Picconia azorica is an endangered endemic species of the Azores whose hard and high density wood is very appreciated for the production of toys, agricultural tools, furniture and religious statuary. Its renewed economic interest represents a good opportunity for establishing conservation programmes. To contribute with information useful for the decision making we performed the genetic analysis of 230 samples from 11 populations collected in three Azorean islands. The majority of the genetic variability was found within populations and no genetic structure was detected between populations and between islands, indicating that the oceanic barriers do not greatly affect gene flow.
Keywords: Azores, endemism conservation, germplasm management, molecular marker, phylogeography, population genetics.
Abstract
Knowledge of the levels and distribution of genetic diversity is important for designing conservation strategies for threatened and endangered species so as to guarantee sustainable survival of populations and to preserve their evolutionary potential. Picconia azorica is a valuable Azorean endemic species recently classified as endangered. To contribute with information useful for the establishment of conservation programmes, the genetic variability and differentiation among 230 samples from 11 populations collected in three Azorean islands was accessed with eight inter-simple sequence repeat markers. A total of 64 polymorphic loci were detected. The majority of genetic variability was found within populations and no genetic structure was detected between populations and between islands. Also the coefficient of genetic differentiation and the level of gene flow indicate that geographical distances do not act as barriers for gene flow. In order to ensure the survival of populations in situ and ex situ management practices should be considered, including artificial propagation through the use of plant tissue culture techniques, not only for the restoration of habitat but also for the sustainable use of its valuable wood.
Introduction
Knowledge of genetic variability, together with information about demography, reproductive biology and dynamics, is very important when establishing any conservation and management programme (Newton et al. 1999; Francisco-Ortega et al. 2000; Juan et al. 2000; Frankham 2003; Jamieson 2007; Silva et al. 2011) that aims to preserve genetic variability within and among populations and consequently safeguard their potential for adaptation (Eriksson 2001; Silva et al. 2011). Information about genetic diversity patterns may also give insight into the evolutionary history of a taxon, providing the means to assess the future risk of diversity erosion (Neel and Ellstrand 2003).
The development of molecular genetic methods has been a significant advance providing tools for answering questions on the diversity among flora and fauna and to help define strategies for conservation purposes. Inter-simple sequence repeat (ISSR) markers offer the possibility of randomly scanning the whole genome (Mariette et al. 2001) as no sequence information or prior genetic studies are required (Zietkiewicz et al. 1994), and have proved to be valuable, efficient and cost-effective tools in the characterization and evaluation of genetic diversity within and between species and populations of several endemic and endangered plant species (Palacios and González-Candelas 1997; Delgado et al. 1999; Rossetto et al. 1999; Li and Ge 2001; Lee et al. 2003; Torres et al. 2003; Bahulikar et al. 2004; Chen et al. 2004; Segarra-Moragues et al. 2005; Guasmi et al. 2012). Inter-simple sequence repeats are DNA sequences delimited by two inverted SSR and are especially useful in detecting diversity in closely related, or even clonal, individuals (Zietkiewicz et al. 1994). The use of just a single PCR primer to amplify ISSR sequences between simple sequence repeat (SSR) provides multilocus patterns that are very reproducible, abundant and polymorphic in plant genomes (Zietkiewicz et al. 1994; Bornet and Branchard 2001; Bornet et al. 2002, 2004), making this technology a good reliable molecular tool to determine the genetic diversity of Azorean endemic and endangered species.
The archipelago of the Azores is located in the North Atlantic Ocean between latitudes 36°55′N and 39°43′N, and longitudes 24°46′W and 31°16′W. It is composed of nine volcanic islands divided into three groups: the eastern (São Miguel and Santa Maria), the central (Terceira, Graciosa, São Jorge, Pico and Faial) and the western group (Flores and Corvo). From the about 200 species native to the Azorean flora only 70 have been described as endemic for the Azores (Schaefer 2003, 2005), representing 7.2 % of the Azorean flora (Borges et al. 2005). The abusive use of Azorean forests for over five centuries, the expansion of pasture and agricultural fields and the introduction of exotic/imported species led to a substantial reduction of the natural native forest populations. Only recently there has been an increased interest in the endemic forest species for reforestation, particularly of rare species and species with a high wood quality (Ferreira and Eriksson 2006).
Picconia azorica (Oleaceae Family), locally named pau-branco, is a xerophytic, evergreen shrub or a small tree, endemic to the Azores archipelago. This species grows up to 8 m tall and has simple, lanceolate to ovate, opposite leaves with entire margins; it flowers from March to July, producing small white flowers in axillary clusters and its fleshy fruits are dark blue drupes (Martins et al. 2011). Although P. azorica has been present in all nine Azorean islands (Frutuoso 1583), currently is scattered in small patches of coastal forests and marginal sites (Schaefer 2003). Clearly, the over-exploitation of its appreciated wood for manufacturing toys, agricultural tools, furniture and religious statuary, as well as human disturbance that promoted habitat degradation, expansion of agricultural land, deforestation and introduction of aggressive exotic species led this species to becoming almost extinct in some islands (Martín et al. 2008). In fact, it is extinct in Graciosa island and near to extinction in São Miguel and Terceira islands (Cardoso et al. 2008; Silva et al. 2010; Ferreira et al. 2011), thus being a priority Azorean endemic species for conservation, listed as endangered (EN B1 + 2c) on the IUCN Red List (2013) and protected according to the Directive Habitats (Annexes II and IV) (European Commission 1992) and the Bern Convention (Annex I) (Council of Europe 1993). Picconia azorica is one of the two residual species of the Picconia genera which became extinct in continental land. However the Azores, together with Madeira and the Canary islands, are considered refuge areas for this genus. Therefore, P. azorica due to its fragile status demands urgent specific management and conservation measures for restoration of depleted natural populations (Martín et al. 2008). Ecological restoration plans to prevent the erosion of P. azorica genetic resources are necessary, primarily because of its endangered status, for its biological and economical relevance and for its major ecological importance. Unfortunately, there are limited numbers of studies on the Azorean endemic forests and no detailed characterization about the biology and management of P. azorica has been made so far (Ferreira et al. 2011). For the genetic characterization of P. azorica, chloroplast markers were used under phylogenetic and phylogeographical perspectives, due to their uniparental inheritance, revealing absence of geographical structure and limited intra-specific genetic diversity (Ferreira et al. 2011), suggesting the need for a sound assessment of the species genetic structure at the nuclear level. Only recently a limited number of SSRs were isolated from P. azorica and used for the characterization of different populations (Martins et al. 2013), showing high levels of intra-population diversity and low genetic differentiation between populations. Simple sequence repeats are codominant markers that reveal a high number of alleles, still the variation detected is pre-determined at the sequence sites and the number of analysed loci in diversity studies is usually low (Mariette et al. 2001). In our study we access the genetic variability and differentiation, as well as phylogeographical patterns within different populations of São Miguel, Terceira and Pico islands of such an endangered endemic Azorean species, by using ISSR markers, as conservation should be based on genetic diversity at the whole-genome level (Mariette et al. 2001). These islands were selected as they are the most populated ones and therefore more susceptible to human habitat disturbance and also because Pico is the island with more area forested with P. azorica and since the earliest colonization of the archipelago an exporter of this valuable wood (Frutuoso 1583).
