Abstract
Post-translational modifications alter protein structure, affecting activity, stability, localization and/or binding partners. Antibodies that specifically recognize post-translationally modified proteins have a number of uses including immuno-cytochemistry and immuno-precipitation of the modified protein to purify protein-protein and protein-nucleic acid complexes. However, antibodies directed at modified sites on individual proteins are often non-specific. Here we describe a protocol to purify polyclonal antibodies that specifically detect the modified protein of interest. The approach uses iterative rounds of subtraction and affinity purification, using stringent washes to remove antibodies that recognize the unmodified protein and low sequence complexity epitopes containing the modified amino acid. Dot and western blots assays are employed to assess antibody preparation specificity. The approach is designed to overcome the common occurrence that a single round of subtraction and affinity purification is not sufficient to obtain a modified protein specific antibody preparation. One full round of antibody purification and specificity testing takes 6 days of discontinuous time.
Keywords: phospho-specific polyclonal antibodies, modification-specific polyclonal antibodies, iterative rounds of subtraction/affinity purification, phospho-CGH-1
Introduction
Covalent modification of amino acids in proteins is a post-translation mechanism that alters the proteome, changing the structure of individual proteins and thus potentially affecting their activity, stability, localization and/or binding partners1-18. In response to changing conditions during development or in the environment, post-translational protein modification can much more rapidly alter the activity of the proteome compared to transcriptional or translational control mechanisms4,14,19-21. While numerous protein modifications are known, for example histone methylation and acetylation, the most widely studied modification is phosphorylation. Protein kinase mediated phosphorylation of substrates is the output of a number of signaling pathways (e.g. Ras-ERK MAP Kinase signaling13,15) and is often part of the molecular mechanism for execution of the signaling cascade and its modulation (e.g. Wnt signaling)22. Mass spectrometry has revolutionized our ability to identify post-translationally modified proteins, through the identification of the modified residue and the surrounding amino acid sequence in which it is embedded16,23-26. Once a modification site has been identified, antibodies can be generated that specifically recognize the modified protein (modified residue plus surrounding amino acids)27,28. Such modified-protein-specific antibodies can be used in a wide range of in vivo studies of the modified protein, some of which are not easily performed by other approaches, such as mass spectrometry27,28. The modified-protein-specific antibodies allow immuno-cytochemical analysis of the modified-protein at high spatial and temporal resolution. For example in complex tissues, the modified-protein-specific antibodies can identify individual cells or cell types that do or do not contain the modified-protein27,29-33 and, at the single cell level, identify individual organelles or subcellular structures that contain the modified-protein32-40. Modified-protein-specific antibodies can be used in high-throughput RNAi screens41 or to test hypotheses related to control of a signaling pathway or utilization of the phosphorylated protein42-45. Importantly, modified-protein-specific antibodies can be used to purify protein-protein, protein-DNA or protein-RNA complexes that contain the modified protein; a widely employed example is the use of antibodies specific to modified histones for analysis of genomic regions that contain the modified histone in chromatin4,14,20,24,46,47.
These approaches require that the antibody is specific to the modified protein being investigated. However modification-specific antibodies are not straightforward to generate and widely-used antibodies can be non-specific, recognizing both the modified and unmodified forms of the target protein or identifying other proteins that contain the modified residue. The modENCODE project, designed to identify the distribution of histone-modifications genome-wide using Chromatin Immunoprecipitation (ChIP) analysis, found that nearly 50 of the 200 commonly available antibodies were either not specific to the histone modification or showed reactivity to non-histone proteins; for example, in the case of regularly used antibodies to H3pS10, preparations were found to cross-react with the unmodified form45. Unknown are the number of attempts by individual laboratories to generate/purify antibodies specific to a modified-protein that failed. Thus, there is a need to develop and refine methods that yield very high quality, specific antibodies for detection of post-translational modifications in vivo. Here we present a protocol that allows purification of highly specific polyclonal antibodies that detect post-translationally modified proteins, which can then be used in multiple distinct assays including immuno-cytochemistry, western blotting, and immune-precipitation.
Development of the protocol
Development of the strategy and protocol grew out of our study on substrates of ERK MAP Kinase that function in C. elegans germ cell biology. We identified candidate substrates using a three-part approach: (1) bioinformatic identification of evolutionarily conserved proteins that contain ERK docking sites; (2) an RNAi screen for enhancement of either a weak loss-of-function mutation in ERK or a weak gain-of-function mutation in Ras; and (3) quantitative in vitro tests of phosphorylation of the candidate proteins by activated EKR228. While this approach identified high-likelihood substrates, it remained to be demonstrated that the gene products were phosphorylated in vivo by ERK. Therefore we turned to the generation of phospho-protein-specific antibodies to the candidate substrates. We used site-directed mutagenesis to map the serine (S) or threonine (T) residue, N-terminal to proline (P), which is typically used as the phospho-acceptor for ERK2, in an in vitro kinase assay. We then generated antibodies to a peptide that contained the phosphorylated S/T and surrounding amino acids that were unique to the substrate. We purified phospho-protein-specific antibodies to candidate substrates DDX-19 and GSK3β and used the antibodies to show that these gene products are phosphorylated in vivo at the identified sites and that the phosphorylation was dependent on ERK activity, as it failed to occur in a null mutant of mpk-1, the C. elegans ERK ortholog27.
The utility of phospho-protein specific antibodies, as well as non-phospho specific antibodies, in studies of modified protein function is illustrated with NOS-3 in Figure 1. NOS-3, a Nanos-related RNA binding protein, was identified as an MPK-1 ERK substrate where phosphorylation disrupts binding to Pumilio-related co-factors FBF-1/-2 leading to relief of translational repression of the fem-3 mRNA27. To understand spatial control of MPK-1 ERK mediated regulation of fem-3 translation we generated and purified antibodies specific for phosphorylated NOS-3 (pNOS-3) and non-phosphorylated NOS-3 (non-pNOS-3) proteins. We used western blotting of C. elegans lysates (Figure 1A) to demonstrate that the anti-pNOS-3 antibody preparation was specific as it showed a single band in wild-type but no staining in extracts from nos-3 null mutant worms (specificity for the NOS-3 protein) and no staining in extracts from mpk-1 ERK null mutant worms (specificity of the modified site for the kinase). Similarly we demonstrated that the anti-non-pNOS-3 antibody (Figure 1B) was specific as it produced a single band in wild-type but no staining in extracts from nos-3 null mutant worms (specificity for NOS-3) while a single band was observed in mpk-1 null (specificity to the non-phosphorylated form of NOS-3). Using immuno-cytochemical staining of the germline tissue with the phospho-specific NOS-3 antibodies we found that only cells containing activated MPK-1 ERK have phosphorylated NOS-3 (Figure 1C) and that phosphorylation was genetically dependent on MPK-127. Antibody staining demonstrates that non-phospho-NOS-3 is present only in the region of the germline tissue that lacks activated MPK-1 ERK (Figure 1D), that phospho- and non-phospho-NOS-3 are present in a mutually exclusive accumulation patterns in the germline tissue and that the sum of the two patterns is identical to the staining observed for total NOS-3 protein26. These results indicate that in cells with activated MPK-1 ERK, there is essentially complete conversion of NOS-3 to the phosphorylated form that no longer binds to co-regulators FBF-1/-2, fully relieving fem-3 translational repression.
Figure 1. Spatial control of NOS-3 phosphorylation in the C. elegans germline tissue.

Phospho- and non-phospho- specific antibodies provide unique reagents that allow analysis of the spatial distribution of protein modifications that would be difficult or impossible to obtain by other methods. The specificity of purified anti-pNOS-3 and purified anti-non-pNOS-3 antibody preparations are shown in A & B, respectively, which are western blots of total lysates from adult wild-type (WT), nos-3 null mutant (nos-3(0)) and mpk-1 ERK null mutant (mpk-1(0)) worms. Purified anti-pNOS-3 antibody, raised against the peptide shown in Table 2, identifies a single protein species in wild-type that is absent in nos-3(0) and in mpk-1(0), which encodes the kinase that generates the modified sites. Purified anti-non-pNOS-3 antibody, raised against the peptide in Table 2 without the phospho-residues, identifies a single band in wild type and in the absence of the kinase (mpk-1(0)) but fails to identify a protein species in nos-3(0). Tubulin western blot sample loading control, bottom (A) and (B). Immuno-cytochemical staining of wild-type adult hermaphrodite germlines show that purified anti-pNOS-3 antibody staining (green in C) is found in proximal germ cells that also contain activated MPK-1 ERK (red, dpMPK-1, in C) while purified anti-non-pNOS-3 antibody staining (green in D) is found in a mutually exclusive population of germ cells that lack activated MPK-1 (red, dpMPK-1, in D). The sum of the pNOS-3 and non-pNOS-3 signal is equivalent to total NOS-3 staining27. Adapted with permission from ref. 27.
In this protocol, we describe the generation of phospho-specific antibodies to the RNA helicase CGH-1, which is orthologous to yeast Dhh1p and human DDX6, to illustrate a number of points in the description of the antibody purification protocol and specificity testing. Interestingly, phosphorylation of CGH-1 causes a change in subcellular localization of the protein, which is detected by immuno-cytochemical staining with the phospho-specific antibodies (see below).
Overview of the workflow
The protocol describes the generation of polyclonal phospho-protein-specific antibodies, where we have significant experience with sites phosphorylated by proline directed S/T kinases (p(S/T)P site) such as MAP Kinases. The protocol can also be applied to purifying polyclonal antibodies specific to many other protein modifications as well as standard fusion proteins (see Applications). The first phase of the procedure is to raise polyclonal antibodies against a peptide that contains the phosphorylation site(s); this polyclonal serum will contain a complex mix that includes antibodies that react specifically to the phospho-protein of interest as well as antibodies that react with the non-phosphorylated protein and antibodies that react with low sequence complexity phospho-epitopes (Table 1). The protocol employs a subtraction procedure to remove undesired antibodies that react with the non-phosphorylated protein, and an affinity purification procedure to enrich for antibodies that react with the complex phospho-peptide epitope, similar to previously described methods48,49. In practice however, we find that while a single round of subtraction can be sufficient to remove antibodies that react with the non-phospho-peptide, a single round of affinity purification is in most cases insufficient to remove antibodies that react with low sequence complexity phospho-epitopes (see Supplemental Figure 3C in ref. 28). Therefore the workflow of the protocol incorporates two methodological approaches to remove undesired antibodies and specificity tests to assess the extent of purification along the way (Figure 2). The first approach employs iterative rounds of subtraction and affinity purification, to remove undesired antibodies. The second approach employs high stringency washes to remove antibodies that bind to low sequence complexity phospho-epitopes. After each round, dot blot and western blot analyses are conducted as specificity tests to determine whether additional rounds of subtraction and/or affinity purification are necessary. Finally, western blots and/or immuno-histochemical staining of phospho-protein gene product null mutants or RNAi knockdown samples are used to validate specificity of the antibody preparation to the phospho-protein of interest.
Table 1. Assortment of Antibody Reactivity Following Immunization with Phospho-Peptide.
| Antibody Reactivity | Amino Acid Sequence | Description |
|---|---|---|
|
|
||
| Peptide Immunogen | X1-X2-X3-X4-p[S/T]-P-X7-X8-X9X10-C | Conceptual phospho-peptide immunogen used to generate phospho-protein specific antibodies and used for affinity purification. |
| Non-phospho Peptide | X1-X2-X3-X4-[S/T]-P-X7-X8-X9X10-C | The corresponding non-phospho-peptide that is used in the subtraction. |
| Desired Antibody Specifcity | X2-X3-X4-p[S/T]-P-X7-X8 | Desired antibody specificity, which is typically of sufficient sequence complexity to specifically recognize the modified protein and is generated from the immunogen in Row 1 and the purification workflow outlined in Figure 2. |
| Total Protein Antibody Specificity | X1-X2-X3-X4 | Total protein antibody specificity, not including the phospho-epitope. |
| P-X7-X8-X9X10 | ||
| Antibody Specificity to Low Complexity Epitope | p[S/T]-P | Antibodies that recognize the phospho-site, but only one or a few adjacent amino acids, where this low sequence complexity recognition epitope will likely cross-react with other phospho-proteins. |
| p[S/T]-P-X7 | ||
| X4-p[S/T]-P | ||
| Non-phospho-specific antibody | X2-X3-X4-[S/T]-P-X7-X8 | Antibodies that specifically recognize the non-phospho-peptide. |
Red, phosphorylated residue; Blue C-terminal cysteine added for coupling peptide to KLH and BSA.
