Abstract
Soft-tissue cells are surprisingly sensitive to the elasticity of their microenvironment, suggesting that traditional culture plastic and glass are less relevant to tissue regeneration and chemotherapeutics than might be achieved. Cells grown on gels that mimic the elasticity of tissue reveal a significant influence of matrix elasticity on adhesion, cytoskeletal organization, and even the differentiation of human adult derived stem cells. Cellular forces and feedback are keys to how cells feel their mechanical microenvironment, but detailed molecular mechanisms are still being elucidated. This review summarizes our initial findings for multipotent stem cells and also the elasticity-coupled effects of drugs on cancer cells and smooth muscle cells. The drugs include the contractility inhibitor blebbistatin, the proliferation inhibitor mitomycin C, an apoptotis-inducing antibody against CD47, and the translation inhibitor cycloheximide. The differential effects not only lend insight into mechano-sensing of the substrate by cells, but also have important implications for regeneration and molecular therapies.
Keywords: Cells, Tissue regeneration, Drug delivery, Matrix elasticity, Extracellular matrix, Atomic force microscopy (AFM), Mesenchymal stem cell (MSC)
1. Introduction
Physical contact of a soft tissue cell with its solid surroundings is integral to anchorage-dependence and other functions of the cell. Contacts or adhesions allow a cell to probe various properties of the extracellular matrix (ECM) as well as other cells, but in order to study such systems methodically, model microenvironments with well-defined parameters are needed. Fig. 1A illustrates a prototypical system that has emerged over the last decade that enables the study of the effects of a solid substrate's elasticity on cells. A tissue cell is shown attached via receptor-engaged contractile ‘stress fibers’ not to rigid plastic or glass but to an elastic substrate of tunable stiffness given by the elastic modulus E. Just as in vivo, adhesive ligands mediate attachment and cells are immersed in serum full of nutrients and growth factors. Such mechano-chemical culture models have indeed revealed the important biophysical interplay of cells and their microenvironment, thereby illuminating the fact that elasticities typical of solid tissue can be as influential to tissue cell structure and function as soluble chemical factors. The results even raise questions about the pathological effects of various forms of fibrosis and sclerosis (e.g. arteriosclerosis, multiple sclerosis)—which literally means ‘hardening’ as in hardening of the arteries. Here we extend recent reviews (e.g. [1]) to some of our recent data on substrate elasticity effects on cells, starting with stem cells before describing some of the modulating effects on drug efficacy.
Fig. 1.

Cells on elastic substrates that model tissue elasticity. A) Sketch of a model in vitro environment of a cell on a substrate of elasticity E, coated with ligands that are specifically recognized by cell adhesion receptors. Force sensing and transduction is mediated by these contacts. Biochemical stimuli are also provided by factors in the surrounding media. B) Elasticity of various solid tissues, and blood as a “fluid tissue”.
1.1. Tissue cells pull hard
Around 1980, at a time when various key cell adhesion and matrix molecules were still being elaborated [2], the developmental biologist A.K. Harris and his coworkers showed by very visual means that many non-muscle cells are highly contractile. They made highly flexible, silicone rubber films to image the strain-induced wrinkles under many types of tissue cells [3]. Fig. 2 shows the types of wrinkling deformations visible beneath adhering and contracting 3T3 fibroblasts, demonstrating that cells directly apply mechanical forces as they interact with their microenvironment. Although such films are still in use today, relating the wrinkling to tissue elasticities has proven difficult, and so connections to tissue function are less clear than might be achieved.
Fig. 2.