Methods
Picconia azorica leaf samples were collected from 11 naturally occurring populations from three Azorean Islands: São Miguel, Terceira and Pico (Table 1). Populations Caloura (CL), Lombo Gordo (LG), Ribeira Quente (RQ) and Tronqueira (TR) were collected in São Miguel Island located in the eastern group of the archipelago. The remaining populations were collected from islands located in the central group: Pico [Alto de São Roque (ASR), Cais do Mourato—Santana (CM), Candelária (CD), Piedade (PD) and Santo Amaro (SA)] and Terceira [Pico do Boi (PB) and Serreta (SER)] [see Supporting Information]. Geographical coordinates of all stations of origin were recorded using a hand-help GPS navigator. Samples were collected randomly across the distribution area with at least a minimal distance of 20 m. Efforts were made that the number of individuals collected was correlated to the dimension of each population. However, some populations were difficult to access (TR) or were very small (CL), in which case sampling was limited. Leaf samples were weighed and stored at −80 °C.
Table 1.
Populations of P. azorica from S. Miguel, Pico and Terceira islands used in this study. N, number of individuals sampled. For population locations, see Supporting Information.
| Island | Population | Code | N | Altitude range (m) |
|---|---|---|---|---|
| S. Miguel | Caloura | CL | 4 | 30–50 |
| Lombo Gordo | LG | 16 | 20–270 | |
| Ribeira Quente | RQ | 37 | 30–100 | |
| Tronqueira | TR | 7 | 560–615 | |
| Pico | Alto de São Roque | ASR | 14 | 240–430 |
| Cais do Mourato – Santana | CM | 37 | 15–115 | |
| Candelária | CD | 17 | 30–220 | |
| Piedade | PD | 27 | 10–320 | |
| Santo Amaro | SA | 24 | 10–50 | |
| Terceira | Pico do Boi | PB | 19 | 645–690 |
| Serreta | SER | 28 | 140–230 |
DNA was extracted by downscaling the protocol described by Fabbri et al. (1995) using 100 mg of leaves, so that extraction could be performed with 800 μL of CTAB extraction buffer in 2 mL microtubes.
A total of 19 anchored ISSR primers from the University of British Columbia (UBC, Vancouver, Canada) were firstly tested in a batch of 48 samples. From these, 8 that produced clear, polymorphic, reproducible bands across two repetitions of each ISSR assay were selected (Table 2) and used for genotyping all the 230 samples. Additionally three samples of Picconia excelsa, from Madeira island, were also genotyped to be used as an outgroup for the phylogenetic analysis of individuals.
Table 2.
Primers used for ISSR amplification of P. azorica.a Melting temperature.
| ISSR |
Bands |
||||
|---|---|---|---|---|---|
| Primer name | Primer sequence | Tm (°C)a | Readable | Polymorphic | % Polymorphic |
| UBC807 | (AG)8T | 52 | 15 | 12 | 80.00 |
| UBC808 | (AG)8C | 54 | 12 | 10 | 83.33 |
| UBC811 | (GA)8C | 54 | 9 | 7 | 77.77 |
| UBC826 | (AC)8C | 54 | 6 | 3 | 50.00 |
| UBC834 | (AG)8YT | 54 | 8 | 6 | 75.00 |
| UBC836 | (AG)8YA | 56 | 4 | 2 | 50.00 |
| UBC840 | (GA)8YT | 54 | 10 | 10 | 100.00 |
| UBC842 | (GA)8YG | 56 | 15 | 14 | 93.33 |
| Average | 10 | 8 | 76.18 | ||
| Total | 79 | 64 | 81.01 | ||
Polymerase chain reactions were carried out in a total volume of 25 μL containing 20 ng DNA, 0.2 μM of primer, 0.2 mM of each dNTPs and 1 U of DreamTaq DNA polymerase (Fermentas) in reaction buffer. Amplification was performed in a UNO II Biometra thermocycler with 5 min denaturation at 94 °C, followed by 35 cycles of 30 s denaturation at 96 °C, 45 s annealing at the appropriate melting temperature (Table 2), 1 min 30 s elongation at 72 °C and a final 20 min elongation step at 72 °C. Amplification products were separated for 3 h at 120 V on a 2 % TAE agarose gel stained with SybrGreen Premium (NZYTech) along with GeneRuler 1 kb Plus DNA ladder (Fermentas) for estimation of the molecular size of the amplified fragments. Gels were recorded under ultraviolet light with a GelDoc XR+ system and analysed with the Image Lab 3.0 software (BioRad). To confirm that fragments were being consistently amplified, one sample was replicated across all runs. Furthermore, to minimize the effects of electrophoresis and staining on band variability two replicate experiments were carried out for each ISSR primer–sample combination. In every case the banding patterns on the first and second amplification/gels were identical. To ensure that neither self-amplification nor contamination was occurring, negative controls were also prepared.
Amplified bands of size ranging from 0.4 to 2 kb were scored manually as present (1) or absent (0) and compiled into a data matrix. As we were studying an Azorean endemic species monomorphic loci were considered for the analysis of genetic parameters as suggested by Ayres and Ryan (1999) and Ellstrand and Elam (1993).
Genetic diversity was measured at individual, population and island levels. At the individual level, a cladogram of all the 233 samples based on the similarity analysis of fingerprint patterns was drawn using the unweighted pair group method arithmetic average (UPGMA) algorithm using Dice's coefficient as implemented in NTSYSpc (Rohlf 1992) and was plotted into a dendrogram.
To learn about the genetic diversity among populations and among islands, we calculated Nei's coefficient (h) (Nei 1972), Shannon's information index (I) (Shannon and Weaver 1949), unbiased Nei's genetic distance (Nei 1978), coefficient of genetic differentiation between populations (GST) (Nei 1973) and gene flow (Nm) (Slatkin and Barton 1989) in the software POPGENE 1.31 (Yeh et al. 1999) with 1000 permutations. Different analysis indexes were used to enhance the significance of the results. The estimates of genetic diversity obtained by Shannon's index (I) and Nei's gene diversity (h) were compared by Pearson's rank coefficient correlation (Sokal and Rohlf 1995) using IBM SPSS statistics 20 software and to compare analysis between marker systems, as these should not be based on the comparison of levels of diversity within populations, but on the comparison of the ranking of different populations by correlation analysis (Mariette et al. 2001).
The number of different alleles (Na) and the number of effective alleles (Ne) (Brown and Weir 1983) were also computed with POPGENE 1.31 (Yeh et al. 1999) to allow comparison of populations with different sample sizes and where the number and distribution of alleles differ (Ojango et al. 2011).
To learn about the partitioning of genetic variabilty among islands, among populations and among individuals (Excoffier et al. 1992; Huff et al. 1993), an analysis of molecular variance (AMOVA) was performed with GenAlEx 6.5 (Peakall and Smouse 2012) by resampling 999 times.
Genetic differentiation among populations (φPT) based on the Euclidean distances (Huff et al. 1993) and among individuals was calculated with GenAlEx 6.5. The former were plotted in a tree using the UPGMA method from the NEIGHBOR module from Phylip 3.695 (Felsenstein 2005) and the latter were used to carry out a principal coordinate analysis (PCoA) in GenAlEx 6.5. The Mantel test computed with GenAlEx 6.5 with 999 replications was used to evaluate correlations between the pairwise Euclidean genetic distance of individuals and their geographical distance, and between pairwise genetic differentiation (φPT) and geographical distances of each population (Huff et al. 1993). This software was also used to estimate the number of migrants (Frankham et al. 2002).