Figure 2. Workflow for phospho-antibody purification.
Workflow for purification of phospho-protein specific antibodies, which includes (A) sera generation, subtraction steps (B) & (C), affinity purification steps (D) – (F) and specificity tests (G) & (H). If the dot blot (Figure 5) and western blot (Figure 3) specificity tests indicate that the anti-phospho-peptide antibody preparation retains reactivity to undesired epitopes (Table 1), then the purified sample undergoes an additional round of purification; if reactivity to the non-phospho-peptide remains, then the preparation is returned to (B) [Step 93; purple arrow while if reactivity to other phospho-proteins remains, then the preparation is returned to (D), Step 95; red arrow. If antibodies to total protein and to low sequence complexity epitopes are present, then both the subtraction and affinity purification are repeated, return to (B),Step 96; blue arrow].
Comparison to other methods
Antibodies that specifically recognized protein modifications such as phospho-tyrosine, were first described in 1981 by Ross et al50. Polyclonal anti-phospho-tyrosine antibodies were generated by immunization with phospho-tyrosine modified KLH followed by affinity purification with phospho-tyrosine modified Sepharose. These purified pan-phospho-tyrosine antibodies were used to demonstrate that numerous tyrosine-phosphorylated proteins are present in RSV transformed mammalian cells. Subsequently, Nairn et al51 generated antibodies to a phosphorylation site on the protein G-substrate, using a phospho-peptide immunogen, and also generated antibodies to the corresponding unphosphorylated site, using a non-phospho-peptide immunogen; the two antibodies were then employed to follow the kinetics of G-substrate phosphorylation in vitro. These initial studies provided the basis of current methods for purification of phospho-protein specific antibodies.
Salient features of current methods for purification of polyclonal phospho-protein specific antibodies 48,49,51-56, 57-63 are summarized as follows. (1) Immunization with a phospho-peptide corresponding to the phosphorylated region of the protein under study. (2) Purification of the resulting antisera, through first removal of antibodies that react to the non-phosphorylated protein by a single round of subtraction against the non-phosphorylated peptide linked to Sepharose, followed by affinity enrichment for antibodies that react to the phosphorylated protein by a single round of binding the antibody preparation to the phospho-peptide linked to Sepharose. (3) In the final step the antibodies bound to the phospho-peptide column are eluted and tested under various experimental conditions.
Ours and other groups have found that a single round of affinity purification is usually insufficient to obtain polyclonal antibodies that are specific to the phosphorylated protein of interest as the preparation shows cross-reactivity towards other cellular/organismal proteins (Figure 3)28,49. The polyclonal antiserum generated following immunization with the phospho-peptide contains a complex mixture (also see below) including antibodies that recognize the phospho-residue (p(S/T)) plus a number of surrounding amino acids (Table 1) such that the epitopes are of sufficient sequence complexity to uniquely identify, in most case, a specific protein in an organismal proteome. However, the complex mixture also contains antibodies that recognize the phospho-residue and only one or a few surrounding amino acids (Table 1), where these epitopes are found on a number of other phosphorylated proteins in an organismal proteome. A single round of affinity purification will enrich for both antibodies that recognize the unique/complex sequence epitope as well as antibodies that recognize low sequence complexity epitopes, which then bind to and cross-react with other cellular phospho-proteins that contain the low sequence complexity epitopes as part of their phospho-sites, resulting in a preparation that is not specific to the protein of interest. Previous protocols have proposed that the solution to the problem of single round purified antibodies cross-reacting with other cellular proteins is to repeat the immunization process starting with a new cohort of rabbits and then perform a single round of subtraction and affinity purification49. However, antibodies that recognize the lower sequence complexity phospho-epitopes are also likely to be generated in additional immunizations. Instead, this protocol employs iterative cycles of affinity purification (and subtraction, if necessary) and high stringency (pH 4.0) washes to enrich for antibodies that recognize the unique/complex sequence epitopes and remove antibodies that bind to the low sequence complexity epitopes (as described above).
Figure 3. Removal of non-specific antibodies from the phospho-CGH-1 preparation through multiple rounds of affinity purification as assessed by western blots of adult C. elegans lysates.
Multiple rounds of affinity purification, with stringent washes, were necessary to generate a polyclonal antibody preparation that is specific to phospho-CGH-1 (band corresponding to pCGH-1 is indicated by *).Whole worm lysates, 200 μg (left) or 100 μg (right)/lane, were resolved on a 10% SDS gel and then probed with 1:500 dilution of the phospho-antibody preparation after one (A), two (B), three (C) and four (D) rounds of affinity purification. Expectations for the size of the phospho-protein and the number of bands (e.g. if there are multiple isoforms from alternative splicing N-terminal and/or C-terminal to the phosphorylation site) will be specific to the protein under study; if there are not existing publications on the gene product then size may be deduced from the protein amino acid length and number of isoforms from ESTs/cDNA sequence and RNA-seq data obtained from databases such as the UCSC Genome Browser.
Monoclonal antibodies specific for a phosphorylation site on the protein of interest are an excellent alternative to purification of polyclonal antibodies for that phospho-protein. Monoclonal antibodies that are specific to a number of modified proteins have been generated; two widely used examples are the monoclonal antibody to activated ERK28 and to phospho Histone H3(Ser10)64-66. Distinct advantages of a phospho-protein specific monoclonal antibody is that the single complex epitope specificity is defined and preparations are highly uniform leading to reproducible results between laboratories. The generation of phospho-protein specific monoclonal antibodies is, however, several fold more expensive than generation and purification of polyclonal antibodies and it is time consuming to screen among many monoclonal antibody secreting clones to identify the one that is specific to the phospho-protein of interest, not cross-reacting with other related lower sequence complexity phospho-sites, with no guarantee of a successful outcome. The generation and purification of polyclonal antibodies specific to a phospho-protein is most useful in the initial characterization phase of a project where the antibody preparation is used to demonstrate that the phosphorylation occurs in vivo, that it occurs through the predicted kinase(s), and is temporal and/or spatially regulated in a biologically interesting way, providing impetus for generation of a monoclonal antibody. Furthermore it provides proof of principle that antibodies which recognize an epitope that specifically identifies the phospho-protein of interest can be produced, supporting the time and expense in generation of the corresponding monoclonal antibody.
Applications
The protocol has been successfully employed to generate a number of phospho-protein specific antibodies27,28 (Arur. S, unpublished data) as well as histone H3 methylation- and acetylation-specific antibodies and thus should be applicable to any modified amino acid that can be incorporated into a chemically synthesized peptide. We have also employed the affinity purification steps to purify polyclonal antibodies to fusion proteins, to detect total FEM-327 and GFP (Arur. S, unpublished data). In all these cases, two or more rounds of affinity purification were necessary to obtain specific antibodies. Similarly, it is likely that numerous research groups have generated polyclonal antibody preparations that failed to show specificity after having undergone one round of affinity purification, and thus were assumed not to contain specific antibodies and were discarded or stored unused in freezers. It is possible that such antiserum, following multiple rounds of affinity purification with stringent washes, may generate specific antibodies, resurrecting discontinued lines of investigation. Therefore, the protocol will be useful to obtain specific polyclonal antibodies to modified proteins and to total proteins, using modified-peptide and peptide or fusion protein immunogens, respectively, from de novo immunizations and potentially from stored frozen antiserum that was thought not to contain specific antibodies.
Limitations
The modified peptide (or the fusion protein) must elicit a strong immune response, usually assessed by ELISA through the custom antibody vendor. It is very difficult to generate a specific antibody preparation from low titer antiserum. High temporal resolution samples can usually be obtained for immuno-cytochemistry and used with the modified protein specific antibody preparation. However such fixed samples may not allow observation of dynamic molecular behaviors that can be obtained from real-time cytological analysis. There are currently no methods to specifically track a modified protein in real-time. Sometimes, sequence context of phospho-epitope is not unique, this usually occurs in cases where the protein may belong to a gene family. In such situations, it is important to devise extensive specificity controls to ensure that the desired protein is indeed being phosphorylated in vivo and not another family member, often times, these experiments are challenging and difficult to conduct due to the inherent redundancy in biology.
Experimental Design
Phospho-peptide design
To be a useful experimental tool, phospho-specific antibodies must specifically bind with high affinity to the phosphorylated protein of interest but not recognize the unphosphorylated form or other phosphorylated proteins. The binding pocket of an antibody can interact with its antigenic determinant (epitope) in the range of up to six to ten amino acids67-69. Six to ten amino acids are of sufficient sequence complexity that it should, in most cases, uniquely identify a specific protein in the proteome of an organism47-49. Because phosphorylation occurs at a specific site(s) in the protein under study, the peptide sequence that is to be used as an immunogen is constrained by this location. BLAST analysis70 should be performed to determine the uniqueness of this site in the proteome of the relevant organism(s). Typical phospho-peptide immunogens that we have used have a centrally located p(S/T)P site with about four amino acids N-terminal and four C-terminal to that site (Table 1). A cysteine (C) is placed at the N- or C-terminus for cross-linking the peptide to carrier proteins for immunization (KLH) and antibody purification (BSA). Table 2 lists examples of peptides we have used successfully to raise phosphoprotein-specific antibodies. In peptide immunogen design, we have not used N- or C-terminal extensions greater than six amino acids flanking a given side of the p(S/T)P site to avoid excessive generation of antibodies to the total protein, and in some cases shorter lengths were used to avoid cross reaction with related protein family members or cysteine residues that can form disulfide bonds with the terminal cysteine used in coupling. For MPK-1 ERK substrates GSK3β and NOS-3, in vitro kinase assays and site-directed mutagenesis suggested that two sites were phosphorylated in vivo26, 27 that were in sufficient proximity that antibodies of high sequence complexity could be generated that simultaneously recognize both modified sites.
Table 2. Phospho Peptides Immunogens Used to Generate Phospho-Peptide Antibody.
| Name | Amino Acid Sequence |
|---|---|
| DDX-19 | DPK* pSPLYS-C and IVIG* pTPGI-C |
| GSK-3 | IIEY* pTPTSRP* pTPQA-C |
| NOS-3 | STENSE* pSPSRSS* pTPKHRKK-C |
| CGH-1 | ERP* pSPIQEKK-C |
| PAR-5 | EILN* pTPEHKK-C |
| CDC-48.2 | LRK* pTPLSAKK-C |
: Phosphorylated Residue
Generation of polyclonal antisera
Screening of animals pre-immunization: We recommend prescreening of the animals to be injected for generation of the sera against whole cell extracts of the tissue or cell type of interest. Obtain 1 ml of pre-immune sera from the animals to be injected from the vendor, and perform western blot analysis with the sera at 1:1000 and 1:5000 against tissue or cell extract of choice. Ideally, the animal to choose for injecting the antigen and generation of the serum should be one that has least amount of preexisting response against the tissue/cell extract.
Typically a10-week immunization schedule was followed for generation of the antisera, which consists of 1 priming injection and three biweekly and 1 booster injection followed by the production bleed. Antisera with a low titer to the phospho-peptide will often be difficult to purify. The antibody response to the phospho-peptide can be assayed via ELISA or dot blot prior to terminal bleeds, usually performed by the vendor. For a given peptide antigen, antibody titers can differ from animal to animal due to differences in the immune response resulting in differences in the concentration of the various antibodies generated. Typically, ELISA titers are compared between the pre-immune serum and the post-immune serum, at dilutions of 1:1000, 1:10,000 and 1:100,000. A high titer of post-immune sera vs preimmune sera (e.g. 20,000 vs 800) is considered a good sign of response specifically to the antigen injected, at the 1:100,000 dilution, or in terms of OD levels, 0.088 (for pre-immune at 1:100,000) to 2.873 (for post-immune, at 1:100,000) are considered good values to proceed with. Usually, these values are an indication that a response has been generated, and thus antibodies can be purified.
