 ± 13.7 |
| Diagnosis after 1987 (n) | 539 | 524 |
| Diagnosis after 1995 (n) | 351 | 357 |
| L1 location (n) | 202 | 204 |
| L2 location (n) | 162 | 160 |
| L3 location (n) | 156 | 156 |
| L4 location (n) | 71 | 71 |
No difference between the two groups was significant
Figure 4.
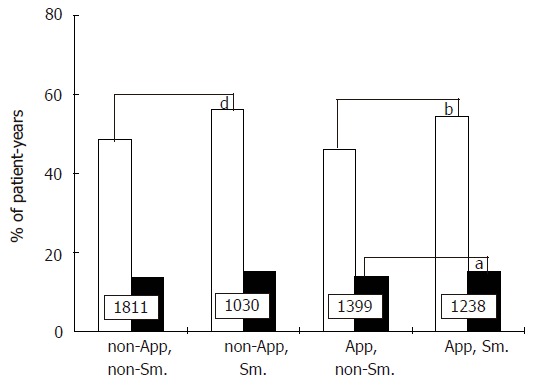
Percentages of patient-years with active disease (grey columns) and hospitalization (black columns), respectively, according to appendectomy and smoking status (App: appendectomized; Sm: smokers). Numbers indicate the number of patient-years in each group. aP < 0.05 vs App, non-Sm.; bP < 0.001 vs App, non-Sm.; dP < 0.001 vs non-App, non-Sm.
DISCUSSION
The present results show that previous appendectomy, while associated with some particularities in the location and behaviour of Crohn’s disease, has no effect upon the course of the disease. Appendectomized patients are more prone to ileal disease and to formation of strictures, and less to distal colonic involvement and penetrating perianal disease. However, previous appendectomy does not change disease severity retrospectively assessed from therapeutic needs or prospectively assessed from year-by-year activity.
The role of appendectomy in Crohn’s disease is difficult to assess because various biases may jeopardize the analysis. First, appendicitis may be the first manifestation of Crohn’s disease. The appendix is frequently involved by Crohn’s disease[17], granulomatous appendicitis may in some cases reveal Crohn’s disease[18,19], and resection of a Crohn’s disease appendix may be followed by a long period of remission[20,21]. However, most pathological studies concluded that idiopathic granulomatous appendicitis is nosologically distinct from Crohn’s disease[22] and that most of these patients do not develop subsequent Crohn’s disease. Moreover, when analyzing a cohort of Crohn’s disease patients, Crohn’s disease confined to the appendix was very unusual, observed in less than 1% of Crohn’s disease patients[23]. Although the pathology reports of the removed appendix were not available in our series, the data suggest that the great majority of our patients had appendectomy performed not for Crohn’s disease. Moreover, the frequency of appendectomy in our series is similar to other series[12] and not different from controls without Crohn’s disease[24,25]. Another difficulty is to accurately define in time the patient population in which the effect of appendectomy may be assessed. On one hand, inclusion of all appendectomized patients whatever the time span between appendectomy and the onset of Crohn’s disease may lead to an overrepresentation of ileal disease cases, as an acute abdominal pain in the lower right quadrant may reveal ileal Crohn’s disease. On the other hand, prolonging the time span between appendectomy and Crohn’s disease may select cases of Crohn’s disease occurring later in life and thus susceptible to share a lesser activity. Actually, in the present study, the comparison of patients subgroups defined by the duration of the interval between appendectomy and onset of Crohn’s disease showed that only patients appendectomized within one year prior to Crohn’s disease diagnosis clearly differed from other subgroups through a higher prevalence of ileal disease, as it could be expected (Figure 1). The characteristics of the subgroup of appendectomized patients one to five years prior to Crohn’s disease diagnosis were intermediate between the two adjacent subgroups. This led us to define the cutoff at five years prior to appendectomy. Such a cutoff is in agreement with the data of Frisch et al[6] who found that, five years after appendectomy, the relative risk of Crohn’s disease was not further elevated. Finally, in the present study, selection bias were minimal, as we did not use a postal questionnaire, but included prospectively all consecutive patients seen over a 10-year period. The criteria used to assess the severity of the disease were objective, and were based on therapeutic needs. Both surgical and immunomodulator history are accepted as useful indicators of the over-all severity of Crohn’s disease because these interventions are both easy to document retrospectively, and their use and the effects of their use may be complementary[14,26]. Moreover, the prospective part of the study confirmed the results of the retrospective analysis.