To investigate the genetic structure and the degree of admixture between each sample and between the 11 Azorean populations, the Bayesian clustering procedure of STRUCTURE (Pritchard et al. 2000) was used by running an admixture model with correlated frequencies between populations. A 10 000 initial burn-in was used, followed by 10 000 MCMC iterations as suggested by Evanno et al. (2005) with 10 independent replicates each. The tested number of clusters varied from 1 to 14 (the number of populations plus three). The most likely number of clusters (K) was estimated by using the maximum value of L(K) and by calculating ΔK (Evanno et al. 2005). A mean of the 10 permuted matrices was estimated using CLUMPP (Jakobsson and Rosenberg 2007) and the LargeKGreedy algorithm. The output of the cluster analysis was visualized with DISTRUCT (Rosenberg 2004).
The number of populations of P. azorica (n) which are necessary to represent 99.99 % of the total genetic diversity among populations (P) was calculated according to the modified equation of Ceska et al. (1997): P = 1−(φPT)n.
Results
Genetic diversity
From 230 Azorean individuals distributed across 11 populations, the eight primers used yielded 79 clearly scorable bands, ranging from four at primer UBC836 to 15 at primers UBC807 and UBC842, with a mean value of 10. Among them 64 were polymorphic (81.0 %) (Table 2). At the island level, São Miguel presented the lowest percentage of polymorphic loci (54.4 %) and Pico presented the highest (63.3 %) (Table 3). At the population level, these values ranged from 17.72 % (CL from São Miguel) to 46.84 % (PB from Terceira). The total genetic diversity obtained by Shannon's information index (I) was 0.2456 and by computing Nei's genetic diversity (h) was 0.1503. While the population CL showed the lowest genetic diversity (I = 0.1131, h = 0.0795), the population PB showed the highest (I = 0.2421, h = 0.1619) (Table 3). All three indexes do not show significant differences (P = 0.455, P = 0.360, P = 0.321, respectively) within populations.
Table 3.
Genetic diversity within 11 populations of P. azorica. N, sample size; n, number of polymorphic loci; P%, percentage of polymorphic loci; Na, number of different alleles; Ne, number of effective alleles; I, Shannon's information index; h, Nei's gene diversity; SD, standard deviation.
| Population | N | n | P% | Na (SD) | Ne (SD) | I (SD) | h (SD) |
|---|---|---|---|---|---|---|---|
| S. Miguel | |||||||
| Caloura | 4 | 14 | 17.72 | 1.177 (0.384) | 1.149 (0.333) | 0.1131 (0.2476) | 0.0795 (0.1750) |
| Lombo Gordo | 16 | 28 | 35.44 | 1.354 (0.481) | 1.242 (0.366) | 0.2008 (0.2858) | 0.1370 (0.1991) |
| Ribeira Quente | 37 | 30 | 37.97 | 1.380 (0.488) | 1.206 (0.338) | 0.1810 (0.2660) | 0.1203 (0.1844) |
| Tronqueira | 7 | 23 | 29.11 | 1.291 (0.457) | 1.161 (0.301) | 0.1456 (0.2446) | 0.0959 (0.1673) |
| Total | 64 | 43 | 54.43 | 1.544 (0.501) | 1.225 (0.328) | 0.2173 (0.2525) | 0.1380 (0.1759) |
| Pico | |||||||
| Piedade | 27 | 33 | 41.77 | 1.418 (0.496) | 1.195 (0.315) | 0.1822 (0.2549) | 0.1183 (0.1749) |
| Santo Amaro | 24 | 25 | 31.65 | 1.317 (0.468) | 1.155 (0.273) | 0.1493 (0.2402) | 0.0970 (0.1613) |
| Alto São Roque | 14 | 27 | 34.18 | 1.342 (0.477) | 1.173 (0.295) | 0.1629 (0.2484) | 0.1062 (0.1687) |
| Cais do Mourato – Santana | 37 | 36 | 45.57 | 1.456 (0.501) | 1.215 (0.305) | 0.2066 (0.2595) | 0.1339 (0.1760) |
| Candelária | 17 | 27 | 34.18 | 1.342 (0.477) | 1.184 (0.296) | 0.1720 (0.2548) | 0.1130 (0.1723) |
| Total | 119 | 50 | 63.29 | 1.633 (0.485) | 1.190 (0.267) | 0.2085 (0.2230) | 0.1260 (0.1524) |
| Terceira | |||||||
| Pico do Boi | 19 | 37 | 46.84 | 1.468 (0.502) | 1.275 (0.358) | 0.2421 (0.2848) | 0.1619 (0.1973) |
| Serreta | 28 | 33 | 41.77 | 1.418 (0.496) | 1.242 (0.355) | 0.2113 (0.2775) | 0.1409 (0.1918) |
| Total | 47 | 47 | 59.49 | 1.595 (0.494) | 1.269 (0.340) | 0.2558 (0.2618) | 0.1645 (0.1826) |
| Total (all islands) | 230 | 64 | 81.01 | 1.810 (0.395) | 1.233 (0.300) | 0.2465 (0.2307) | 0.1503 (0.1640) |
Population genetic structure
The phenogram of Picconia spp. (Fig. 1) based on Dice's coefficient showed a high genetic similarity among all P. azorica genotypes with a mean value of 91.2 % and a clear differentiation of this species from P. excelsa from Madeira island with a similarity <76.2 %. In the tree a distribution of the samples with no obvious clustering according to the population and/or island of provenience was observed.
Figure 1.
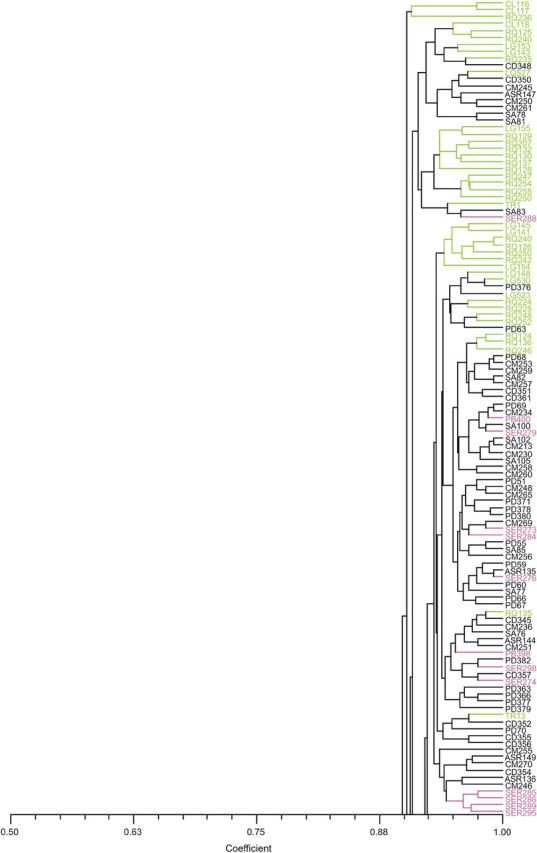
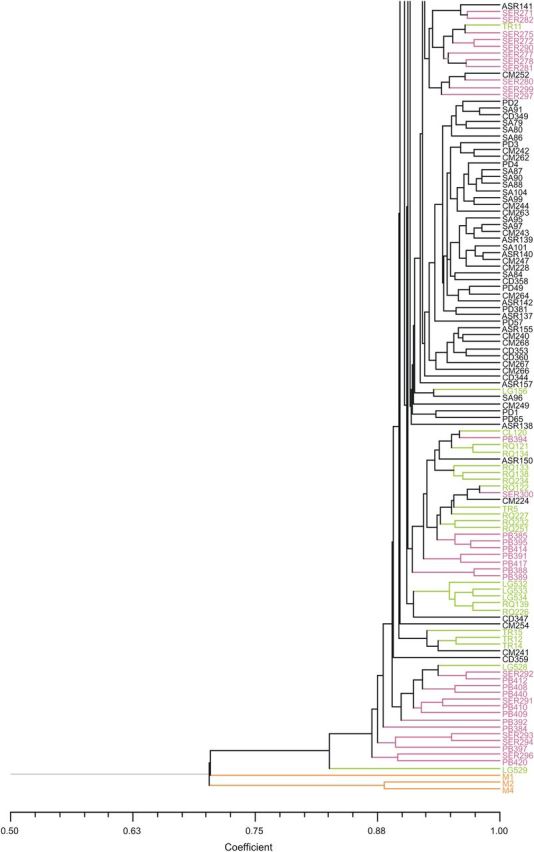
Relationship of 230 P. azorica genotypes from three islands in the Azores: São Miguel (green), Pico (black) and Terceira (pink); and three P. excelsa samples from Madeira island (orange). UPGMA tree based on Dice's coefficient.