Immunization and the various antibody species that are generated
Following immunization with the 2 mg of KLH-conjugated phospho-peptide, polyclonal antibodies will be generated having a diverse array of reactivities, shown schematically in Table 1. These include: (i) antibodies with the desired specificity that react with sufficiently complex sequence epitopes, including the p(S/T) residue, such that it uniquely identifies the phosphorylated protein under study and binds with high affinity; (ii) antibodies that react with only flanking residues, but not the p(S/T) residue, and thus may have specificity to the total protein, both the phosphorylated and non-phosphorylated forms; (iii) antibodies that react with the p(S/T) residue and only one or a few surrounding amino acids and thus represent low-sequence complexity epitopes that problematically will cross-react with a number of other phosphorylated proteins present in the proteome and should bind the immunogen with lower affinity; and (iv) antibodies that react specifically with the non-phosphorylated form of the protein, which presumably arise from dephosphorylation of the peptide immunogen early after immunization.
Peptide column preparation (Steps 1– 46)
Antibodies that specifically bind to the complex sequence phospho-epitope represent a small proportion of the antibodies present in the immune serum and thus efficient recovery is important. The protocol employs two peptide columns, a non-phospho-peptide column to subtract antibodies that recognize total protein and a phospho-peptide column for affinity purification. A Sepharose bead is typically linked to a single peptide (or protein)76-78. By contrast a single BSA molecule can have up to nine peptides conjugated through cysteine residues. By first coupling the peptide to BSA and then coupling the peptide-BSA complex to Sepharose, we can obtain an approximately nine-fold increase in the number of peptides bound per Sepharose bead which increases the capacity of the column and the higher local peptide density may help to anchor the antibodies to the beads. The protocol describes the production of the peptide Sepharose columns in two stages. In the first stage (Steps 1 – 6), homobifunctional maleimide based chemistry is used to crosslink the added N- or C-terminal cystine of the phospho- or non-phospho-peptide to maleimide activated BSA, forming stable thioether linkages. The peptide∷BSA complex is then purified using a desalting column (Steps 7 – 17) and the efficiency of coupling the peptide to BSA assessed by SDS-PAGE (Steps 18 – 22) (Figure 4). In the second stage (Steps 26-46), the peptide∷BSA complex is coupled to the column matrix with amine-based chemistry79, using cyanogen bromide activated Sepharose (CNBr-Sepharose). Prior to coupling, the peptide∷BSA complex is dialyzed into a basic pH solution (Steps 23-25) required for the reaction and to remove any amine-based buffers (Tris, EDTA) that will inactivate the CNBr-Sepharose. After coupling, the column is stringently washed with repeated cycles of alkaline buffer then acidic buffer (Steps 42 – 44) to eliminate any peptide∷BSA complex that is loosely associated with the CNBr-Sepharose through ionic interactions, which would be eluted in the later elution step and then bind to the purified antibody, blocking its activity. Finally, the column is washed with a Tris based high salt buffer (Blocking Buffer) to block any reactive sites that may have remained uncoupled on the Sepharose resin (Steps 45-46).
Figure 4. Testing the efficiency of peptide conjugation to activated BSA.
If the affinity purification fails to recover antibodies that recognize the phospho-peptide (Figure 2 and Table 1), this may occur due to a failure to efficiently conjugate the phospho-peptide to the BSA. Conjugation can be assessed by electrophoresis (10% SDS-PAGE) of the unconjugated BSA material (left lane), non-phospho-peptide conjugated BSA (middle lane) and phospho-peptide conjugated BSA (right lane) followed by Coomassie staining. If the conjugation has gone to completion, the conjugated peptide-BSA complex should run at a higher molecular weight/retarded mobility (middle and right lanes) compared to the unconjugated BSA (left lane), with the phospho-peptide-BSA running slightly slower than the non-phospho-BSA complex. Adapted with permission from ref. 28.
Workflow – subtraction (Steps 47-57) and affinity purification (Steps 58-73)
To obtain phospho-protein-specific antibodies, the protocol employs a subtraction step to remove antibodies that will cross-react with total protein, including the non-phosphorylated form (Table 1), and an affinity purification step to remove antibodies that will react with low sequence complexity phosphorylation sites (Table 1) and thus are not specific to the phosphorylated protein under study. The workflow for the purification is shown in Figure 2, where the crude antiserum is sequentially passed over two columns (prepared as described above). The first column contains the non-phospho-peptide that will bind and subtract away antibodies that react with the total protein (Table 1), while antibodies that react with the phospho-peptide will not bind and be present in the flow-through fraction. The second column contains the phospho-peptide and is used for affinity purification. The flow-through fraction from the non-phospho-peptide column contains two distinct populations that associate with the phospho-peptide - antibodies that bind to complex sequence epitopes, at high affinity, that are specific to the phospho-protein under study (Table 1) and antibodies that bind low sequence complexity epitopes, containing only one or a few residues other than the p(S/T) residue (Table 1). Stringent washes are employed to remove antibodies that bind the low sequence complexity epitopes, which are expected to bind with lower affinity than those that bind to the complex sequence epitopes. Sequential washes with alkaline (pH 9.5) and acidic (pH 4.0) buffers are employed; the alkaline buffer stabilizes the interaction between the antibody and the phospho-peptide71,72 while the acidic, pH 4.0, wash will destabilize the low affinity antibody interactions with the phospho-peptide resulting in their elution from the column47,73-75. The stringent alkaline and acidic washes are repeated 3 or 4 times in an effort to remove more antibodies with low affinity or low sequence complexity recognition. The antibodies that recognize high-complexity epitopes that bind at high affinity are then eluted with strong acidic conditions, glycine buffer at pH 2.2, and immediately neutralized.
Workflow – Phospho-peptide and phospho-protein specificity tests and iterative rounds of subtraction and affinity purification (Steps 74-96)
Two specificity tests are then performed on the preparation that has undergone one round of subtraction and affinity purification. First, we employ a simple dot blot procedure to determine which elution fractions contain IgG activity (Steps 74-82). Alternatively, if a fraction collector is employed that is connected to a UV 280 nm readout machine, then the protein reading in the fractions can be automatically visualized, and the fractions in the OD peak pooled. Using the dot blot assay, the eluted antibody is tested for reaction to the phospho-peptide and to the non-phospho-peptide (Steps 83-93; Figure 5). If the purified antibody reacts with the non-phospho-peptide, in addition to the phospho-peptide, then antibodies that recognize the total protein (Table 1) remain in the preparation. Thus to remove the antibodies that recognize the total protein, the preparation must undergo a second round of subtraction, passed again over the non-phospho-peptide column (Figure 2, G → B). Second, employing a western blot assay with lysates from wild type cells/organisms that are undergoing signaling that generates the phospho-modification, the antibody preparation is tested for specificity to the phosphoprotein under study (Steps 94-96; Figure 3). If the purified antibody shows western blot bands in addition to the relevant gene product, then the antibody preparation is reacting to other proteins, which contain the p(S/T) site plus one or a few amino acids that are similar to the complex phospho-epitope (Table 1). Thus a second round of affinity purification with stringent washes is used to further remove antibodies that react with the lower sequence complexity phospho-epitopes (Figure 2, H → D). If antibodies to total protein and to low sequence complexity epitopes are present, then both the subtraction and affinity purification are repeated (Figure 2, H → B). In practice we find that the major impediment to obtaining a phospho-protein-specific preparation are antibodies that react with the lower sequence complexity phospho-epitopes (Table 1, Figure 3)28 as also observed by others49. While removal of antibodies that react with the non-phospho-protein is usually accomplished with one or two rounds of subtraction (Figure 5), removal of antibodies against the lower sequence complexity epitopes, as assessed by non-specific bands on the western blot (Figure 3), often requires four rounds of affinity purification with stringent washes (shown for pCGH-1 here and for affinity purification of anti-pDDX-19 antibodies in Supplemental Figure 3C of ref. 28). Thus the use of multiple rounds of affinity purification, with stringent washes, and subtractions, are key to obtaining an antibody preparation that is specific to the phospho-protein of interest.
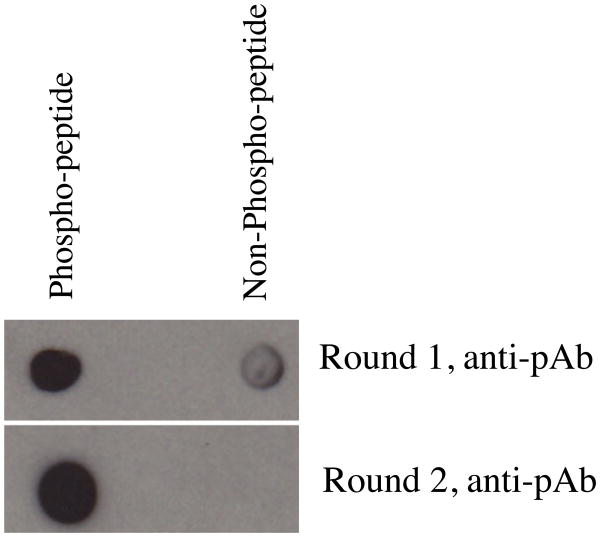
Figure 5. Dot blot to test specificity towards the CGH-1 phospho- and non-phospho-peptides.
10 ng each for the phospho- and non-phospho-peptide was spotted on nitrocellulose and probed with 1:1000 dilution of the anti-phospho CGH-1 peptide column eluate (from Figure 2). Row 1 shows that after the first round of antibody purification, the antibody retains residual activity towards the non-phospho-peptide. Row 2 shows that the activity towards the non-phospho-peptide is lost after second round of purification.
Genetic-based specificity tests (Step 97)
The gold standard for determining in vivo specificity of the antibody preparation are genetic based assays (Figures 1A and 6). First, a null mutant or RNAi-mediated depletion of the protein containing the phosphorylation site (CGH-1) is used in western blots (Figure 6), and/or in immuno-cytological staining27, to assess if the signal from the purified antibody is specific to the protein under study. If the gene product null or RNAi knockdown show inappropriate bands in a western blot or signal in immuno-hystochemistry, then the preparation shows cross-reaction to other gene products/modified proteins and should undergo an additional round of affinity purification (Figure 2, H → D; repeat Steps 58 – 73). Note that if there is a paralog(s) or homolog(s) with sequence identity in the phosphorylation site and surrounding sequences, as assessed from BLAST analysis, then the expectations for results from western blots and immuno-cytochemistry of wild type and null mutant/knockdown should be modified accordingly and additional genetics-based experiments may need to be employed to assess specificity. Second, if the enzyme that produces the protein modification is known, then RNAi knockdown or a null mutant of the modifying enzyme (e.g. MPK-1 ERK) should reduce or eliminate the signal from the modified protein specific antibody preparation (pCGH-1) but not affect the amount of total protein (Figures 1 and 6).
Figure 6. Genetic tests for specificity of the purified anti-pCGH-1 polyclonal antibody preparation.
Western blot of wild-type (WT), mpk-1(0) and cgh-1(0) adult whole worm lysates probed with anti-CGH-1 total antibody (gift of David Greenstien, Univ of Minnesota) (top), the anti-pCGH-1 antibody preparation following four rounds of affinity purification (middle) and anti-tubulin antibody (bottom). Probing with anti-total CGH-1 reveals a closely spaced doublet (* upper band, # lower band) in wild-type where both bands are absent in cgh-1(0). Probing with anti-pCGH-1 reveals a single band (*) in wild-type, which is absent incgh-1(0), indicating that the anti-pCGH-1 antibody preparation is specific for CGH-1. In mpk-1(0), the anti-pCGH-1 preparation fails to detect a band, indicting that the antibody is specific to MPK-1 ERK mediated phosphorylation of CGH-1. The slower migrating band (*) in wild-type recognized by anti-total-CGH-1 is likely phospho-CGH-1 as it is absent from the mpk-1(0) lysate and co-migrates with the single band recognized by the anti-pCGH-1 antibody preparation (data not shown) while the bottom band (#) is likely unphosphorylated CGH-1 as it is present in the mpk-1(0) lysate and absent following staining with the anti-pCGH-1 antibody preparation.
Purification of non-phospho-protein specific antibodies (Steps 98 – 103) and total protein antibodies (Steps 117 – 142)
In some situations it is useful to generate polyclonal antibodies that are specific for the unmodified non-phospho-protein (e.g. NOS-3, Figure 1B, D)27. In this situation, the order of purification is reversed from the workflow shown in Figure 2. The crude antiserum is first passed over the phospho-peptide column, removing antibodies that react with the phospho-epitopes and total protein (Table 1). The flow–through is then passed over the non-phospho-peptide column, washed stringently to remove antibodies to low sequence complexity epitopes and then eluted. Specificity tests are performed as described above, with non-phospho-protein specificity the desired goal27 (Figure 1B). Much like with purification of the phospho-specific antibody fraction, the non-phospho antibody sometimes also needs to be purified reiteratively. For example, the non-phospho-NOS-3 antibody was purified via subtraction from the phospho fraction followed by affinity purification in three complete cycles, wherein the first cycle consisted of subtraction followed by affinity purification, as did the second and the third cycle.