Crohn’s disease patients with prior appendectomy differed from non-appendectomized Crohn’s disease patients in many aspects. There were more often females, smokers, of low-to-moderate socioeconomic status, and of Caucasian origin. In fact, these particularities might be more a consequence of appendectomy as such, than related to a particular Crohn’s disease phenotype. Indeed, an epidemiological national survey in 1978-82 in France showed that appendectomy, but not appendicitis, was more frequent in females than in males[24], and an association has been shown between appendectomy and passive or active smoking[27]. Other epidemiological studies from UK have also reported an increased rate of incidental appendectomies in women [28]. Similarily, the contrast between a higher proportion of Caucasian patients and a lower proportion of patients of high socio-economic status fits better with what is known about epidemiology of appendicitis than with IBD[29,30]. In the Western world, appendectomy is performed more often in whites than in blacks[31], whereas data from UK suggest that the incidence of IBD in immigrants is similar to that of Europeans[32]. Changes in hygiene and water supply increased the risk of appendectomy[33] which in the last four decades has preferentially concerned populations of low-to-moderate socio-economic status, whereas patients with IBD seem to be better educated[34]. We believe that the overrepresentation of ileal disease in appendectomized Crohn’s disease patients may be at least in part a consequence of the high prevalence of smokers in this group, smoking being associated with ileal Crohn’s disease and not colonic Crohn’s disease[35]. Alternative explanations of the more frequent ileal involvement in appendectomized patients would be a larger use of explorative procedures such as barium follow-through or colonoscopy because patients with previous appendectomy are more prone to small bowel obstruction[36,37] and non specific abdominal pain[38]. The occurrence of abdominal pain may give the opportunity to find out a paucisymptomatic ileitis which could have been ignored otherwise.
Comparisons of disease behaviour between appendectomized patients and controls revealed some subtle differences within each disease location group. The former patients were more exposed to stricture formation and less to penetrating anal disease. Actually, within the colon only group (L2 group), disease location tended to be more distal in non-appendectomized patients, perianal lesions were more frequent, and it could be expected that perianal perforating complications occurred more frequently. The increased prevalence of intestinal strictures in patients with prior appendectomy, although weak, seems more relevant. It may be hypothesized that the presence of intra-abdominal adherences and/or vasculature modifications secondary to prior laparotomy has a role in stricture formation.
The most important and unequivocal result of the present study is the lack of effect of prior appendectomy on the severity of Crohn’s disease. We observed an increased risk of surgery in appendectomized patients, but multivariate analysis revealed that this was related to confounding factors. Riegler et al[12] have reported an increased risk of surgery in 41 Crohn’s disease patients with previous appendectomy when compared to 88 non-appendectomized patients, but patients appendectomized within one year prior to diagnosis of Crohn’s disease were included in the analysis. In the study by Andersson, compared to Crohn’s disease controls, Crohn’s disease patients with a history of perforated appendicitis had a higher incidence of intestinal resections, those with appendectomy for other diagnoses had a lower incidence, but the incidence for the whole group with prior appendectomy was similar to controls[7]. Radford-Smith et al[3] have reported similar results in a retrospective analysis of 335 patients with Crohn’s disease, of whom 36 had had prior appendectomy. Thus the effect of prior appendectomy on the risk of surgery seems nil or weak, or not related to appendectomy per se. Besides, therapeutic needs in terms of steroid and immunosuppressants requirements were very similar in appendectomized and non-appendectomized patients, whatever the disease location. Finally, the prospective follow-up of our appendectomized patients compared with controls matched for gender, age, and disease location, clearly demonstrated the lack of effect of prior appendectomy on the disease activity. This study was powered enough to support a firm conclusion, since concomitantly among the same patients, the deleterious effect of current smoking was highly significant.
In conclusion, this study shows that prior appendectomy is associated with a distinct phenotype of Crohn’s disease. However these particularities may be attributable more to appendectomy than to Crohn’s disease. Besides, in contrast to smoking, appendectomy has no effect on Crohn’s disease severity. Taken together, these data indicate that appendectomy and Crohn’s disease share common environmental or genetic characteristics whereas appendectomy per se does not exert any immune modulating effect. The response of IBD to the only environmental factors clearly documented so far, smoking and appendectomy, is quite different in UC and in Crohn’s disease.