The AMOVA analysis that considered the three islands showed that 84 % of the total variation occurred within populations and only 8 and 8 % occurred among populations and among islands respectively (Table 4). Individually for each island, the AMOVA analysis indicated that most of the molecular variation in Terceira exists among individuals within populations (83 %), with lesser amounts among populations (17 %). Higher values of molecular variation among individuals within populations were obtained for São Miguel (89 %) and Pico (96 %).
Table 4.
Analysis of molecular variance (AMOVA) for 11 populations of P. azorica distributed across three Azorean islands.
| Source of variation | d.f. | Sum of squares | Variance components | % total variance |
|---|---|---|---|---|
| Among islands | 2 | 113.316 | 0.535 | 8 |
| Among populations | 8 | 124.546 | 0.517 | 8 |
| Within populations | 219 | 1250.547 | 5.710 | 84 |
Pairwise unbiased Nei's genetic distance (Table 5) showed among populations within Pico values <0.013 and within Terceira populations a genetic distance of 0.034. For São Miguel population pairs CL-RQ, LG-RQ and RQ-TR showed values of genetic distance <0.033 while population pairs CL-LG, CL-TR and LG-TR showed values >0.051.
Table 5.
Genetic differentiation and unbiased Nei's genetic distance among populations of P. azorica. Genetic differentiation (φPT) is listed below the diagonal and unbiased Nei's genetic distance is listed above the diagonal.
| Population | Caloura | Lombo Gordo | Ribeira Quente | Tronqueira | Piedade | Santo Amaro | Alto São Roque | Cais do Mourato | Candelária | Serreta | Terra Brava |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caloura | – | 0.0514 | 0.0255 | 0.0563 | 0.0576 | 0.0838 | 0.0791 | 0.0679 | 0.0611 | 0.0592 | 0.0784 |
| Lombo Gordo | 0.184 | – | 0.0244 | 0.0634 | 0.0339 | 0.0472 | 0.0451 | 0.0366 | 0.0393 | 0.0399 | 0.0621 |
| Ribeira Quente | 0.082 | 0.080 | – | 0.0323 | 0.0256 | 0.0496 | 0.0427 | 0.0332 | 0.0348 | 0.0367 | 0.0487 |
| Tronqueira | 0.190 | 0.236 | 0.157 | – | 0.0271 | 0.033 | 0.0379 | 0.0269 | 0.0305 | 0.0297 | 0.0437 |
| Piedade | 0.281 | 0.119 | 0.158 | 0.155 | – | 0.0134 | 0.0126 | 0.0095 | 0.0086 | 0.0164 | 0.0535 |
| Santo Amaro | 0.339 | 0.177 | 0.226 | 0.169 | 0.068 | – | 0.0073 | 0.0109 | 0.0085 | 0.0246 | 0.0653 |
| Alto São Roque | 0.279 | 0.124 | 0.174 | 0.140 | 0.033 | 0.016 | – | 0.011 | 0.0109 | 0.0178 | 0.0571 |
| Cais do Mourato | 0.296 | 0.132 | 0.179 | 0.144 | 0.036 | 0.049 | 0.000 | – | 0.0097 | 0.0184 | 0.0512 |
| Candelária | 0.247 | 0.138 | 0.151 | 0.085 | 0.045 | 0.035 | 0.022 | 0.038 | – | 0.0179 | 0.0595 |
| Serreta | 0.280 | 0.176 | 0.207 | 0.162 | 0.105 | 0.165 | 0.066 | 0.112 | 0.091 | – | 0.0336 |
| Terra Brava | 0.194 | 0.221 | 0.192 | 0.117 | 0.201 | 0.282 | 0.178 | 0.212 | 0.189 | 0.138 | – |
Among all populations, genetic distance values <0.04 were detected for all population pairs, except for population pairs LG–SA, LG–ASR, RQ–SA and RQ–ASR (0.047, 0.045, 0.050, 0.043, respectively) and for populations CL from São Miguel and PB from Terceira. While population CL showed genetic distances >0.058 for all pairwise comparisons with populations from Pico and Terceira, population PB showed values >0.044 for all pairwise comparisons with populations from São Miguel and Pico.
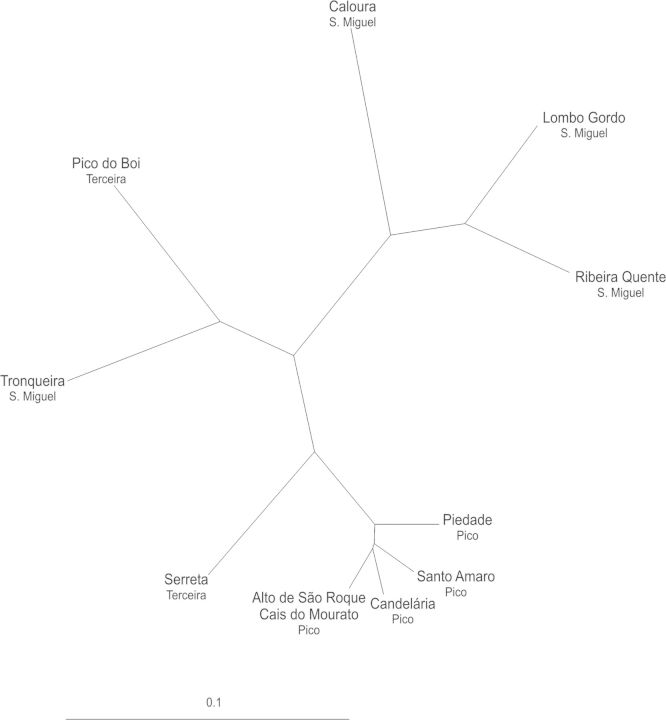
The pairwise values of genetic differentiation (φPT) (Table 5) between populations from São Miguel island varied between 0.080 for population pair RQ–LQ and 0.236 for population pair TR–LG; for Pico island ranged from 0.000 for population pair ASR–CM to 0.068 for population pair SA–PD; and for Terceira island it was 0.138 (PB–SER). Between islands the highest value (0.339) was observed between populations CL from São Miguel and SA from Pico. The determined values of φPT among islands showed levels of genetic differentiation of 0.118 (Pico–Terceira), 0.131 (São Miguel–Terceira) and 0.128 (Pico–São Miguel). The UPGMA dendrogram obtained with φPT pairwise values (Fig. 2) grouped the populations into four main clusters, one corresponding to populations RQ, LG and CL all from São Miguel island, another containing one population from Terceira (PB) and a population from São Miguel (TR), a third one containing population SER from Terceira and a fourth one with all populations from Pico (CM, ASR, CD, SA and PD).