In many cases it is useful to generate antibodies that are specific for total protein (both the modified and unmodified forms) (e.g., total CGH-1, Figure 6). However, antibodies that will react with total protein from the phospho-peptide immunogen (Table 1) are problematic as they have specificity to shorter peptide sequences and thus are likely to react with non-target proteins. Instead, we have used either recombinant fusion protein isolated from E. coli, which will lack eukaryotic modifications, or peptide immunogens that correspond to a separate part of the protein than the region where the modification resides. The protocol describes coupling of the recombinant proteins to Sepharose (Steps 117 – 130) and affinity purification (Steps 131 – 142).
Concentration and storage of the purified antibody preparation (Steps 111 – 116)
Once the purified antibody preparation has passed the relevant specificity tests described above and is ready for use, it should be concentrated to about 1 mg/ml and placed into a stabilization buffer (final concentration - 2.5% BSA, 0.01% Tween-20, and 25% glycerol). For long-term storage, keep the antibody at -80°C, however, working stocks can be maintained at 4°C up to 3 months. A good starting dilution for the final antibody in various applications such as western blotting, immunoprecipitation and immunofluorescence is 1:1000 (for western blotting), 10-15 μg of antibody per mg of total protein extract for immunoprecipitation, and 1:200 for immunofluorescence. However, dilutions should be optimized depending on the results obtained.
Regeneration of columns (Steps 104 – 110)
For reuse or storage of the columns, either for additional rounds of purification or for first round purification of crude serum, residual antibodies bound to the column must be eluted and the pH brought back to neutral (to prevent degradation of the peptide∷BSA complex coupled to the Sepharose). We have reused columns up to six times, although this will be highly dependent on duration of storage. Storage should not be more than three months from the time of making the column. Longer storage times result in decreased efficacy of purification.
Scaling of the protocol and materials (Table 3)
Table 3. Scaling Up Phospho-Antibody Purification.
| Serum to be used | Peptide (mg) | Maleimide activated BSA (mg) | Amount of Cyanogen Bromide | Bed Volume Obtained | Fractions collected (1ml) |
|---|---|---|---|---|---|
| 1ml | 2mg | 2mg | 0.3g | 1ml | 10 |
| 2ml | 2mg | 2mg | 0.3g | 1ml | 20 |
| 2-4ml | 4mg | 4mg | 0.6g | 2ml | 20-40 |
| 3-6ml | 6mg | 6mg | 0.9g | 3ml | 30-50 |
The amount of serum that will be used to purify the phospho-peptide specific antibody will determine the size of the peptide column that is made, the amount of starting peptide, the amount of maleimide activates BSA, the amount of CNBr-activated Sepharose and the resulting column bed volume and the number of fractions collected. The Procedure here assumes that 1-2 ml of crude serum will be used; if a larger amount is to be used, then scale up each component as indicated (Table 3).
Materials
Reagents
Reagents as part of kits used in the protocol
Conjugation Buffer (Imject Maleimide Activated BSA kit, Pierce, Catalog # 77667)
Purification Buffer (Imject Maleimide Activated BSA kit, Pierce, Catalog # 77667)
Salts
Sodium Chloride (NaCl), Sigma-Aldrich, Catalog # S7653
Potassium Chloride (KCl), Sigma-Aldrich, Catalog # P9333
Sodium Phosphate (Na2HPO4), Sigma-Aldrich, Catalog # S7907
Potassium Phosphate (KH2PO4), Sigma-Aldrich, Catalog # P9791
Tris Base, Sigma-Aldrich, Catalog # T6791
Glycine, Sigma-Aldrich, Catalog # G8898
Coomassie Brilliant Blue, Sigma-Aldrich, Catalog # B0149
Sodium bi-Carbonate (NaHCO3), Sigma-Aldrich, Catalog # S7795
Sodium Azide, Sigma-Aldrich, Catalog # S8032
Corrosive Organic Solvents
Methanol, Fischer Scientific, Catalog # BP-1105
Galcial Acetic Acid, Fischer Scientific, Catalog # BP2401-212
Sodium Hydroxide, Sigma-Aldrich, Catalog # S8045
Hydrochloric Acid, Fischer Scientific, Catalog # A144C-212
Dimethyl Sulfoxide (DMSO), Sigma-Aldrich, Catalog # D8418
Detergents
Tween-20, Sigma-Aldrich, Catalog # P9416
Sodium Lauryl Sulfate, Sigma-Aldrich, Catalog # L6026
Others
Glycerol, Fischer Scientific, Catalog # BP2291
Non-Fat dry Milk (Nestle Carnation)
Cyanogen Bromide Activated Sepharose, Sigma-Aldrich, Catalog # C5338
Bovine Serum Albumin for western blotting; Sigma Aldrich, Catalog # A2153
Bovine Serum Albumin for antibody stabilization; Sigma Aldrich, Catalog # A7888
Beta mercaptoethanol, Sigma Aldrich, Catalog # M6250
Reagents for detection of antibody
Nitro-cellulose membrane, Thermo Scientific Pierce, Catalog # PI-88013
Secondary antibody conjugated to Horse Raddish peroxidase (HRP), Invitrogen, Goat anti Rabbit HRP, Catalog # A10547
ECL reagent for HRP, Millipore Immobilon, Catalog # WBKLS0500
Phospho-peptides (and corresponding non-phospho-peptides)
Custom peptides (designed as described in the Experimental Design) can be made in house or obtained from commercial venders; it is recommended that at least 20 mg of the phospho-peptide and 20 mg of the non-phospho-peptide be synthesized, followed by HPLC purification to >90% purity. We have stored the peptides in powdered form from the manufacturer at -20°C and weighed out material as needed.
CRITICAL. The peptide powder is hydroscopic. Warm the vial containing the peptide powder to room temperature prior to opening.
CRITICAL. Avoid, if possible, internal cysteine in the peptide immunogen as it may form a di-sulfide bond with the terminal cysteine, reducing efficiency of conjugation to KLH and BSA. If an internal cysteine is unavoidable, then the terminal cysteine should be placed on the adjacent end.
Polyclonal antisera
Custom polyclonal antisera (e.g. rabbit) are generated with the 2 mg of KLH-conjugated sera phospho-peptide through commercial vendor or in house (as described in the Experimental Design). We have used several different vendors such as Open Biosystems, Covance and Yenzyme. We have worked with Yenzyme for 90% of our antibodies; however, any company of choice can be used.
Reagent Setup
PBS
For 1 L of 1× PBS: Dissolve 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4 and 0.24 g of KH2PO4 in 800 ml of de-ionized water. Once all the salts are dissolved, make up the volume to 1000 ml and test pH. The pH of the solution should be 7.4. Store at room temperature of 22°C. Shelf life: one year.
2× SDS Sample Buffer
Add 0.5 M Tris-HCL (2.5ml) pH 6.8, Glycerol (2 ml) 10% SDS (4 ml) and 0.1% Bromophenol Blue (0.5ml). Bring the volume to 10 ml. Store at room temperature. Shelf life: one year. Add 1% beta mercaptoethanol to the 2× SDS sample immediately before use. Shelf life: 1 week.
10% SDS
For 1L: Dissolve 100 g of Sodium Lauryl Sulfate in 800 ml of de-ionized solution. Heat the solution to about 37°C to facilitate dissolution. Care must be taken to ensure that the solution does not over bubble. Make up the volume to 1000 ml. Store at room temperature. Shelf life: one year.
5× Tris-Glycine SDS
For 1L: Dissolve 15.1 g of Tris Base and 94 g of Glycine in 800 ml of de-ionized water. Once the solution has fully dissolved, add 50 ml of 10% SDS and dissolve gently to avoid excessive bubbling. Make up the volume of the solution to a 1000 ml. Store at room temperature. Shelf life: one year.
Coomassie Brilliant Blue
Dissolve 1 g of Coomassie Brilliant Blue powder in 200 ml Methanol, 3.5 ml Glacial Acetic Acid made up to a final volume of 500 ml with de-ionized water. Store at room temperature. Shelf life: one year. CAUTION – strong acid and should be used in a fume hood, with goggles, gloves and lab coat. Pipette out the acid using a glass pipette to avoid corrosion of plastic.
Coupling Buffer (also called Alkaline Wash Buffer when used for washing peptide columns)
0.1 M NaHCO3, 0.5 M NaCl, pH to 9.5 with 50% NaOH.
For 1 L: Dissolve 8.4 g NaHCO3, 29.2 g NaCl in 800 ml of de-ionized water. Set the pH to 9.5. Make the volume to 1000 ml. Store at room temperature. Shelf life: one year. CAUTION – strong base should be used in a fume hood, with goggles, gloves and lab coat. Pipette out the acid using a glass pipette to avoid corrosion of plastic.
Column Wash Buffer (also called Acidic Wash Buffer when used for washing peptide columns)
0.1 M Sodium Acetate, pH 4.0, 0.5 M NaCl
For 1 L: Dissolve 5.25 ml of Glacial Acetic Acid and 29.2 g NaCl, in 800 ml of de-ionized water. Adjust pH with 50% NaOH. Store at room temperature. Shelf life: one year. CAUTION – strong base should be used in a fume hood, with goggles, gloves and lab coat. Pipette out the acid using a glass pipette to avoid corrosion of plastic.
Blocking Buffer
0.5 M NaCl, 20 mM Tris pH 8.0, 0.05% Tween-20
For 1L: Dissolve 29.2 g of NaCl, 2.42 g of Tris Base in 800 ml of de-ionized water. Set the pH of the solution to 8.0 with HCl. Add 50 μlof Tween-20 to the solution.
Add 1% sodium azide (1 gram in 100 ml of de-ionized water) to the entire amount at 0.02%, or to a smaller aliquot. Shelf life: one month, store at room temperature.
CAUTION – HCL is a strong acid and should be used in a fume hood, with goggles, gloves and lab coat. Pipette out the acid using a glass pipette to avoid corrosion of plastic.
CAUTION – sodium azide is toxic and should be used in a fume hood, with goggles, gloves and lab coat. Pipette out the acid using a glass pipette to avoid corrosion of plastic.
100 mM NaCl
For 1 L: Dissolve 5.84 g of NaCl in 1000ml of de-ionized water. Store at room temperature. Shelf life: one year.
10 mM HCl
Add 86 μl of concentrated HCl to 100 ml of de-ionized water. Make fresh for each use. Store at room temperature. CAUTION: Pipette the concentrated HCl with glass pipettes in a chemical hood.
10 mM Tris (pH 7.5), 0.5 M NaCl
For 1L: Dissolve 1.41 g of Tris Base and 29.2 g of NaCl into 800 ml of de-ionized water. Set the pH to 7.5 using HCl. Make up the volume of the solution to 1000ml. Shelf life: one year Store at room temperature.
0.2 M Glycine-HCl (pH 2.2), Elution Buffer
For 1L: Dissolve 15.04 g of Glycine in 800 ml of de-ionized water.
Set the pH of the solution with HCl.
Filter (through a 0.45μ filter) sterilize the solution for long term storage at room temperature.
1 M Tris Base
For 1L: Dissolve 121.2 g of Tris Base in 1000 ml of de-ionized water. Shelf life: one year. Store at room temperature.
CRITICAL: Do not pH this solution. The solution will be at a pH of 10, and the free hydroxyl's will aid in neutralization of the 0.2M Glycine-HCl (pH 2.2) elution buffer.
Western Blot Blocking Buffer (WBBB)
1× PBS-T with 5% Non-fat dry milk. For phospho-antibodies on western blots, use 5% BSA instead of non-fat dry milk for blocking.
For 1L: In 1000 ml of 1× PBS, dissolve 1 ml of Tween-20. Stir the solution thoroughly until all the Tween-20 is well dissolved. Weigh of 5 g of Non-Fat Dry Milk (we find best results with Nestle Carnation) or 5 g of BSA. Add either 5 g of non-fat dry milk or 5g of BSA to the 100 ml of 1× PBS, with 0.1% Tween and dissolve. Make fresh each time.