Footnotes
S- Editor Wang J L- Editor Zhang JZ E- Editor Bai SH
References
- 1.Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18:481–496. doi: 10.1016/j.bpg.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Radford-Smith GL, Edwards JE, Purdie DM, Pandeya N, Watson M, Martin NG, Green A, Newman B, Florin TH. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn's disease. Gut. 2002;51:808–813. doi: 10.1136/gut.51.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosnes J, Carbonnel F, Beaugerie L, Blain A, Reijasse D, Gendre JP. Effects of appendicectomy on the course of ulcerative colitis. Gut. 2002;51:803–807. doi: 10.1136/gut.51.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koutroubakis IE, Vlachonikolis IG, Kapsoritakis A, Spanoudakis S, Roussomoustakaki M, Mouzas IA, Kouroumalis EA, Manousos ON. Appendectomy, tonsillectomy, and risk of inflammatory bowel disease: case-controlled study in Crete. Dis Colon Rectum. 1999;42:225–230. doi: 10.1007/BF02237133. [DOI] [PubMed] [Google Scholar]
- 6.Frisch M, Johansen C, Mellemkjaer L, Engels EA, Gridley G, Biggar RJ, Olsen JH. Appendectomy and subsequent risk of inflammatory bowel diseases. Surgery. 2001;130:36–43. doi: 10.1067/msy.2001.115362. [DOI] [PubMed] [Google Scholar]
- 7.Andersson RE, Olaison G, Tysk C, Ekbom A. Appendectomy is followed by increased risk of Crohn's disease. Gastroenterology. 2003;124:40–46. doi: 10.1053/gast.2003.50021. [DOI] [PubMed] [Google Scholar]
- 8.Duggan AE, Usmani I, Neal KR, Logan RF. Appendicectomy, childhood hygiene, Helicobacter pylori status, and risk of inflammatory bowel disease: a case control study. Gut. 1998;43:494–498. doi: 10.1136/gut.43.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feeney MA, Murphy F, Clegg AJ, Trebble TM, Sharer NM, Snook JA. A case-control study of childhood environmental risk factors for the development of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14:529–534. doi: 10.1097/00042737-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 10.López Ramos D, Gabriel R, Cantero Perona J, Moreno Otero R, Fernández Bermejo M, Maté Jiménez J. Association of MALTectomy (appendectomy and tonsillectomy) and inflammatory bowel disease: a familial case-control study. Rev Esp Enferm Dig. 2001;93:303–314. [PubMed] [Google Scholar]
- 11.Sicilia B, López Miguel C, Arribas F, López Zaborras J, Sierra E, Gomollón F. Environmental risk factors and Crohn's disease: a population-based, case-control study in Spain. Dig Liver Dis. 2001;33:762–767. doi: 10.1016/s1590-8658(01)80693-9. [DOI] [PubMed] [Google Scholar]
- 12.Riegler G, Caserta L, Esposito I, De Filippo FR, Bossa F, Esposito P, Russo MI, Carratù R. Worse clinical course of disease in Crohn's patients with previous appendectomy. Eur J Gastroenterol Hepatol. 2005;17:623–627. doi: 10.1097/00042737-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Lennard-Jones JE. Classification of IBD. Scand J Gastroenterol. 1989;24(S170):2–4. doi: 10.3109/00365528909091339. [DOI] [PubMed] [Google Scholar]
- 14.Cosnes J, Carbonnel F, Beaugerie L, Le Quintrec Y, Gendre JP. Effects of cigarette smoking on the long-term course of Crohn's disease. Gastroenterology. 1996;110:424–431. doi: 10.1053/gast.1996.v110.pm8566589. [DOI] [PubMed] [Google Scholar]
- 15.Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, et al. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm Bowel Dis. 2000;6:8–15. doi: 10.1097/00054725-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Stangl PC, Herbst F, Birner P, Oberhuber G. Crohn's disease of the appendix. Virchows Arch. 2002;440:397–403. doi: 10.1007/s004280100532. [DOI] [PubMed] [Google Scholar]
- 18.Bronner MP. Granulomatous appendicitis and the appendix in idiopathic inflammatory bowel disease. Semin Diagn Pathol. 2004;21:98–107. doi: 10.1053/j.semdp.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Tucker ON, Healy V, Jeffers M, Keane FB. Granulomatous appendicitis. Surgeon. 2003;1:286–289. doi: 10.1016/s1479-666x(03)80047-1. [DOI] [PubMed] [Google Scholar]