Figure 2.
UPGMA radial tree showing the genetic relationships based on pairwise φPT values among 11 populations of P. azorica, occurring in three islands from the Azores.
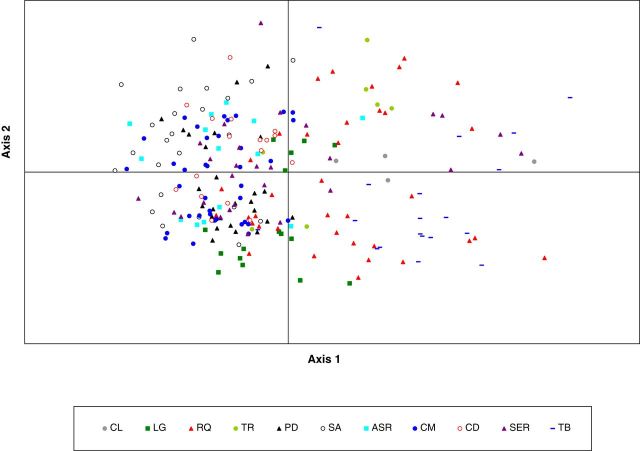
In the PCoA based on the matrix of individual genotypes, only 44.16 % of the total variance was explained by the first two axes (Axis 1 = 23.82 %; Axis 2 = 20.34 %) (Fig. 3) where most of the samples from Pico island cluster together on the left side of the plot reflected the genetic differentiation detected by φPT.
Figure 3.
Two-dimensional representation of the first two axes of the principal component analysis from the matrix of genetic distances of 230 samples from 11 populations. Percentage of variance accumulates on the first two axes = 44.46 % (Axis 1 = 23.82 %; Axis 2 = 20.34 %). For population names see Table 1.
The coefficient of genetic differentiation between populations (GST) was 0.0940 and the level of gene flow (Nm) calculated based on GST was estimated to be 4.818, suggesting a high gene flow between P. azorica populations and islands. The number of migrants calculated as described by Frankham et al. (2002) showed higher values among populations from Pico than among populations from the other two islands. The lowest value obtained among populations from Pico (3.438) was higher than the highest value obtained in the remaining islands (2.859). The estimated number of migrants between São Miguel and Terceira was 1.656, between São Miguel and Pico was 1.705 and between Pico and Terceira was 1.874.
Insignificant correlation between the genetic distance of individuals and geographical distances (r2 = 0.047, P = 0.001) was shown by the Mantel test. For pairwise φPT and geographical distances a slightly positive correlation was obtained (r2 = 0.242, P = 0.005).
The Bayesian analysis carried out in STRUCTURE for all populations demonstrated that there were no predominant genetic clusters. Average mean values of ln P(K) did not show any substantial increases when K varied from 1 to 14 [see Supporting Information]. Similar results were obtained when the analysis was performed separately for each island.
According to the genetic differentiation φPT value (0.17), the conservation of six populations is the minimum necessary in order to preserve 99.99 % of the total diversity of the 11 analysed populations.
Discussion
Inter-simple sequence repeat markers have been used in population genetic studies of plant species as they effectively detect very low levels of genetic variation (Zietkiewicz et al. 1994). They are also potentially useful for analysing biogeographical patterns among populations within species (Bussell et al. 2004). With these purposes eight ISSR markers were successfully used to study the Azorean endemic plant species P. azorica, using 230 samples collected on populations across the Azorean islands of São Miguel, Terceira and Pico so as to provide knowledge of the levels and distribution of genetic diversity within these islands to help in designing conservation strategies for this endemic species.
The number of scored bands and the percentage of polymorphic loci obtained in this study can be considered enough for estimating genetic diversity within populations. Nybom (2004) who conducted an analysis on a compilation of different DNA markers for estimating intraspecific genetic diversity in plants calculated an average of 54.9 polymorphic markers per study. The values of genetic diversity for all populations obtained by Shannon's index (I) and Nei's gene diversity (h) were higher for the former but highly correlated (r2 = 0.010; P = 0.988). The estimated total diversity of I = 0.2465, h = 0.1503 was not expected as endemic and narrowly distributed plants usually show lower levels of genetic diversity and higher levels of genetic structure compared with their relatives with wider distribution areas (Hamrick and Godt 1989; Nybom 2004). The fact that the species is wind pollinated and zoochory (Arteaga et al. 2006; Dias et al. 2007) together with its long life cycle may explain the detected level of diversity. Long-lived species generally have a higher potential for long-range gene movement (Nybom and Bartish 2000). Similar results were obtained for this species when other populations were characterized with SSR molecular markers (Martins et al. 2013), which showed a total genetic diversity (HT) of 0.7. It is not surprising that Martins et al. (2013) obtained such a high level of total genetic diversity, as one of the SSR markers used detected a total of 31 alleles in 443 samples, indicating the presence of alleles at very low frequencies. Also other species from the Oleae complex, Olea europaea ssp. cerasiformis and O. europaea ssp. Guanchica living in insular habitats, in Madeira and the Canary Islands, respectively, also showed relatively high levels of total genetic diversity (García-Verdugo et al. 2009). Nevertheless, care should be taken when comparing data between studies as genetic diversity depends on numerous factors, namely life history, breeding system, growth life forms, geographical range and type of molecular method used (Powell et al. 1996; Nybom 2004). For example, the molecular markers used by Ferreira et al. (2011) are usually applied to identify genetic variances among different taxon and therefore limited intra-specific genetic diversity, and the absence of genetic structure was detected. Also Martins et al. (2013) analysed a wider geographical range by studying populations from eight islands and the number of samples per populations varied considerably. According to Nybom (2004), the number of plants per population has a positive effect on gene diversity values, probably because larger sample sizes may increase data quality.
The total genetic diversity was similar across islands, being slightly higher for Terceira, which also showed the higher genetic differentiation among populations due to the uneven distribution of alleles. Among all populations, the detected genetic diversity is evenly distributed with values higher for CM (I = 0.207, h = 0.134) from Pico, and lower for CL (I = 0.113, h = 0.080) and TR (I = 0.146, h = 0.096) from São Miguel. Population CM is one of the largest in terms of individuals and area of distribution; population CL is a very small isolated population highly disturbed by human activities; and TR is composed of a few dispersed individuals in a preserved natural forest of high altitude the conditions of which are considered to be out of the optimum range for the species (Schaefer 2003). When using the Pearson correlation analysis, we did find correlation (P = 0.05) between population size (log transformed) and genetic diversity when all populations were analysed, but when the smallest population (CL) was removed from the analysis correlations were not detected. Therefore, the diversity index from this population can be influenced by its reduced size (N = 4). The same analysis performed between islands and considering all populations did not find any correlation between population size and genetic diversity. Therefore, we have no indication that habitat fragmentation resulted in a pronounced loss of genetic diversity within P. azorica populations. If populations are small and isolated from one another with increased habitat fragmentation, the genetic flow could be capable of influencing the genetic structure and decreasing differentiation among populations (Ellstrand and Elam 1993). Our gene diversity data are strongly correlated (P = 0.023; r2 = 0.859) with the data obtained with SSR markers (Martins et al. 2013) for five of the common populations analysed. Population Santo Amaro (n = 24) from our study which corresponds to population Praínha (n = 2) from Martins et al. (2013) was not included in this analysis due to the discrepant number of samples analysed. The reasons for the highest diversity observed in PB population from Terceira, although occurring at high altitude, could be due to the difficulty in access and therefore less or no human disturbances. These factors have been described as the most suitable for successful long-distance dispersal in Macaronesia (Vargas 2007), and could have minimized human disturbances. Also the phenogram based on Dice's coefficient showed a high genetic similarity among all P. azorica genotypes with no obvious clustering according to population and/or island. The only clear differentiation is between P. azorica from the Azores archipelagos and P. excelsa from Madeira island.