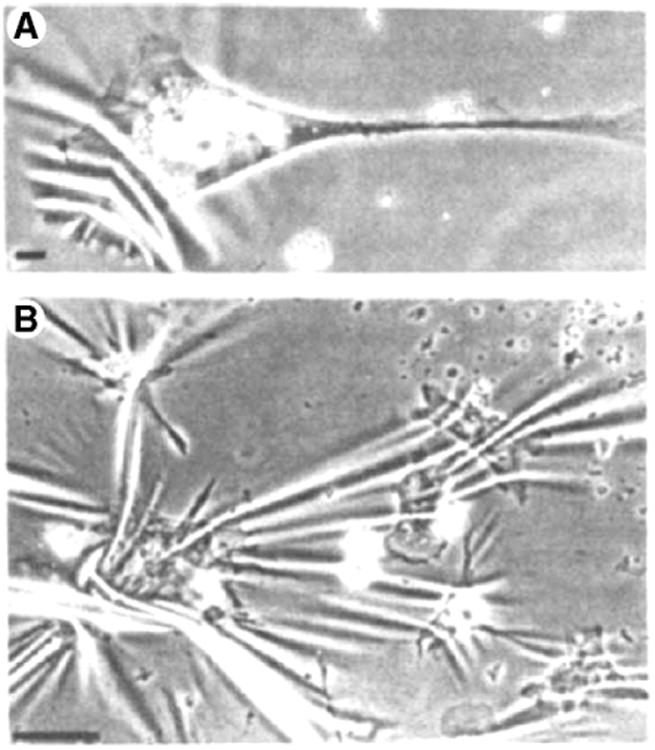
Cells exert force on the underlying substrate. (Top) A 3T3 fibroblast on a silicone rubber substrate deforms the film, causing wrinkles. Scale bar is 10 μm. (Bottom) Group of chick heart fibroblasts forming a more complex wrinkle pattern showing that strain transmission through the substrate propagates to neighboring cells. Scale bar is 100 μm. (Reprinted by permission from Macmillan Publishers Ltd: Nature (Harris, A.K., D. Stopak, and P. Wild, Fibroblast Traction as a Mechanism for Collagen Morphogenesis. Nature, 1981 290(5803): p. 249–251.), copyright (1981)).
Lurking within the studies of film wrinkling was a deeper, biologically important question of feedback: does the cell-induced strain exert an influence back on the cell? Cell biophysicist Y.L. Wang and coworkers provided perhaps the first clear and surprising answer. They replaced the wrinkling films with thick polyacrylamide (PA) gels in which the Young's modulus E could be tuned by polymer crosslinking, and then they showed that gel elasticity directly influences how cells spread, migrate, contract, and organize key intracellular structures such as focal adhesions [4]. This seminal work was somewhat limited by inaccurate measurements of E (that continue in many labs today and so workers in the field should beware), however, it subsequently led to an elaboration of “traction maps” that exploited displacements of gel-embedded beads – rather than wrinkles – to map out cell-induced strains in the elastic substrate [5]. A number of other biologically important feedback-based “cell on gel” effects were derived from such cell-matrix mechanics [1], but these have been placed increasingly in a tissue context with more accurate measurements of E that not only clarify the level of stress or traction exerted by the cells but also allow important comparisons to tissue microenvironments.
Cells ‘grip’ a substrate by attachment to adhesive ligands through transmembrane receptors that are often linked to the cytoskeleton. Synthetic matrices such as PA gels must therefore be covalently coated with ECM proteins, but while ligand density is important, it cannot override the key modulating effects of substrate elasticity [1]. In such studies, gels are attached to glass coverslips to maintain a well-defined gel surface, and to minimize detachment, shift, or gel shrinkage in prolonged cell culture. Furthermore, a basic law in polymer physics is that an increase in, physical or chemical, crosslinking increases the stiffness of a gel. With PA gels, increasing the concentrations of acrylamide monomer or bis-acrylamide crosslinker indeed increases the elastic modulus (or Young's modulus) in the range of E∼0.1–100kPa (Table 1). This is a relatively large range compared to most other polymer systems and can in principle lead to gel mimics of very soft tissues such as brain (∼0.5kPa), intermediate stiffness tissues such as muscle (∼10kPa, transverse and in a relaxed state), and rigid tissues such as pre-mineralized collagenous bone (≥30kPa) as depicted in Fig. 1B. While the cited elasticities are typical of normal tissues, disease states often lead to changes in tissue mechanical properties. Perhaps the classic example is the rigidification in tumors that forms the basis for breast cancer self-examination, but further examples include several forms of neuro-degeneration, such as multiple sclerosis and amyotrophic lateral sclerosis that involve ‘hardening’ of otherwise soft neuronal tissue. Suitably tuned elastic gel substrates might serve as better models to study such diseases, especially the coupling to soluble factors and perhaps drug treatments as elaborated below.