CAUTION: Tween-20 is very viscous and rises into the pipette tip very slowly.
Western Block Wash Buffer
1× PBS with 0.1% Tween-20. Make fresh each time.
Equipment Setup
Balance (Fine Balance), Sartorium Mechatronics Corp, Catalog # SECURA513
Rotator Shaker, Thermo Scientific, Catalog # 2309-1CEFSQ
Cold room (room set at 4°C)
Econo-Pac columns, Biorad Life Sciences Technologies, Catalog # 732-2032
Spectrophotometer, Thermo Scientific, Catalog # 4001-000
ECL gel/blot developer, LiCor Odssey, ODYSSEY© Fc
Slide-A-Lyzer Dialysis Cassettes, 10K MWCO from Thermo Scientific, Catalogue # 66380
Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-10 membrane, Millipore, Catalogue # UFC901024.
Hydrion 5.5-8.0 Plastic pH Strips, Fischer Scientific Catalog # 13-640-516
Procedure
Generating column for antibody purification - conjugation of peptides to Maleimide activated BSA. TIMING 2-4 hours
Weigh out 2 mg of the peptide (either the non-phospho-peptide, for the subtraction, or the phospho-peptide for the affinity purification) and dissolve in 200 μl of conjugation buffer.
If some peptide remains undissolved (solution is turbid), add DMSO to a final concentration of 1% to the mixture to increase solubilization, and continue rotation at room temperature for 1 hour.
Centrifugethe mix at 8000 g at 4°C for 2 minutes and collect the supernatant for use in Step 5; the precipitate is undissolved peptide and should be discarded. If not removed this peptide will disrupt conjugation efficiency and also clog the desalting column (below).
Add 200-500 μl of conjugation buffer to the maleimide∷BSA tube.
Add the dissolved peptide (supernatant from Step 3) to the tube from Step 4.
Rotate the tubes on invert rotator at room temperature for 2 hour.
CRITICAL STEP: Both peptide and BSA should be fully dissolved before proceeding.
Desalting the peptide∷BSA conjugated mix. TIMING 30 minutes-1 hour
7. Add 60 ml of deionized water to 1 bottle of purification salts.
8. Dissolve the salts in water by vortexing the mix.
9. Add DMSO to a final concentration of 1%, if DMSO was used in Step 2.
10. Add 500 μl to 1 ml of this purification buffer to one desalting column and let drain by gravity.
11. Add 500 μl of purification buffer each time to the column and repeat Step 10 three times.
12. Label ten 1.5 ml eppendorf tubes and have ready for fraction collection.
13. Add the peptide∷BSA mix from Step 6 to the top of the column and drain the flow through and discard.
14. Add 500 μl of the purification buffer to the center of the column (on top) and collect the fraction in one of the tubes from Step 12.
15. Repeat Step 14 nine additional times to collect the 10 fractions.
16. Measure the absorbance of each of the fractions at 280 nm to assess for presence of peptide∷BSA mix. Combine all the fractions with a positive OD number.
17. Determine the peptide∷BSA concentration either using the 280 nm wavelength and the Warburg-Christian method for protein detection80, or BCA protein quantitation method81, or any preferred method of protein quantitation. It is essential to know the protein concentration per ml for subsequent antibody purification step, Step 47.
Assessing the efficiency of peptide∷BSA conjugation. TIMING 5-6 hours
18. Add 10 μl of 2× SDS sample buffer to 10 μl of peptide∷BSA mix from Step 16.
19. Boil at 100°C for 1 minute in aboiling water bath.
20. Centrifuge the mix at 8000 g at 22°C for 2 minutes and let sit at room temperature for 5 minutes.
21. Load the entire amount on 10% SDS-PAGE gel and run with a protein marker at 80V (for more details see ref. 82).
22. Stain the gel for protein visualization with Coomassie Blue (for more details see ref. 82). The conjugated peptide-BSA complex runs at a higher molecular weight (Figure 4) compared to the unconjugated BSA. ? TROUBLESHOOTING
Dialysis of peptide∷BSA complex into Coupling Buffer for column preparation. TIMING 16 hours at 4°C
23. Add the peptide∷BSA complex (maximum of 5 ml volume) to the 10kDA slide-a-lyzer cassettes.
24. Place cassette in 4-liter beaker, with stir bar, containing Coupling buffer and dialyze over night at 4°C.
25. The next morning, remove the liquid from the slide-a-lyzer; there should be no precipitate formation. Remove 5 μl of the dialyzed peptide∷BSA complex and check the pH of the solution with pH paper to ensure that the mixture is at a pH of 9.0. If pH is lower, add more coupling buffer until the pH is at 9.0. Store the solution at 4°C until use.
Coupling of non-phospho- and phospho-peptide-BSA to sepharose. TIMING 1 day
26. Weigh out 0.3 g of CNBr-Sepharose for a 1 ml bed volume column (Table 3, row 1), in a disposable 15 ml polypropylene conical tube.
27. Add 7 ml of 10 mM HCl to the CNBr-Sepharose powder
28. Mix on an invert rotator at room temperature for 60 minutes at room temperature.
29. Set up a Biorad Econopac Column by breaking off the bottom and attaching a small stopcock provided with the columns.
30. Add 10ml of 10 mM HCl to the column and let drip through by gravity, and repeat 3 times.
31. Add the swollen CNBr-Sepharose resin (from Step 28) to the column, keep the stop cock closed. Open the stop cock and add the remaining 90 ml of 10 mM HCl and allow to drip through with gravity.
32. Add 10 ml of Coupling Buffer to the column and let drip through.
CRITICAL STEP: This Step activates the resin, so should only be carried out when the peptide∷BSA solution is ready to use.
33. Close the stopcock at the bottom before the last of the buffer drips through. Remove 10 μl of the sephaorse with a pipette and save in an eppendorf tube for processing at Step 40.
34. Add the dialyzed peptide∷BSA mixture from Step 25 to the column. Given the capacity of the CNBr-Sepharose, use no more that 2 mgs of the peptide∷BSA mix with 1 ml of resin
35. Fill up the column with Coupling Buffer.
36. Cap the top of the column.
37. Gently invert-rotate the column with the resin, peptide∷BSA mixture and the buffer at room temperature for 1 hour.
CAUTION: In all cases where columns are being inverted-rotated, (a) the closed stopcock at the bottom and the cap on the top should also be sealed with a Parafilm wrap to prevent inadvertent loss of liquid and (b) care should be taken to ensure that the resin does not stick to the side of the column and dry out, by adding more of the buffer used to load the column. In this case, drying out will cause less peptide∷BSA to be coupled to the column resulting in a column that has a lower capacity for binding to the antibodies.
38. Continue mixing of the contents of the column at 4°C over night on a rotating shaker or drum.
39. Remove the stop cock at the bottom and the cap at the top and allow the peptide∷BSA to drip through. Save 100 μl of the flow through for assessment of coupling efficiency between the peptide∷BSA mix and the sepharose in Step 40.
40. Take 10μl each of theflow through (that drips through with gravity) from Step 39, and the unconjugated Sepharose beads (Step 33). To each add 2× SDS Sample Buffer and boil for 1 min at 100°C in a boiling water bath.
41. Resolve samples from Step 40 on an SDS PAGE (as in ref 28) to assess the extent of conjugation of the peptide∷BSA complex to the Sepharose beads. Stain the gel with Coomassie blue for visualization of conjugated peptide∷BSA. The sepharose conjugated to peptide-BSA complex will reveal protein bands on the gel, whereas sepharose unconjugated to protein beads will not reveal any protein bands on the gel. ? TROUBLESHOOTING
42. Add 10 ml of Alkaline Wash Buffer to the column from Step 39. Let it drip through and discard.
43. Add 10 ml of Acidic Wash Buffer to the column. Let it drip through and discard.
44. Repeat Steps 42 and 43 five more times.
45. Add 10 ml of Blocking Buffer to the column. Let it drip through and discard. Repeat this 5 times.
46. Add 0.2% sodium azide in 4 ml of Blocking Buffer to the column for future storage. The column can be stored up to 3 months at 4°C.
CAUTION: For all uses of the peptide columns, at no point should the column be allowed to completely dry. Always leave a little bit of buffer behind.
Subtraction of the non-phospho-peptide antibodies from the crude serum. TIMING 1 day
47. Thaw 1-2 ml of crude immune serum (for a 1 ml column) on ice.
48. Remove large debris by spinning in a clinical centrifuge or microfuge at 4°C at 2000 g for 3 minutes and remove small particulate matter using a 0.45 μm syringe filter.
49. Dilute the serum 1:10 with chilled 100 mM NaCl. Keep on ice.
50. Remove the non-phospho-peptide column from 4°C.
51. Add 20 ml of 100 mM NaCl to the column and let drip by gravity and discard flow through.
52. Close the stopcock and add the diluted serum to the column, and ensure that there is not much space on top.
53. Cap and Parafilm wrap the column.
54. Incubate the column on a rotator shaker at 4°C over night, with gentle agitation.
55. The next morning, collect the flow-through from the column.
CRITICAL STEP: The flow-through is the set of antibodies that did not bind to the non-phospho peptide column and represents, at least in part, the antibodies that are specific to the phospho-peptide, and will next be passed over the phospho-peptide column.
56. Add 5 ml of 100 mM NaCl to the column and collect the flow-through.
57. Combine the flow-throughs from Steps 55 and 56 and place on ice at 4°C.
Affinity purification of phospho-peptide antibodies from fraction subtracted with the non-phospho column. TIMING 1 day
58. Remove the phospho-peptide column from 4°C.
59. Let the storage solution drip through the column after opening the stopcock and cap.
60. Add 20 ml of 100 mM NaCl to the column and let drip by gravity and discard flow through.
61. Close the stopcock and add the flow-through collected from the non-phospho-peptide column (from Step 57) to the phospho-peptide column. Cap and Parafilm wrap the column.
62. Gently invert-rotate the column on a rotator shaker or drum overnight at 4°C.
63. The next morning, continue the incubations at room temperature for 1 hour then let the flow-through drip through the column and collect.
64. Save the flow-through from the phospho-peptide column at 4°C; if necessary, it can be tested to determine the efficacy of the binding of the antibodies to the phospho-peptide column.
Stringent washing of the phospho-peptide column. TIMING 1 hour
65. Add 10 ml of 10mM Tris (pH 7.5), 0.5M NaCl to the column and let drip through the column and discard.
66. Repeat three times.
67. Add 10 ml of Alkaline Wash Buffer (pH 9.5) to the column. Let it drip through and discard.
68. Add 10 ml of Acidic Wash Buffer (pH 4.0) to the column. Let it drip through and discard.
69. Repeat Steps 67 and 68 three additional times.
Elution of the anti-phospho-peptide antibodies TIMING: 30 minutes to 1 hour
70. To fifteen 1.5ml eppendorf tubes add 100μl of 1M Tris Base. Label the tubes 1 through 15.
71. Add 1 ml of Elution Buffer (pH2.2) to the column from Step 69.
72. Collect the elution with appropriately marked tube, and mix by inversion to neutralize the sample. Place on ice. Repeat Steps 71 and 72 fourteen more times.
73. Test 1-2 μl of each fraction with pH paper to verify neutralization. ?TROUBLESHOOTING
Identifying fractions with IgG activity. TIMING 2 hours
74. Spot 1 μl of neutralized eluate from each fraction collected in Step 72 to a strip of nitro-cellulose paper in a linear array. (Note that PVDF should not be used).
75. Let the spots dry completely at room temperature.
76. Place the membrane in a flat plastic dish.
77. Add Western Blot Blocking Buffer (WBBB) and place on a horizontal platform rotator/shaker at room temperature for 30 minutes.
78. After 30 minutes, discard the WBBB and wash the membrane 3 times in WBBB for 5 minutes each, discarding the liquid after each wash.
79. Incubate the membrane in WBBB containing secondary antibody conjugated to Horse Radish Peroxidase (HRP) or Alkaline Phosphatase (AP) (as needed) for 30 minutes at room temperature.
Critical STEP: The secondary antibody should be specific to species of the antibody being purified (in the case of the rabbit anti-pCGH-1, it is an anti-rabbit IgG secondary).
80. Wash the membrane three times for 10 minutes each with WBWB.
81. Develop with the ECL reagent as per manufacturer's instructions. ? TROUBLESHOOTING
82. Pool all the fractions that are positive for IgG in Step 81 (Figure 7).
Figure 7. Dot blotting to identify fractions containing anti-peptide antibody.