For the endemic P. azorica, the results of AMOVA revealed that for each island most of the genetic diversity was found within populations (83 % for Terceira, 88 % for São Miguel and 96 % for Pico), a trend commonly reported in outcrossing and/or perennial species (Hamrick et al. 1992). Within and among islands, a low level of genetic differentiation was detected. Similar patterns of low differentiation among populations have also been reported in other insular trees of Macaronesia as Morella faya and Morella rivas-martinezzi (González-Pérez et al. 2009), where most of the genetic variability was found within populations (92 and 86 %, respectively). The genetic differentiation detected is a result of the high gene flow between populations and islands, corroborated by the number of migrants calculated, indicating that geographical distance was not found to be responsible for the reduction in gene flow between the different locations. Also levels of gene flow above one migrant between populations per generation were determined by Martins et al. (2013). Moreover, the lower value of total genetic diversity detected for Pico island reflects the continuous distribution of plants (Wright 1949) and a possible combination of founder and bottleneck effects, as Pico is the youngest island from Azores, and is caused by human interference that this species experienced.
The Mantel test failed to reveal isolation by distance, as no genetic structure was observed for the species. Also the Bayesian approach implemented in STRUCTURE corroborates these results, indicating that geographical distances that separate the central and eastern islands do not seem to have acted as barriers preventing gene flow (maximum interisland distance roughly 246 km). A possible explanation could be that several bird species, including the two Azorean endemic bird taxa Columba palumbus azorica and Pyrrhula murina, feed on its fruits (Dias et al. 2007) and as a result may have given an important contribution to P. azorica dissemination within and among islands, although the contribution of P. murina can be considered limited since it only exists in São Miguel. Regarding the results obtained here, it may also be considered that wind flow could provide further opportunities for long-distance dispersal of pollen.
Due to the over-exploitation of P. azorica and the habitat degradation that led to its extinction in Graciosa island, this species has been considered as a priority Azorean endemic species for conservation and measures for restoration of depleted natural populations should be taken. Moreover, P. azorica is part of the habitat of the second most threatened bird in Europe—the Azores bullfinch (P. murina) protected under the Birds directive (Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the conservation of wild birds) and therefore knowledge about P. azorica can indirectly contribute to the preservation of other species. However in order to guarantee sustainable survival of populations and to preserve their evolutionary potential, knowledge of the levels of genetic diversity and their distribution is important for designing conservation strategies for threatened and endangered species (Hamrick and Godt 1989; Francisco-Ortega et al. 2000). Population establishment and long-term persistence as well as long-term evolutionary potential of restored populations is ensured by within-population genetic diversity (McKay et al. 2005), as loss of genetic diversity can lead to a decrease in the species' ability to survive environmental changes and demographical fluctuations both in short and in long term (Ellstrand and Elam 1993). Although the conservation of six populations is considered the minimum necessary in order to preserve 99.99 % of the total diversity of the analysed populations, management should aim to conserve as many of the small populations as possible. Concentrating conservation efforts only on the few large populations would result in the likelihood of loss of genetic variability for the species.
For long term, the most suitable strategy for the conservation of P. azorica is the protection and restoration of its habitat. Also artificial propagation of the species for timber use should be considered as the best preservation guaranteeing its ex situ conservation and sustainable survival, thus enhancing the in situ conservation. This could be sustainably achieved by propagation of seedlings and vegetative micropropagation of cuttings (D. Mendonâça et al., submitted) and the reintroduction of the micropropagated plants into their populations of origin. Its valuable wood has been used in former times for the construction of toys, agricultural tools, furniture and religious statuary, and recent studies on its technological features support its use (Caetano-Ferreira et al. 2012). The establishment of a breeding programme for wood quality together with plant tissue culture techniques and micropropagation would for sure avoid the destruction of the small populations and the extinction of the species, fostering its use.
Conclusions
Population genetic analysis with ISSR markers in the endangered endemic species P. azorica detected a high within-population genetic diversity but low genetic differentiation between populations and between islands which can be explained by its life history traits, dispersal and gene flow. Neither isolation of some habitats nor population size affected genetic variability within the studied populations. The obtained data are important for establishing guidelines and priorities for germplasm and genetic diversity conservation, with in situ and ex situ conservation, together with renewed economic interest for its utilization, following reforestation programmes, representing good opportunities for conservation of this tree species.
Sources of Funding
This project was supported by the Azorean Government (Secretary for Natural Resources). LA IBB-CBA is supported by the Portuguese Foundation for Science and Technology (FCT, PEst-OE/EQB/LA0023/2013) and the Azorean Regional Science Fund (FRC). The authors thank A. Arraiol for providing samples of Picconia excelsa from Madeira Island. The following authors were supported by FRC: M.S.L. (M3.1.7/F/023/2011), D.M. (M3.1.7/F/010A/2009), S.X.B. (M3.1.7/F/026/2011) and A.R.B. (M3.1.2/F/039/2011), and by FCT: C.M. (SFRH/BPD/78059/2011).
Contributions by the Authors
A.C.M. conceived the experiment. D.M., S.X.B. and C.M. collected the samples. M.S.L., A.R.B., C.M. and C.B. extracted the DNA. M.S.L. and A.R.B. performed the PCR and data analysis together with D.M. and S.X.B. M.S.L., D.M., S.X.B., A.R.B. and A.C.M. wrote the manuscript. All authors read the manuscript and agreed to submit it.
Conflicts of Interest Statement
None declared.
Supporting Information
The following Supporting Information is available in the online version of this article –
Figure S1. Location of the samples collected from São Miguel Island.
Figure S2. Location of the samples collected from Pico Island.
Figure S3. Location of the samples collected from Terceira Island.
Figure S4. Bayesian clustering performed by STRUCTURE for a set of 230 P. azorica genotypes from 11 different populations. (A) ΔK calculated as ΔK = m|L″(K)|/s[L(K)]; (B) ln Pr(G|K) values presented as a function of the number of clusters; (C) graphical presentations of different samples. Each sample is represented by a single vertical line broken into K colour segments, with lengths proportional to the estimated membership of the inferred cluster. Individuals are grouped into populations. For populations names see Table 1.
Acknowledgements
The authors thank A. Arraiol for providing samples of Picconia excelsa from Madeira Island.
Literature Cited
- Arteaga MA, González G, Delgado JD, Arévalo JR, Fernández-Palacios JM. Offspring spatial patterns in Picconia excelsa (Oleaceae) in the Canarian laurel forest. Flora. 2006;201:642–651. [Google Scholar]
- Ayres DR, Ryan FJ. Genetic diversity and structure of the narrow endemic Wyethia reticulate and its congener W. bolanderi (Asteraceae) using RAPD and allozyme techniques. American Journal of Botany. 1999;86:344–353. [PubMed] [Google Scholar]
- Bahulikar RA, Lagu MD, Kulkarni BG, Pandit SS, Suresh HS, Rao MKV, Ranjekar PK, Gupta VS. Genetic diversity among spatially isolated populations of Euryanitida Korth (Theaceae) based on inter-simple sequence repeats. Current Science. 2004;86:824–831. [Google Scholar]
- Borges PAV, Cunha R, Gabriel R, Martins AF, Silva L, Vieira V, editors. 2005. A list of terrestrial fauna (Mollusca and Arthropoda) and flora (Bryophyta, Pteridophyta and Spermattophyta) from the Azores. Direcção Regional do Ambiente and Universidade dos Açores, Horta, Angra do Heroísmo and Ponta Delgada.