Table 1. Synthetic and natural substrates used for two dimensional cell culture.
| Material | Origin | Elasticity E [kPa] |
|---|---|---|
| Polyacrylamide (PA) | Synthetic | 0.1–100 |
| PDMS | Synthetic | 10–1000 |
| Collagen | Natural | 0.001–1 |
| Fibronectin | Natural | 0.001–1 |
| Matrigel | Natural | <1 |
| Alginate | Natural | 0.1–150 |
| Hyaluronic acid (HA) | Natural | 0.1–150 |
Collagen I coated substrates that are stiff – with E ranging from 20kPa gels to 1GPa glass coverslips – will generally promote maximal cell spreading, drive localized enrichments of cell adhesion proteins (i.e. ‘focal adhesions’), and foster cytoskeleton assembly with stress fiber formation. In contrast, cells on very soft gels tend to be rounder and less spread, exhibiting few stable focal adhesions and a more diffuse and less organized cytoskeleton. All of these aspects of adhesion influence the tractions or stresses exerted by the cells. By monitoring bead displacement in gels, estimates of tractions from classical elasticity computations imply cell stresses can be up to ∼1–10kPa, and while these stresses are generally directed radially inward by contractile stresses, they are heterogeneous [5]. PA gel systems can be functionalized with various densities of ligands, and are otherwise inert, linearly elastic, and stable in long term culture. For these reasons and more, PA gels continue to be a key material of choice for the study of cells on elastic matrices; but other synthetic hydrogel systems are also being exploited to measure cell-generated forces and/or monitor cell responses to substrate elasticity. For instance, matrices of polydimethylsiloxane (PDMS), polyethylene glycol (PEG), and polyelectrolyte multilayers (PEM) have shown similar effects as PA gels [6–9]. PDMS micro-pillar arrays are likewise being used to measure the traction forces of cells [10,11], although such systems convolute substrate elasticity effects with topology or geometry effects.
The synthetic polymers cited above allow one to learn new – and arguably important – biology, but such materials might not be optimal for in vivo applications. Natural ECMs can be made or modified from fibronectin, fibrinogen [12], collagen [13], or a mixture known as matrigel [14,15] composed of collagen, laminin, and growth factors co-extracted from murine tumors. These ECMs are biocompatible in many ways and possibly allow for therapeutic use in the body as discussed below, although care must be taken when using animal-derived proteins, especially from different species (‘xenobiotics’) [16]. Despite important advantages with ECMs such as the ability to embed cells and present a 3-dimensional fibrillar network that resembles an in vivo microenvironment, the elasticity of these natural scaffolds is less ‘tunable’. It therefore seems important to select the proper material based on both the desired receptor–ligand interactions (detailed below) and the cell-directing mechanical properties of the ECM.
In addition to gels of natural ECM proteins, polysaccharides and their derivatives are also being developed to study ‘cell on gel’ effects. Alginate, a seaweed-derived product, has been labeled with two distinct fluorescent dyes by Mooney and coworkers so that Fluorescence Resonance Energy Transfer (FRET) between the dyes will occur at locations where cell adhesions cluster the material and increase the local fluorophore density [17]. Glycosaminoglycans are linear polysaccharides with additional carboxylic and acetamido side groups, making them particularly good candidates for biomaterial applications, especially as they are commonly found in mammalian connective tissue. In particular, hyaluronic acid (HA) has already been used extensively for wound healing purposes, and can be covalently cross-linked (using an efficient chemical modification scheme) to form stable but soft hydrogels [18]; we have recently extended this chemistry with minor modification to achieve stable and stiff gels that cover the range of E achieved with PA gels (Table 1). Since HA is polysaccharide-based and does not contain any protein, it is readily purified and free of xenobiotics that can prompt an immune response; it has thus been safely injected into animals and humans [19,20]. Table 1 summarizes the origin and elasticity of some of the cited cell matrices.
2. Accurate micro-measurements of substrate elasticity
A number of experimental approaches allow one to measure the mechanical properties of matrices used for cell culture studies. However, techniques that measure culture samples on the micron length scale are perhaps the most appropriate since cells feel their matrices on this length scale rather than on length scales of millimeters or more. Compared to macroscopic methods, micro-scale methods can best assess matrix homogeneity or heterogeneity—both laterally and through the depth of a gel. Atomic force microscopy (AFM) and related nano-indenter devices are perfectly suited for micro-scale measurements as these instruments use a well-defined indenting tip to deform the substrate and measure the requisite force. While the AFM developed in the 1980's by Binnig et al. [21] is often used as a ‘stylus’ for topographical imaging of samples at nanometer scale resolution, the many commercial instruments available now also generally allow precise mechanical measurements with a wide choice of indenter shape, cantilever stiffness (i.e. spring constant), and control over indentation rate and depth [22]. Important to biological applications, an AFM can be operated in a liquid environment, which has fostered its success in measuring the mechanical properties of hydrogel matrices [8], as well as living cells [23] and even single protein molecules [24].