Following elution and neutralization of the bound antibody from the affinity column dot blotting is used to identify which fractions contain anti-IgG reactivity. 1 μl of each fraction from the phospho-peptide column elution (Step 72) is spotted on nitrocellulose, dried and then probed with appropriate secondary antibody (anti-rabbit) and developed with ECL. Positive signal for each fraction indicates that the fraction has IgG. All the positive fractions should be pooled and taken further.
Testing of purified antibodies for phospho-peptide specificity. TIMING 3 hours
83. Dissolve 10 μg/μl of the phospho and non-phospho peptide each in sterile de-ionized water.
84. Spot 1 μl each of the non-phospho and the phospho peptide from Step 83, in serial dilutions of 1 μg, 100 ng, 10 ng, 1 ng and 0.1 ng onto the same strip of nitrocellulose.
85. Let the spots dry.
86. Incubate the membrane in WBBB for 30 minutes at room temperature.
87. Discard the blocking buffer and wash the membrane three times for five minutes each in WBWB.
88. Dilute the pooled, neutralized phospho-antibody column eluate from Step 721:1000 in WBBB and add 10ml to the nitrocellulose membrane.
89. Incubate the membrane on a rotator at room temperature for 1 hour.
90. Discard the solution containing the diluted primary antibody.
91. Wash the membrane 3 times in WBWB for 5 minutes each.
92. Proceed as detailed in Step 81.
93. If the purified phospho-antibody preparation has reactivity to the non-phospho-peptide in the dot blot (Figure 5), then pass the pooled antibody fractions (Step 82) over the non-phospho-peptide column for an additional round of subtraction (Steps 50-57, Figure 2, G → B); the number of fractions pooled in Step 82 will depend on the efficiency of elution – make-up to a total volume of 15 ml by adding 100 mM NaCl prior to loading on to the non-phospho-peptide column in Step 52 (as a replacement for loading the diluted serum). ?TROUBLESHOOTING
CRITICAL STEP: Columns must be regenerated (Steps 104-110) before use in additional rounds of purification.
Testing of purified antibodies for phospho-protein specificity. TIMING: 1 day
94. If, as expected, the purified phospho-antibody preparation detects the phospho-peptide in the dot blot (Step 93; Figure 5) then test it for specificity (i.e. effectiveness of removal of antibodies that recognize low sequence complexity phospho-epitopes (Table 1)), using western blots prepared from total extracts of cells or organisms where the phosphorylation event is known or predicted to occur (as described in refs. 27 and 28; Figure 3).
95. If non-specific bands are detected in the western blot from Step 94 (Figure 3), purify the phospho-antibody preparation over the phospho-peptide column again repeating the stringent washes and elution (Steps 58-82, Figure 2, H→ D) by pooling fractions from Step 82, adjusting volume up to 15 ml with 100 mM NaCl and loading on to the phospho-peptide column in Step 61 (as a replacement for loading the flow through) and continue to Step 82 again.
96. If the first round antibodies detect both non-phospho-peptide in the dot blot from Step 93 and have multiple bands on whole cell extract western blot from Step 94, then re-purify the phospho-antibody, i.e. perform subtraction against the non-phospho-peptide and affinity purification against the phospho-peptide (repeat Steps 50-82; Figure 2, H → B), bypooling fractions from Step 82, adjusting volume up to 15 ml with 100 mM NaCl and loading on to the non-phospho-peptide column in Step 52 (as a replacement for loading the diluted serum) and continue to Step 82 again.
Genetic tests for phospho-antibody gene product specificity and kinase specificity (TIMING 1 day, not including time to generate biological samples to be analyzed)
97. A key test for specificity of a phospho-protein antibody preparation is to assess if signal from the purified antibody is specific to the gene product under study. The phospho-protein specific antibody should display no signal above background in gene product null mutants, or a strongly reduced signal following RNAi knockdown, in western blots (Figures 1A and 6) and by immuno-histochemistry (Figure 9) (as described in refs. 27 and 28). If inappropriate bands are observed in a western blot of a gene product null mutant sample then the antibody should undergo an additional round of affinity purification (Figure 2, H→ D; repeat Steps 58-82). An additional specificity test is to determine if the signal from the purified antibody is specific to the kinase that phosphorylates the site, if known. The phospho-protein specific antibody should display no signal above background in the kinase null mutants, or a strongly reduced signal following RNAi knockdown (as described in refs. 27 and 28; Figures 1A and 6).
Figure 9. Phosphorylation of CGH-1 alters protein subcellular localization.
Immuno-cytochemical staining of anti-total CGH-1 antibody (A) and purified anti-pCGH-1 antibody (B – D) to wild type (A & B), cgh-1(tn691ts) (C) and cgh-1(0) (D) mutant adult germlines, shown as an enlargements of the proximal pachytene region that contains activated MPK-1 ERK (Figure 8). Anti-total CGH-1 staining (A) highlights P-granules (arrows) that surround nuclei, as well as cytoplasmic and weaker nuclear staining. In contrast, anti-pCGH-1 staining (B) does not highlight P-granules while the cytoplasmic and weak nuclear staining remains. The anti-pCGH-1 antibody fails to stain cgh-1(0) (D) indicating that the antibody is specific for CGH-1 and fails to stain cgh-1(tn691ts) (C) which is a missense mutation in the Pro (Pro68Leu) that is C-terminal to phosphorylated Ser67 and thus not expected to be phosphorylated in the mutant.
Purification of non-phospho-protein specific antibodies. TIMING 2 hours
98. Prepare crude serum as described in Steps 47-49, remove the phospho-peptide column from 4°C, and perform Steps 51-56.
99. Collect the flow through from the phospho-peptide column and add to the non-phospho-peptide column.
100. Perform affinity purification with stringent washes following Steps 58-69.
101. Elute and neutralize the non-phospho-peptide specific antibody and identify antibody containing fractions as described (Steps 70-82).
102. Assess the specificity of the non-phospho-peptide antibody via dot blots (Steps 83-92) and western blotting (Step 94).
103. If the eluted non-phospho-peptide antibody preparation detects the phospho-peptide, then follow Steps 98-101 again and repurify the antibody.
Regeneration of the peptide columns. TIMING 30 minutes
104. Add 2 ×10 ml of 100mM glycine, pH 2.2 (Elution Buffer) to the column and let it drip through and discard.
105. Add 2 ×10 ml of 10mM Tris (pH 8.8) to the column and let it drip through and discard.
106. Add 2 ×10 ml of 10mM Tris (pH 7.5) to the column and let it drip through and discard.
107. Repeat Step 106 once more.
108. Add 10 ml of 10mM Tris (pH 7.5), 0.5M NaCl to the column and let drip through and discard.
109. Fill column with 10mM Tris (pH 7.5), 0.5M NaCl.
110. Add 0.2% sodium azide in 4 ml of Blocking Buffer to the column, close the stopcock, cap, wrap with Parafilm and store at 4°C.
Concentration of purified polyclonal antibody and storage. TIMING 1 hour for concentration and 16 hours for dialyzing the antibody
111. Add 10 ml of pooled purified phospho-antibody from Step 82 to a 10kDA Amicon Concentrator (Millipore).
112. Spin the tubes at 4000g for 45 minutes in a high-speed centrifuge at 4°C.
113. With extreme care and without disturbing the membrane draw out the reduced, concentrated antibody from top of the membrane, usually 100 – 400 μl of antibody containing solution.
114. Determine antibody concentration using a spectrophotometer.
115. Dialyze the antibody into 1× PBS overnight at 4°C using the Dialyzer cassettes.
116. Add to a final concentration of 2.5% BSA, 0.01% Tween-20 and 25% glycerol from a 10% BSA stock to the concentrated dialyzed antibody for long-term storage.
Purification of polyclonal antibodies to fusion proteins; making the columns with recombinant protein. Timing: 2 hours
117. Dialyze 500 μg/ml of purified recombinant protein into coupling buffer. Typically 500 μg-1 mg of a fusion protein is used for coupling to 1 ml of Sepharose.
118. Perform Steps 26-28.
119. Add the swollen resin to a Biorad EconoPac Column as described in Steps 29 and 30.
120. Add 20 ml of 10 mM HCl to the column, and let it drip through.
121. Add 5 ml of Coupling Buffer to the column and let it drip through.
122. Add 1 ml of the recombinant protein solution from Step 117 to the column from Step 121.
123. Perform Steps 35 and 36.
124. Incubate the column with the protein and Sepharose at 4°C overnight on a rotating shaker.
125. The next morning, at room temperature, let the protein solution drip through from the column.
126. Add 10 ml of Coupling Buffer to the column and let drip through.
127. Add 10 ml of Column Wash Buffer to the column and let drip through.
128. Perform Steps 65-69.
Caution: Do not allow the solution to collect in the column at any point by plugging the column. Allow for steady flow rate.
129. Add 50 ml of blocking buffer to the column, and let it drip through.
130. Perform Step 46 for column storage and future use.
Purification of antibodies from crude antisera to the fusion protein. Timing: 1 hour
131. Thaw 1-2 ml crude immune serum from -70°C.
132. Perform Steps 48-49.
133. Remove the affinity column (from Step 130) from 4° C and pre-wash the column with 20 ml of 100mM NaCl.
134. Add the diluted crude serum from Step 132 to the column, and care should be taken to close the column with the stopcock.
135. Perform Steps 53-54.
136. The next morning open the stop-cock and the cap and let solution drip through and discard.
137. Add 4 ×20 mL of 10 mM Tris pH 7.5, 0.5 M NaCl to the column and let drip through and discard.
138. Perform stringent washes as in Steps 65-69.
139. Elute bound antibodies as described in Steps 70-73? TROUBLESHOOTING
140. Regenerate the column as described in Steps 104-110.
141. Perform western blot to total protein (and genetic specificity tests) as in steps 94-97. If not specific, repeat affinity purification, stringent washes and elution as described in Steps 58-73.
142. Store the concentrated antibodies in 2.5% BSA, 0.01% Tween-20, and 25% glycerol at -80C. These antibodies can be stored for several years at -80°C.
Anticipated Results
To generate modified-protein specific polyclonal antibodies, multiple rounds of subtraction and affinity purification are usually necessary. As shown by dot blot (Figure 5), two rounds of subtraction for the anti-pCGH-1 antibody were necessary to remove antibodies that bound to the unmodified peptide. Four rounds of affinity purification with stringent washes were required to remove antibodies that reacted with proteins other than pCGH-1, as assessed by western blots of wild-type adult hermaphrodites lysates (Figure 3). Genetic tests employing western blots (Figure 6) confirmed that the purified antibody preparation was specific to CGH-1, as no signal was detected from cgh-1 null mutant lysates, and that the phosphorylation event required MPK-1 ERK, as the pCGH-1 band was not detected in the mpk-1 null mutant. Immuno-cytochemistry of whole germline tissue shows that there is complete overlap between the cells that stain with purified pCGH-1 antibody and those that contain activated MPK-1 ERK (Figure 8), as expected given that ERK is the kinase that phosphorylates CGH-1 on Ser67. Intriguingly, phosphorylation of CGH-1 alters the subcellular localization of the protein (Figure 9), illustrating one of the unique attributes of a modification specific antibody reagent. Total CGH-1 is present in the cytoplasm of germ cells, as well as on peri-nuclear P-granules19,83,84, germline granules that are thought to function in regulation of mRNA trafficking, translation and stability. We find that MPK-1 ERK phosphorylated CGH-1, while still present in the cytoplasm, is apparently absent from P-granules (Figure 9), illustrating a unique use of phospho-protein specific antibodies. Overall the protocol is robust, as we have purified phospho-protein specific antibodies for 14 proteins (S.A., Andy Golden and T.S. unpublished data) and total protein specific antibodies for 3 proteins (ref. 27, and S. A. unpublished data), made from either fusion proteins or peptides.
Figure 8. Staining for pCGH-1 and activated MPK-1 ERK overlap in the germline tissue.
Immuno-cytochemical staining of a wild-type adult C. elegans hermaphrodite germline for DNA (DAPI in blue, top), anti-activated ERK67 (dpMPK-1 in red, middle) and anti-phospho-CGH-1 (pCGH-1 in green, bottom), using the antibody preparation following four rounds of affinity purification. pCGH-1 is only observed in germ cells that contain activated MPK-1, one of a number of criteria which indicates that the affinity purified pCGH-1 preparation is specific (also see Figures 3 and 6).