- 20.Prieto-Nieto I, Perez-Robledo JP, Hardisson D, Rodriguez-Montes JA, Larrauri-Martinez J, Garcia-Sancho-Martin L. Crohn's disease limited to the appendix. Am J Surg. 2001;182:531–533. doi: 10.1016/s0002-9610(01)00811-x. [DOI] [PubMed] [Google Scholar]
- 21.Huang JC, Appelman HD. Another look at chronic appendicitis resembling Crohn's disease. Mod Pathol. 1996;9:975–981. [PubMed] [Google Scholar]
- 22.Dudley TH, Jr Dean PJ. Idiopathic granulomatous appendicitis, or Crohn's disease of the appendix revisited. Hum Pathol. 1993;24:595–601. doi: 10.1016/0046-8177(93)90238-c. [DOI] [PubMed] [Google Scholar]
- 23.Wettergren A, Munkholm P, Larsen LG, Meinecke B, Langholz E, Jess P, Binder V. Granulomas of the appendix: is it Crohn's disease. Scand J Gastroenterol. 1991;26:961–964. doi: 10.3109/00365529108996249. [DOI] [PubMed] [Google Scholar]
- 24.Tiret L, Rotman N, Hatton F, Fagniez PL. [Digestive surgery in France. A national epidemiologic survey (1978-1982)] Gastroenterol Clin Biol. 1988;12:354–360. [PubMed] [Google Scholar]
- 25.Caserta L, de Filippo FR, Riegler G. Relationship between anamnestic evidence of appendectomy and onset and clinical course of Crohn's disease. Am J Gastroenterol. 2002;97:207–208. doi: 10.1111/j.1572-0241.2002.05409.x. [DOI] [PubMed] [Google Scholar]
- 26.Brant SR, Panhuysen CI, Bailey-Wilson JE, Rohal PM, Lee S, Mann J, Ravenhill G, Kirschner BS, Hanauer SB, Cho JH, et al. Linkage heterogeneity for the IBD1 locus in Crohn's disease pedigrees by disease onset and severity. Gastroenterology. 2000;119:1483–1490. doi: 10.1053/gast.2000.20245. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery SM, Pounder RE, Wakefield AJ. Smoking in adults and passive smoking in children are associated with acute appendicitis. Lancet. 1999;353:379. doi: 10.1016/S0140-6736(05)74951-5. [DOI] [PubMed] [Google Scholar]
- 28.Primatesta P, Goldacre MJ. Appendicectomy for acute appendicitis and for other conditions: an epidemiological study. Int J Epidemiol. 1994;23:155–160. doi: 10.1093/ije/23.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg E, Lindquist B, Holmquist L, Hildebrand H. Inflammatory bowel disease in children and adolescents in Sweden, 1984-1995. J Pediatr Gastroenterol Nutr. 2000;30:259–264. doi: 10.1097/00005176-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Ekbom A. The epidemiology of IBD: a lot of data but little knowledge. How shall we proceed. Inflamm Bowel Dis. 2004;10 Suppl 1:S32–S34. doi: 10.1097/00054725-200402001-00007. [DOI] [PubMed] [Google Scholar]
- 31.Mort EA, Weissman JS, Epstein AM. Physician discretion and racial variation in the use of surgical procedures. Arch Intern Med. 1994;154:761–767. [PubMed] [Google Scholar]
- 32.Jayanthi V, Probert CS, Pinder D, Wicks AC, Mayberry JF. Epidemiology of Crohn's disease in Indian migrants and the indigenous population in Leicestershire. Q J Med. 1992;82:125–138. [PubMed] [Google Scholar]
- 33.Barker DJ, Osmond C, Golding J, Wadsworth ME. Acute appendicitis and bathrooms in three samples of British children. Br Med J (Clin Res Ed) 1988;296:956–958. doi: 10.1136/bmj.296.6627.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. 1990;31:1037–1040. doi: 10.1136/gut.31.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russel MG, Volovics A, Schoon EJ, van Wijlick EH, Logan RF, Shivananda S, Stockbrügger RW. Inflammatory bowel disease: is there any relation between smoking status and disease presentation European Collaborative IBD Study Group. Inflamm Bowel Dis. 1998;4:182–186. doi: 10.1097/00054725-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Ahlberg G, Bergdahl S, Rutqvist J, Söderquist C, Frenckner B. Mechanical small-bowel obstruction after conventional appendectomy in children. Eur J Pediatr Surg. 1997;7:13–15. doi: 10.1055/s-2008-1071041. [DOI] [PubMed] [Google Scholar]
- 37.Andersson RE. Small bowel obstruction after appendicectomy. Br J Surg. 2001;88:1387–1391. doi: 10.1046/j.0007-1323.2001.01869.x. [DOI] [PubMed] [Google Scholar]
- 38.Tingstedt B, Johansson J, Nehez L, Andersson R. Late abdominal complaints after appendectomy--readmissions during long-term follow-up. Dig Surg. 2004;21:23–27. doi: 10.1159/000075378. [DOI] [PubMed] [Google Scholar]