As depicted in Fig. 3A, a probe-tipped AFM cantilever is pushed into the substrate while cantilever bending is measured by a reflected laser beam. A wide range of materials with a Young's modulus E from 1MPa (very rigid) down to 100Pa (very soft) can be accurately measured using cantilevers that bend at the tip with a spring constant as small as 10–100pN/nm. Most of the newer commercially available scanning AFMs also offer the possibility to raster across a sample in force-mode to map the sample's surface elasticity with sub-micrometer resolution.
Fig. 3.

Measurement of micro-elasticity of matrices by AFM. A) A cantilever with a pyramidal tip (opening angle α) is translated down (Δz) into the sample causing a deflection d monitored by the photodiode, yielding the indentation δ=Δz–d. The required force F=k Δd is defined by the spring constant k multiplied by the deflection d. B) The Young's modulus E is determined by analyzing the resulting force-indentation curves with a modified Hertz model. Black curve denotes data points; red solid line is the best fit resulting from the modified Hertz model. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Force-indention curves fitted with a variant of the classic Hertz model [25] yield the Young's modulus E. A pyramidal tip is commonly used for imaging, but it also works for elasticity measurements and is most simply approximated as a conical probe with an opening angle α, so that the appropriate equation to determine E is:
where F is the force to bend the cantilever (i.e. F=kcd in Fig. 3B), δ is the indentation into the gel, and ν is the gel's Poisson ratio that is separately measured (or estimated typically as 0.3–0.5). Fig. 3B shows measured data points (black) for indentation δ vs. position z and the respective best fit for E of the modified Hertz model (solid red line). While all of the above elasticities for various materials correspond to low frequency or low rate quasi-static elasticity and should be compared when possible to large samples subjected to classical measurements such as tensile tests (e.g. hanging a weight on a rectangular sample), micro-methods also exist to measure the frequency-dependent rheology of substrates. Such measurements will no doubt be important in the future to understand the time scale over which cells feel their substrate. Based on current views, it seems unlikely that cells pull on their substrates or respond at MHz or higher frequencies, but timescales from milliseconds to hours are all potentially relevant to the cell on gel response. Focal adhesion proteins, for example, bind and dissociate from focal adhesions on time scales of ∼1–10s [26].
Among other commonly used methods to determine the mechanical properties of gels are standard macroscopic rheology techniques (e.g. disk rheometer) or scanning probe microscope (SPM) modes of AFM [27], that can determine not only the static elastic response of gels but also the dynamic viscous behavior described by the storage modulus (elastic solid) and loss modulus (viscous fluid) as well as non-linearities with strain. The latter method even allows for assessing the micro rheology of the gel. A comparative study of several types of bio-matrix gels and PA gels [28] has shown that whereas PA gels exhibit elasticity independent of the applied strain, biological gels of collagen or fibrin or cytoskeletal proteins – such as actin, vimentin, or neurofilaments – all display a strain-hardening behavior. Further developments of materials and continued study of cell responses should probably assess such non-linearities.
2.1. Micro-elasticity of tissue and organs
The methods described above can also be applied to accurate determinations of the stiffness of different tissues in native conditions. As mentioned earlier, the elasticity of tissue in an organism ranges from very soft (∼0.1–1kPa, e.g. brain tissue) [29,30] to extremely stiff or rigid (30kPa and far above, e.g. osteoid and fully mineralized bone, respectively). We have had a particular interest in striated muscle and various muscle diseases, and we and others have used AFM to measure their transverse elasticity. At the molecular scale, relaxed myofibrils from rabbit skeletal muscle have an intermediate modulus of E≈ 11.5±3.5kPa that increases almost 10-fold in the fully contracted ‘rigor’ state (84.0± 18.1kPa) [31]. The relaxed results appear relatively consistent with measurements for the passive transverse elasticity of rat skeletal muscle of 15.6±5.4kPa under in vivo compression [32]. Both of these values agree with our measurements for the passive elasticity of EDL muscle from normal mice with E ≈ 12±4kPa [33] and the elasticity of 1-week old C2C12 cells E ≈ 12–15kPa [34]. In light of tissue or organ regeneration, it is also important to determine the elasticity of diseased or regenerating tissue. For example, we find that the muscle from dystrophic mice has an increased stiffness of about 18±6kPa [33]. Likewise, myofibroblast-rich granulation tissue formed during wound closure has E ≈ 50±30kPa that is 3–5-fold higher than normal tissue [35]. We have also measured myocardium, which in healthy rats yields E=18±2kPa and a threefold increase to E=55±15kPa for infarcted myocardium [36]. In general, rigor and disease can significantly stiffen tissue.