Timing
The total time for the purification procedure from start to finish is 5 days and 7 hours, due to multiple over night incubation steps.
Steps 1-6; Conjugation of peptides to Maleimide activated BSA. TIMING 2-4 hours.
Steps 7-17; Desalting the peptide∷BSA conjugated mix. TIMING 30 minutes-1 hour.
Steps 18-22; Assessing the efficiency of peptide∷BSA conjugation. TIMING 5-6 hours.
Steps 23-25; Dialysis of peptide∷BSA complex into Coupling Buffer. TIMING 16 hours at 4°C.
Steps 26-46; Coupling of non-phospho- and phospho-peptide-BSA to sepharose. TIMING 1 day.
Steps 47-57; Subtraction of the non-phospho-peptide antibodies from the crude serum. TIMING 1 day.
Steps 58-64; Affinity purification of phospho-peptide antibodies from fraction subtracted with the non-phospho column. TIMING 1 day.
Steps 65-69; Stringent washing of the phospho-peptide column. TIMING 1 hour.
Steps 70-73; Elution of the anti-phospho-peptide antibodies TIMING: 30 minutes to 1 hour
Steps 74-82; Identifying fractions with IgG activity. TIMING 2 hours
Steps 83-93; Testing of purified antibodies for phospho-peptide specificity. TIMING 3 hours.
Steps 94-96; Testing of purified antibodies for phospho-protein specify. TIMING: 1 day.
Step 97; Genetic tests for phospho-antibody gene product specificity and kinase specificity. TIMING 1 day
Steps 98-103; Purification of non-phospho-protein specific antibodies. TIMING 2 hours.
Steps 104-110; Regeneration of the peptide columns. TIMING 30 minutes
Steps 111-116; Concentration of purified polyclonal antibody and storage. TIMING 1 hour for concentration and 16 hours for dialyzing the antibody.
Steps 117-130; Purification of polyclonal antibodies to fusion proteins; making the columns with recombinant protein. Timing: 2 hours.
Steps 131-142; Purification of antibodies from crude antisera to the fusion protein. Timing: 1 hour.
TROUBLESHOOTING
Troubleshooting advice can be found in Table 4.
Table 4. Troubleshooting.
| Step | Problem | Possible Reason | Solution |
|---|---|---|---|
| Generation of custom polyclonal antisera. | Peptide yielded no phospho-antibodies/very low number of phospho-antibodies. | The peptide designed had an internal cysteine that was used for conjugation. | An internal cysteine should not be used to conjugate the peptide to hapten for injection, since that will mask the phospho-epitope and result in antibodies that will be very difficult, if not impossible to resolve. Thus, in cases where there may be a cysteine right next to the phospho-epitope, there are better chances of obtaining an antibody of interest by making slightly longer peptides (11-14 mers) and obtaining them from the vendor in a reduced state, such that they do not form internal di-sulfide bonds. |
| Generation of custom polyclonal antisera. | The sera produced low titer against the phospho-antigen. | The region of the peptide used was low complexity | Anecdotally, we have found that different species of animals sometimes give different results in titer generation. Thus, using more than one animal species may be useful. Or, use 5-10 fold more sera with the same volume of the column (step 5 on) for purification |
| 22 | Conjugaton of BSA with peptide did not go to completion. | Inefficient or failed conjugation can occur if the quantity of peptide used is incorrect, if the peptide and/or BSA is not fully dissolved or if a wrong solution is used in the conjugation. | Ensure that the peptide is dissolved completely. |
| The peptide did not dissolve efficiently. | Add DMSO to the peptide (at 10%) dissolve. Spin out what has not dissolved. Then re-dissolve this in fresh conjugation buffer, with 10% DMSO and repeat until there is no precipitate left. Pool all the dialyzed samples. | ||
| Conjugation Buffer was not used to dissolve the peptide | Use the conjugation buffer supplied with the kit | ||
| Conjugation Buffer has precipitates | Check the date on which the kit was bought and ensure that it has not been at 4C for too long. | ||
| The conjugation reaction was not allowed to continue for 2 hours at room temperature | Incubate the peptide∷BSA mix at room temperature for 2 hours | ||
| 22, 41, 64 | Conjugation of peptide∷BSA complex did not go to completion/was not in right ratio with Sepharose. | The peptide∷BSA complex was in excess to the Sepharose beads. | Ensure that the peptide∷BSA complex is added in a ration of 2mg of the compelx to 1mg of Sephaorse. |
| The peptide∷BSA complex and Sephaorse mix dried in the column. | Ensure that there is enough coupling buffer such that the beads do not dry out. | ||
| 73, 139 | The pH of the collected Glycine eluted antibodies is not neutral. | The 1M Tris used for neutralization was pHed to a pH of 8. | Use 1M Tris that has not been pHed. This allows for the hydroxyl ions to neutralize the strong acidic Glycine solution. |
| 82, 93 | There are no IgG fractions upon elution from the phospho-peptide column | Peptide design was flawed, refer to generation of custom polyclonal antisera. | |
| Instead of using the flow through from the non-phospho peptide column, the elution from the non-phospho peptide column was used for binding to the phospho-peptide column. | Ensure that the FLOW THROUGH from the non-phospho peptide column is the one used to pass over the phospho-peptide column. | ||
| The phospho-peptide column was not washed thoroughly and coupled during preparation with the coupling buffer and wash buffer, resulting in some phospho-peptides coming off the column during antibody elution. | Prepare the phospho-peptide column again, with care taken to follow each step of the protocol. | ||
| The secondary used for blotting is old, and does not work. | Use a positive control sample to ensure that the secondary antibody works. | ||
| ECL kit used was left out at room temperature and the reagent is not optimal. | Use a positive control sample to ensure that the ECL reagent is optimally functioning. | ||
| 93 | The phospho-peptide antibody preparation also recognizes the non-phosph-peptide. | Refer to Figure 1 for possible options for the types of antibodies that can be generated from the phospho-peptide immunogen. | Pass the antibody back over the non-phospho-peptide column, collect flow through that is then passed over the phospho-peptide column. |
Acknowledgments
Work in the T.S. laboratory on this project was supported by National Science Foundation Grant 0416502 and National Institutes of Health Grant GM085150. Work in S.A. laboratory on this project was supported by National Institute for Health Grant GM98200, Institutional Research Grant from CCSG to UT MD Anderson Cancer Center and the Center for Genetics and Genomics, UT MD Anderson Cancer Center. We thank Drs. Andy Golden (NIH/NIDDK), David Greenstein (University of Minnesota) and Caroline Spike (University of Minnesota) for extensive comments on the manuscript.
Footnotes
Author Contributions: SA and TS designed, implemented and wrote the protocol.
Competing Financial Interests: The authors declare that they have no competing financial interests.
References
- 1.Cleghon V, et al. Opposing actions of CSW and RasGAP modulate the strength of Torso RTK signaling in the Drosophila terminal pathway. Mol Cell. 1998;2:719–727. doi: 10.1016/s1097-2765(00)80287-7. [DOI] [PubMed] [Google Scholar]
- 2.Cleghon V, et al. Drosophila terminal structure development is regulated by the compensatory activities of positive and negative phosphotyrosine signaling sites on the Torso RTK. Genes Dev. 1996;10:566–577. doi: 10.1101/gad.10.5.566. [DOI] [PubMed] [Google Scholar]
- 3.Cutler RE, Jr, Morrison DK. Mammalian Raf-1 is activated by mutations that restore Raf signaling in Drosophila. Embo J. 16:1953–1960. doi: 10.1093/emboj/16.8.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deem AK, Li X, Tyler JK. Epigenetic regulation of genomic integrity. Chromosoma. 121:131–151. doi: 10.1007/s00412-011-0358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao JJ, et al. The proteasome regulates bacterial CpG DNA-induced signaling pathways in murine macrophages. Shock. 34:390–401. doi: 10.1097/SHK.0b013e3181d884ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gayko U, Cleghon V, Copeland T, Morrison DK, Perrimon N. Synergistic activities of multiple phosphotyrosine residues mediate full signaling from the Drosophila Torso receptor tyrosine kinase. Proc Natl Acad Sci U S A. 1999;96:523–528. doi: 10.1073/pnas.96.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu V, Zobel CL, Lambie EJ, Schedl T, Kornfeld K. Caenorhabditis elegans lin-45 raf is essential for larval viability, fertility and the induction of vulval cell fates. Genetics. 2002;160:481–492. doi: 10.1093/genetics/160.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs D, Beitel GJ, Clark SG, Horvitz HR, Kornfeld K. Gain-of-function mutations in the Caenorhabditis elegans lin-1 ETS gene identify a C-terminal regulatory domain phosphorylated by ERK MAP kinase. Genetics. 1998;149:1809–1822. doi: 10.1093/genetics/149.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan DR, Morrison DK, Wong G, McCormick F, Williams LT. PDGF beta-receptor stimulates tyrosine phosphorylation of GAP and association of GAP with a signaling complex. Cell. 1990;61:125–133. doi: 10.1016/0092-8674(90)90220-9. [DOI] [PubMed] [Google Scholar]
- 10.Kornfeld K, Hom DB, Horvitz HR. The ksr-1 gene encodes a novel protein kinase involved in Ras-mediated signaling in C. elegans. Cell. 1995;83:903–913. doi: 10.1016/0092-8674(95)90206-6. [DOI] [PubMed] [Google Scholar]
- 11.Lackner MR, Kornfeld K, Miller LM, Horvitz HR, Kim SK. A MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis elegans. Genes Dev. 1994;8:160–173. doi: 10.1101/gad.8.2.160. [DOI] [PubMed] [Google Scholar]
- 12.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 14.Ohsawa R, Adkins M, Tyler JK. Epigenetic inheritance of an inducibly nucleosome-depleted promoter and its associated transcriptional state in the apparent absence of transcriptional activators. Epigenetics Chromatin. 2009;2:11. doi: 10.1186/1756-8935-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ory S, Morrison DK. Signal transduction: implications for Ras-dependent ERK signaling. Curr Biol. 2004;14:R277–278. doi: 10.1016/j.cub.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Ory S, Zhou M, Conrads TP, Veenstra TD, Morrison DK. Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr Biol. 2003;13:1356–1364. doi: 10.1016/s0960-9822(03)00535-9. [DOI] [PubMed] [Google Scholar]
- 17.Ritt DA, Monson DM, Specht SI, Morrison DK. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol. 2010;30:806–819. doi: 10.1128/MCB.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, et al. Key inflammatory signaling pathways are regulated by the proteasome. Shock. 2006;25:472–484. doi: 10.1097/01.shk.0000209554.46704.64. [DOI] [PubMed] [Google Scholar]
- 19.Jud MC, et al. Large P body-like RNPs form in C. elegans oocytes in response to arrested ovulation, heat shock, osmotic stress, and anoxia and are regulated by the major sperm protein pathway. Dev Biol. 2008;318:38–51. doi: 10.1016/j.ydbio.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T, et al. Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res. 2011;21:227–236. doi: 10.1101/gr.115519.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikeladze-Dvali T, et al. Analysis of centriole elimination during C. elegans oogenesis. Development. 2012;139:1670–1679. doi: 10.1242/dev.075440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 23.Argenzio E, et al. Proteomic snapshot of the EGF-induced ubiquitin network. Mol Syst Biol. 7:462. doi: 10.1038/msb.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blagoev B, et al. A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat Biotechnol. 2003;21:315–318. doi: 10.1038/nbt790. [DOI] [PubMed] [Google Scholar]