- Bornet B, Branchard M. Nonanchored inter simple sequence repeat (ISSR) markers: reproducible and specific tools for genome fingerprinting. Plant Molecular Biology Reporter. 2001;19:209–215. [Google Scholar]
- Bornet B, Muller C, Paulus F, Branchard M. High informative nature of inter simple sequence repeat (ISSR) sequences amplified with tri- and tetra-nucleotide primers from cauliflower (Brassica oleracea var. botrytis L.) DNA. Genome. 2002;45:890–896. doi: 10.1139/g02-061. [DOI] [PubMed] [Google Scholar]
- Bornet B, Antoine E, Bardouil M, Baut CM-L. ISSR as new markers for genetic characterization and evaluation of relationships among phytoplankton. Journal of Applied Phycology. 2004;16:285–290. [Google Scholar]
- Brown AHD, Weir BS. Measuring genetic variability in plant populations. In: Tanksley SD, Orton TJ, editors. Isozymes in plant genetics and breeding, Part A. Amsterdam: Elsevier Science Publishers; 1983. pp. 219–239. [Google Scholar]
- Bussell JD, Waycott M, Chappill JA. Arbitrarily amplified DNA markers as characters for phylogenetic inference. Perspectives in Plant Ecology, Evolution and Systematics. 2004;7:3–26. [Google Scholar]
- Caetano-Ferreira R, Lo Monaco A, Picchio R, Schirone A, Vessella F, Schirone B. Wood anatomy and technological properties of an endangered species: Picconia azorica (Oleaceae) IAWA Journal. 2012;33:375–390. [Google Scholar]
- Cardoso PB, Borges PAV, Costa AC, Cunha RT, Gabriel R, Frias Martins AM, Silva L, Homem N, Martins M, Rodrigues P, Martins M, Mendonça E. A perspectiva arquipelágica; Açores. In: Martín JL, Arechavaleta M, Borges PAV, Faria B, editors. As cem espécies ameaçadas prioritárias em termos de gestão na região europeia biogeográfica da Macaronésia. Canarias, Spain: Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias; 2008. pp. 421–450. [Google Scholar]
- Ceska JF, Affolter JM, Hamrick JL. Developing a sampling strategy for Baptisia arachnifera based on allozyme diversity. Conservation Biology. 1997;11:1133–1139. [Google Scholar]
- Chen S, Xia T, Chen S, Zhou Y. RAPD profiling in detecting genetic variation in endemic Coelonema (Brassicaceae) of Quinghai–Tibet plateau of China. Biochemical Genetics. 2004;43:189–201. doi: 10.1007/s10528-005-1511-4. [DOI] [PubMed] [Google Scholar]
- Council of Europe. 1993. Convention on the Conservation of European Wildlife and Natural Habitats, Annex I: Strictly Protected Flora Species. Council of Europe, Strasbourg, France.
- Delgado P, Piñero D, Chaos A, Perez NN, Alvarez-Buylla ER. High population differentiation and genetic variation in the endangered Mexican pine, Pinus rzedowskii (Pinaceae) American Journal of Botany. 1999;86:669–676. [PubMed] [Google Scholar]
- Dias E, Araújo C, Mendes JF, Elias RB, Mendes C, Melo C. Espécies florestais das ilhas - Açores. In: Silva JS, editor. Árvores e florestas de Portugal. Público, Comunicação Social, SA/ Fundação Luso-Americana/ Liga para a Protecção da Natureza; 2007. pp. 199–254. [Google Scholar]
- Ellstrand NC, Elam DR. Population genetic consequences of small population size: implications for plant conservation. Annual Review of Ecology and Systematics. 1993;24:217–242. [Google Scholar]
- Eriksson G. Conservation of noble hardwoods in Europe. Canadian Journal of Forest Research. 2001;31:577–587. [Google Scholar]
- European Commission. 1992. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and on Wild Fauna and Flora. European Commission, Brussels. Annexes II and IV.
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction sites. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri A, Hormaza JI, Polito VS. Random amplified polymorphic DNA analysis of olive (Olea europaea L.) cultivars. Journal of the American Society for Horticultural Science. 1995;120:538–542. [Google Scholar]
- Felsenstein J. 2005. PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle.
- Ferreira M, Eriksson G. A programme for the management of forest tree genetic resources in the Azores islands. Silva Lusitana. 2006;14:59–73. [Google Scholar]
- Ferreira RC, Piredda R, Bagnoli F, Bellarosa R, Attimonelli M, Fineschi S, Schirone B, Simeone MC. Phyleogeography and perspectives of an endangered Macaronesian endemic: Picconia azorica (Tutin) Knobl. (Oleaceae) European Journal of Forest Research. 2011;130:181–195. [Google Scholar]
- Francisco-Ortega J, Santos-Guerra A, Kim SC, Crawford DJ. Plant genetic diversity in the Canaries: a conservation perspective. American Journal of Botany. 2000;87:909–919. [PubMed] [Google Scholar]
- Frankham R. Genetics and conservation biology. Comptes Rendus Biologies. 2003;326:22–29. doi: 10.1016/s1631-0691(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Frankham R, Ballou JD, Briscoe DA. Introduction to conservation genetics. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Frutuoso G. Saudades da Terra. Ponta Delgada: Azores; 1583. [Google Scholar]
- García-Verdugo C, Fay MF, Granado-Yela C, Rubio de Casas R, Balaguer L, Besnard G, Vargas P. Genetic diversity and differentiation processes in the ploidy series of Olea europaea L.: a multiscale approach from subspecies to insular populations. Molecular Ecology. 2009;18:454–467. doi: 10.1111/j.1365-294X.2008.04027.x. [DOI] [PubMed] [Google Scholar]
- González-Pérez MA, Sosa PA, Rivero E, González-González EA, Naranjo A. Molecular markers reveal no genetic differentiation Myrica rivas-martinezzi and M. faya (Myricaceae) Annals of Botany. 2009;103:79–86. doi: 10.1093/aob/mcn222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasmi F, Elfalleh W, Hannachi H, Fères K, Touil L, Marzougui N, Triki T, Ferchichi A. The use of ISSR and RAPD markers for genetic diversity among South Tunisian Barley. ISRN Agronomy. 2012 Article ID 952196. [Google Scholar]
- Hamrick JL, Godt MJW. Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahler AL, Weir BS, editors. Plant population genetics, breeding and genetic resources. Sunderland, MA: Sinauer Associates; 1989. pp. 43–63. [Google Scholar]
- Hamrick JL, Godt MJW, Sherman-Broyles S. Factor influencing levels of genetic diversity in woody plant species. New Forests. 1992;6:95–124. [Google Scholar]
- Huff DR, Peakall R, Smouse PE. RAPD variation within and among natural populations of outcrossing buffalograss Buchloe dactyloides (Nutt) Engelm. Theoretical and Applied Genetics. 1993;86:927–934. doi: 10.1007/BF00211043. [DOI] [PubMed] [Google Scholar]
- IUCN. 2013. IUCN Red list of threatened species. Version 2013.2 http://www.iucnredlist.org. (13 January 2014)
- Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Jamieson IG. Has the debate over genetics and extinction of island endemics truly been resolved? Animal Conservation. 2007;10:139–144. [Google Scholar]