- 25.Pandey A, et al. Analysis of receptor signaling pathways by mass spectrometry: identification of vav-2 as a substrate of the epidermal and platelet-derived growth factor receptors. Proc Natl Acad Sci U S A. 2000;97:179–184. doi: 10.1073/pnas.97.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulze WX, Deng L, Mann M. Phosphotyrosine interactome of the ErbB-receptor kinase family. Mol Syst Biol. 2005;1 doi: 10.1038/msb4100012. 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arur S, et al. MPK-1 ERK controls membrane organization in C. elegans oogenesis via a sex-determination module. Dev Cell. 2011;20:677–688. doi: 10.1016/j.devcel.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arur S, et al. Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc Natl Acad Sci U S A. 2009;106:4776–4781. doi: 10.1073/pnas.0812285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus S, Seger R. Determination of ERK activity: antiphospho-ERK antibodies, in vitro phosphorylation, and in-gel kinase assay. Methods Mol Biol. 2004;250:29–48. doi: 10.1385/1-59259-671-1:29. [DOI] [PubMed] [Google Scholar]
- 30.Kumar JP, et al. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- 31.Perlson E, et al. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Z, et al. Detection of partially phosphorylated forms of ERK by monoclonal antibodies reveals spatial regulation of ERK activity by phosphatases. FEBS Lett. 2000;468:37–42. doi: 10.1016/s0014-5793(00)01191-1. [DOI] [PubMed] [Google Scholar]
- 34.Chuderland D, Seger R. Calcium regulates ERK signaling by modulating its protein-protein interactions. Commun Integr Biol. 2008;1:4–5. doi: 10.4161/cib.1.1.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadwiger G, Dour S, Arur S, Fox P, Nonet ML. A monoclonal antibody toolkit for C. elegans. PLoS One. 2010;5:e10161. doi: 10.1371/journal.pone.0010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinfeld H, Seger R. The ERK cascade as a prototype of MAPK signaling pathways. Methods Mol Biol. 2004;250:1–28. doi: 10.1385/1-59259-671-1:1. [DOI] [PubMed] [Google Scholar]
- 37.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Wolf I, et al. Involvement of the activation loop of ERK in the detachment from cytosolic anchoring. J Biol Chem. 2001;276:24490–24497. doi: 10.1074/jbc.M103352200. [DOI] [PubMed] [Google Scholar]
- 39.Yao Z, Seger R. The ERK signaling cascade--views from different subcellular compartments. Biofactors. 2009;35:407–416. doi: 10.1002/biof.52. [DOI] [PubMed] [Google Scholar]
- 40.Zehorai E, Yao Z, Plotnikov A, Seger R. The subcellular localization of MEK and ERK--a novel nuclear translocation signal (NTS) paves a way to the nucleus. Mol Cell Endocrinol. 2010;314:213–220. doi: 10.1016/j.mce.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Friedman A, Perrimon N. A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature. 2006;444:230–234. doi: 10.1038/nature05280. [DOI] [PubMed] [Google Scholar]
- 42.Ahn NG, Seger R, Bratlien RL, Krebs EG. Growth factor-stimulated phosphorylation cascades: activation of growth factor-stimulated MAP kinase. Ciba Found Symp. 1992;164:113–126. doi: 10.1002/9780470514207.ch8. discussion 126-131. [DOI] [PubMed] [Google Scholar]
- 43.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 44.Gabay L, Seger R, Shilo BZ. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997;124:3535–3541. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- 45.Harris D, et al. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHbeta-subunit promoter. Endocrinology. 2002;143:1018–1025. doi: 10.1210/endo.143.3.8675. [DOI] [PubMed] [Google Scholar]
- 46.Egelhofer TA, et al. An assessment of histone-modification antibody quality. Nat Struct Mol Biol. 2011;18:91–93. doi: 10.1038/nsmb.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie C, Liang Z, Chang S, Mohan C. Use of a novel elution regimen reveals the dominance of polyreactive antinuclear autoantibodies in lupus kidneys. Arthritis Rheum. 2003;48:2343–2352. doi: 10.1002/art.11092. [DOI] [PubMed] [Google Scholar]
- 48.Ayyar BV, Arora S, Murphy C, O'Kennedy R. Affinity chromatography as a tool for antibody purification. Methods. 2012;56:116–129. doi: 10.1016/j.ymeth.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Goto H, Inagaki M. Production of a site- and phosphorylation state-specific antibody. Nat Protoc. 2007;2:2574–2581. doi: 10.1038/nprot.2007.374. [DOI] [PubMed] [Google Scholar]
- 50.Ross AH, Baltimore D, Eisen HN. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981;294:654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- 51.Nairn AC, Detre JA, Casnellie JE, Greengard P. Serum antibodies that distinguish between the phospho- and dephospho-forms of a phosphoprotein. Nature. 1982;299:734–736. doi: 10.1038/299734a0. [DOI] [PubMed] [Google Scholar]
- 52.Boldicke T, et al. A new peptide-affinity tag for the detection and affinity purification of recombinant proteins with a monoclonal antibody. J Immunol Methods. 2000;240:165–183. doi: 10.1016/s0022-1759(00)00167-8. [DOI] [PubMed] [Google Scholar]
- 53.Cook C, Hazen B, Pang D. Luer tip quick antibody purification by affinity chromatography. Am Biotechnol Lab. 1990;8:16–18. [PubMed] [Google Scholar]
- 54.Dansithong W, Paul S, Kojima Y, Kamiya K, Shinozawa T. A simple method for midkine purification by affinity chromatography with a heavy chain variable domain (VH) fragment of antibody. J Biochem Biophys Methods. 2001;48:77–84. doi: 10.1016/s0165-022x(00)00145-7. [DOI] [PubMed] [Google Scholar]
- 55.Hocking GR, et al. Lymphocyte fluorescence polarization changes after phytohemagglutinin stimulation in the diagnosis of colorectal carcinoma. J Natl Cancer Inst. 1982;68:579–583. [PubMed] [Google Scholar]
- 56.Kreutz FT, Wishart DS, Suresh MR. Efficient bispecific monoclonal antibody purification using gradient thiophilic affinity chromatography. J Chromatogr B Biomed Sci Appl. 1998;714:161–170. doi: 10.1016/s0378-4347(98)00180-7. [DOI] [PubMed] [Google Scholar]
- 57.Czernik AJ, et al. Production of phosphorylation state-specific antibodies. Methods Enzymol. 1991;201:264–283. doi: 10.1016/0076-6879(91)01025-w. [DOI] [PubMed] [Google Scholar]
- 58.Meggio F, Perich JW, Reynolds EC, Pinna LA. A synthetic beta-casein phosphopeptide and analogues as model substrates for casein kinase-1, a ubiquitous, phosphate directed protein kinase. FEBS Lett. 1991;283:303–306. doi: 10.1016/0014-5793(91)80614-9. [DOI] [PubMed] [Google Scholar]
- 59.Meggio F, Perich JW, Reynolds EC, Pinna LA. Phosphotyrosine as a specificity determinant for casein kinase-2, a growth related Ser/Thr-specific protein kinase. FEBS Lett. 1991;279:307–309. doi: 10.1016/0014-5793(91)80174-2. [DOI] [PubMed] [Google Scholar]
- 60.Perich JW. Synthesis of O-phosphoserine- and O-phosphothreonine-containing peptides. Methods Enzymol. 1991;201:225–233. doi: 10.1016/0076-6879(91)01020-3. [DOI] [PubMed] [Google Scholar]
- 61.Perich JW. Synthesis of O-phosphotyrosine-containing peptides. Methods Enzymol. 1991;201:234–245. doi: 10.1016/0076-6879(91)01021-s. [DOI] [PubMed] [Google Scholar]
- 62.Saez JC, et al. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- 63.Archuleta AJ, Stutzke CA, Nixon KM, Browning MD. Optimized protocol to make phospho-specific antibodies that work. Methods Mol Biol. 2011;717:69–88. doi: 10.1007/978-1-61779-024-9_4. [DOI] [PubMed] [Google Scholar]
- 64.Blanquet PR, Mariani J, Fournier B. Temporal assessment of histone H3 phospho-acetylation and casein kinase 2 activation in dentate gyrus from ischemic rats. Brain Res. 2009;1302:10–20. doi: 10.1016/j.brainres.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 65.Veras E, Malpica A, Deavers MT, Silva EG. Mitosis-specific marker phospho-histone H3 in the assessment of mitotic index in uterine smooth muscle tumors: a pilot study. Int J Gynecol Pathol. 2009;28:316–321. doi: 10.1097/PGP.0b013e318193df97. [DOI] [PubMed] [Google Scholar]
- 66.Tapia C, et al. Two mitosis-specific antibodies, MPM-2 and phospho-histone H3 (Ser28), allow rapid and precise determination of mitotic activity. Am J Surg Pathol. 2006;30:83–89. doi: 10.1097/01.pas.0000183572.94140.43. [DOI] [PubMed] [Google Scholar]
- 67.Burks EA, Chen G, Georgiou G, Iverson BL. In vitro scanning saturation mutagenesis of an antibody binding pocket. Proc Natl Acad Sci U S A. 1997;94:412–417. doi: 10.1073/pnas.94.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davies J, Riechmann L. Affinity improvement of single antibody VH domains: residues in all three hypervariable regions affect antigen binding. Immunotechnology. 1996;2:169–179. doi: 10.1016/s1380-2933(96)00045-0. [DOI] [PubMed] [Google Scholar]
- 69.Davies J, Riechmann L. Single antibody domains as small recognition units: design and in vitro antigen selection of camelized, human VH domains with improved protein stability. Protein Eng. 1996;9:531–537. doi: 10.1093/protein/9.6.531. [DOI] [PubMed] [Google Scholar]
- 70.McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004;32:W20–25. doi: 10.1093/nar/gkh435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bernatowicz MS, Matsueda R, Matsueda GR. Preparation of Boc-[S-(3-nitro-2-pyridinesulfenyl)]-cysteine and its use for unsymmetrical disulfide bond formation. Int J Pept Protein Res. 1986;28:107–112. doi: 10.1111/j.1399-3011.1986.tb03236.x. [DOI] [PubMed] [Google Scholar]
- 72.Bernatowicz MS, Matsueda GR. Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent. Anal Biochem. 1986;155:95–102. doi: 10.1016/0003-2697(86)90231-9. [DOI] [PubMed] [Google Scholar]
- 73.Ejima D, Yumioka R, Tsumoto K, Arakawa T. Effective elution of antibodies by arginine and arginine derivatives in affinity column chromatography. Anal Biochem. 2005;345:250–257. doi: 10.1016/j.ab.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Karim AS, Johansson CS, Weltman JK. Maleimide-mediated protein conjugates of a nucleoside triphosphate gamma-S and an internucleotide phosphorothioate diester. Nucleic Acids Res. 1995;23:2037–2040. doi: 10.1093/nar/23.11.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taipa MA, Kaul RH, Mattiasson B, Cabral JM. Recovery of a monoclonal antibody from hybridoma culture supernatant by affinity precipitation with Eudragit S-100. Bioseparation. 2000;9:291–298. doi: 10.1023/a:1011183904966. [DOI] [PubMed] [Google Scholar]
- 76.Schall CA, Wiencek JM. Stability of nicotinamide adenine dinucleotide immobilized to cyanogen bromide activated agarose. Biotechnol Bioeng. 1997;53:41–48. doi: 10.1002/(SICI)1097-0290(19970105)53:1<41::AID-BIT7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 77.Bollin E, Jr, Sulkowski E. Physico-chemical characterization of hamster interferon. Prep Biochem. 1979;9:339–358. doi: 10.1080/00327487908061699. [DOI] [PubMed] [Google Scholar]
- 78.Werber MM. Nucleophilicity of isourea linkages in substituted agaroses. A direct method for the determination of alkylamino groups coupled to CNBr-activated agarose. Anal Biochem. 1976;76:177–183. doi: 10.1016/0003-2697(76)90276-1. [DOI] [PubMed] [Google Scholar]
- 79.Wilchek M, Oka T, Topper YJ. Structure of a soluble super-active insulin is revealed by the nature of the complex between cyanogen-bromide-activated sepharose and amines. Proc Natl Acad Sci U S A. 1975;72:1055–1058. doi: 10.1073/pnas.72.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meldrum NU, Tarr HL. The reduction of glutathione by the Warburg-Christian system. Biochem J. 1935;29:108–115. doi: 10.1042/bj0290108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol Biol. 1994;32:5–8. doi: 10.1385/0-89603-268-X:5. [DOI] [PubMed] [Google Scholar]
- 82.Sambrook J, Russell DW. The condensed protocols from Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; 2006. [Google Scholar]
- 83.Boag PR, Nakamura A, Blackwell TK. A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development. 2005;132:4975–4986. doi: 10.1242/dev.02060. [DOI] [PubMed] [Google Scholar]
- 84.Navarro RE, Blackwell TK. Requirement for P granules and meiosis for accumulation of the germline RNA helicase CGH-1. Genesis. 2005;42:172–180. doi: 10.1002/gene.20136. [DOI] [PubMed] [Google Scholar]