- Juan C, Emerson BC, Oromí P, Hewitt GM. Colonization and diversification: towards a phylogeographic synthesis for the Canary Islands. Trends in Ecology and Evolution. 2000;15:104–109. doi: 10.1016/s0169-5347(99)01776-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim Y, Kim W. Lack of allozyme and ISSR variation in the rare endemic tree species, Berchemiaberchemiaefolia (Rhammnaceae) in Korea. Annals of Forest Science. 2003;60:357–360. [Google Scholar]
- Li A, Ge S. Genetic variation and clonal diversity of Psammochloa villosa (Poaceae) detected by ISSR markers. Annals of Botany. 2001;87:585–590. [Google Scholar]
- Mariette S, Chagné D, Lézier C, Pastuszka P, Raffin A, Plomion C, Kremer A. Genetic diversity within and among Pinus pinaster populations: comparison between AFLP and microsatellite markers. Heredity. 2001;86:469–479. doi: 10.1046/j.1365-2540.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- Martín JL, Arechavaleta M, Borges PAV, Faria B. A perspectiva macaronésica. In: Martín JL, Borges PAV, Faria B, editors. Top 100-As cem espécies ameaçadas prioritárias em termos de gestão da região europeia biogeográfica da Macaronésia. Canarias, Spain: Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canárias; 2008. pp. 389–420. [Google Scholar]
- Martins J, Moreira O, Silva L, Moura M. Vegetative propagation of the endangered Azorean tree, Picconia azorica. Arquipelago Life and Marine Sciences. 2011;28:39–46. [Google Scholar]
- Martins JM, Moreira OCB, Sardos J, Maciel MGB, Silva L, Moura MMT. Population genetics and conservation of the Azorean tree Picconia azorica. Biochemical Systematics and Ecology. 2013;49:135–143. [Google Scholar]
- McKay JK, Christian CE, Harrison S, Rice KJ. ‘How local is local?’—A review of practical and conceptual issues in the genetics of restoration. Restoration Ecology. 2005;13:432–440. [Google Scholar]
- Neel MC, Ellstrand NC. Conservation of genetic diversity in the endangered plant Eriogonum ovalifolium var. vineum (Polygonaceae) Conservation Genetics. 2003;4:337–352. [Google Scholar]
- Nei M. Genetic distance between populations. The American Naturalist. 1972;106:283–292. [Google Scholar]
- Nei M. Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences of the USA. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978;89:583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC, Allnutt TR, Gillies ACM, Lowe AJ, Ennos RA. Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends in Ecology and Evolution. 1999;14:140–145. doi: 10.1016/s0169-5347(98)01555-9. [DOI] [PubMed] [Google Scholar]
- Nybom H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Molecular Ecology. 2004;13:1143–1155. doi: 10.1111/j.1365-294X.2004.02141.x. [DOI] [PubMed] [Google Scholar]
- Nybom H, Bartish IV. Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:93–114. [Google Scholar]
- Ojango JM, Mpofu N, Marshall K, Andersson-Eklund L. Quantitative methods to improve the understanding and utilisation of animal genetic resources. In: Ojango JM, Malmfors B, Okeyo AM, editors. Animal genetics training resource, version 3, 2011. Nairobi, Kenya: 2011. Uppsala, Sweden: Swedish University of Agricultural Sciences. [Google Scholar]
- Palacios C, González-Candelas P. Analysis of population genetic structure and variability using RAPD markers in the endemic and endangered Limonium dufourii (Plumbaginaceae) Molecular Ecology. 1997;6:1107–1121. doi: 10.1046/j.1365-294x.1997.00283.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse P. GenAlEX 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Molecular Breeding. 1996;2:225–238. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlf FJ. Numerical taxonomy and multivariable analysis system (Version 2.1) New York: Applied Biostatistics Inc; 1992. [Google Scholar]
- Rosenberg NA. DISTRUCT: a program for the graphical display of population structure. Molecular Ecology Notes. 2004;4:137–138. [Google Scholar]
- Rossetto M, Jezierski G, Hopper SD, Dixon KW. Conservation genetics and clonality in two critically endangered eucalypts from the highly endemic south-western Australian flora. Biological Conservation. 1999;88:321–331. [Google Scholar]
- Schaefer H. Chorology and diversity of the Azorean flora. Dissertationes Botanicae. 2003;374:1–130. [Google Scholar]
- Schaefer H. Endemic vascular plants of the Azores: an updated list. Hoppea. 2005;66:275–283. [Google Scholar]
- Segarra-Moragues JG, Palop-Esteban M, González-Candelas F, Catán P. On the verge of extinction: genetics of the critically endangered Iberian plant species, Bordereachouardii (Discoreaceae) and implications for conservation management. Molecular Ecology. 2005;14:969–982. doi: 10.1111/j.1365-294X.2005.02482.x. [DOI] [PubMed] [Google Scholar]
- Shannon CE, Weaver W. The mathematical theory of communication. Urbana: University of Illinois Press; 1949. [Google Scholar]
- Silva L, Moura M, Schäefer H, Rumsey F, Dias EF. List of vascular plants (Tracheobionta) In: Borges PAV, Costa A, Cunha R, Gabriel R, Gonçalves V, Martins AF, Melo I, Parente M, Raposeiro P, Rodrigues P, Santos RS, Silva L, Vieira P, Vieira P, editors. A list of the terrestrial and marine biota from the Azores. Cascais: Princípia; 2010. pp. 117–163. [Google Scholar]
- Silva L, Elias RB, Moura M, Meimberg H, Dias E. Genetic variability and differentiation among populations of the Azorean endemic gymnosperm Juniperus brevifolia: baseline information for a conservation and restoration perspective. Biochemical Genetics. 2011;49:715–734. doi: 10.1007/s10528-011-9445-5. [DOI] [PubMed] [Google Scholar]
- Slatkin M, Barton NH. A comparison of three indirect methods for estimating average levels of gene flow. Evolution. 1989;43:1349–1368. doi: 10.1111/j.1558-5646.1989.tb02587.x. [DOI] [PubMed] [Google Scholar]
- Sokal R, Rohlf FJ. Biometry: the principles and practice of statistics in biological research. New York: W.H. Freeman and Company; 1995. [Google Scholar]
- Torres E, Iriondo JM, Pérez C. Genetic structure of an endangered plant, Antirrinum microphyllum (Scrophulariaceae): alloenzyme and RAPD analysis. American Journal of Botany. 2003;90:85–92. doi: 10.3732/ajb.90.1.85. [DOI] [PubMed] [Google Scholar]
- Vargas P. Are Macaronesian islands refugia of relict plant lineages? A molecular survey. In: Weiss SJ, Ferrand N, editors. Phyleogeography in southern European refugia: evolutionary perspectives on the origins and conservation of European biodiversity. Berlin: Springer; 2007. pp. 297–314. [Google Scholar]
- Wright S. The genetical structure of populations. Annals of Eugenics. 1949;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Yang RC, Boyle T. 1999. POPGENE VERSION 1.31: Microsoft Window-based free Software for Population Genetic Analysis, 1999 ftp://ftp.microsoft.com/Softlib/HPGL.EXE .
- Zietkiewicz E, Rafalski A, Labuda D. Genome fingerprinting by simple sequence repeat (SSR) anchored polymerase chain reaction amplification. Genomics. 1994;20:176–183. doi: 10.1006/geno.1994.1151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.