2.2. Ligands for cell adhesion
‘Cell on gel’ effects do not mitigate the importance of matrix ligand—they add to them. For successful cell adhesion, components of the ECM such as collagen or laminin must be used and these are recognized of course by adhesion receptors such as the integrin superfamily (and dystroglycans, etc.), which establish a cell-matrix anchorage for the cell. Depending on cell type, different ligands might be more applicable. Fibroblasts, for example, secrete collagen and fibronectin, to reestablish the ECM during wound healing. Since collagen I is the most abundant collagen type in the body, it is frequently used as a ligand for multiple cell types ranging from fibroblasts to smooth muscle cells. Other ligands such as collagen II and collagen IV, fibronectin, and laminin are often used for more specialized assays and cell types. Adhesion to ECM through integrins initiates a signal cascade that often fosters further cell adhesion; thus choosing the right ligand is important for mimicking the native environments of different types of cells. Table 2 provides a short list of relevant ligands engaged in cell anchorage with their respective matrix binding partners in their resident tissue.
Table 2. Subset of extracellular ligands and their respective binding proteins [37].
| Ligand | Matrix association | Cell receptor | Tissue location |
|---|---|---|---|
| Collagen I | Collagen, fibronectin | Integrin | ECM, tendons, bone, skin |
| Collagen IV | Laminin | Integrin | Basal lamina |
| Fibronectin | Collagen, fibrin, heparin, fibullin, syndecan | Integrin | ECM, especially wound healing process |
| Elastin | Tropoelastin, fibrillin | ECM, especially arteries | |
| Laminin | Laminin, collagen IV | Integrin | Epithelium, endothelium, basal lamina |
Beyond simply selecting a particular ligand, the ligand density is also often critically important for cell adhesion and the ability of cells to respond to mechanical cues. Ligand density can influence cell motility in non-linear ways as shown in classic experiments that identify an intermediate density of ligand for maximal crawling speed (on rigid glass); modeling with classical reaction kinetics by Lauffenburger et al. suggests that a high ligand density strongly attaches the rear of a cell to the substrate, so that myosin contractility cannot detach the rear [38]. These studies have recently been extended to determine the combined elasticity- and ligand-dependent motility, with high ligand density again limiting mechanically-dependent cell motility responses [39]. Other metrics of cell response, such as cell spreading area, cytoskeletal organization, and contractility, also appear to be ligand density dependent [40,41]. The results generally demonstrate the non-linearity of ECM responses, and reasonable fits with simple thermodynamic (or steady state) models appear insightful.
2.3. Differentiation and other cellular processes on gels and matrices
As highlighted thus far, the stiffness of the underlying substrate influences the extent to which cells spread, establish adhesive contact, and assemble cytoskeletal structures. Mesen-chymal stem cells (MSCs) provide striking evidence that matrix elasticity influences higher cell functions such as differentiation [42]. Naive MSCs plated on collagen-coated PA gels are initially round, but within hours of plating, they attach and form adhesions to the substrates. Fig. 4 illustrates the differences in cell morphology that are suggestive of specific cell lineages after plating cells for just 24 h. A dominant fraction of each population of low passage MSCs spreads as shown (Fig. 4A plot). Importantly, treatment with mitomycin C, a DNA crosslinking agent that generally inhibits proliferation, does not block this initial spreading step for the majority of cells and further indicates that this is a population-wide shift towards morphologies of various lineages. After 1–4 weeks on elastic substrates (Fig. 4B), cells in identical media conditions are found to express key markers of early neurogenic, myogenic, and osteogenic lineages on gels that correspond respectively to tissue elasticities of brain, muscle, and nascent (pre-mineralized) bone. The neurogenic cytoskeletal protein β3-tubulin was expressed most prominently in the axon-like extensions of cells on the soft 1kPa gels. The myogenic transcription factor MyoD peaked on the stiffer 11kPa substrates, and MyoD even appeared prominent in a fraction of the cell nuclei. Likewise, the osteogenic transcription factor CBFα1 was up-regulated on the stiffest, 34kPa, matrices. These observations of protein markers were substantiated and elaborated further with transcript profiles [42].
Fig. 4.

Mesenchymal stem cell (MSC) differentiation on elastic substrates. A) Morphology of naive, low passage MSCs (upper panel) 24 h after plating on PA gels of different stiffness closely matches that of cell lineages found within each microenvironment. Naive MSCs are initially small and round but a dominant fraction indicated here (lower panel) develops increasingly branched, spindle, or polygonal shapes within days of plating when grown on matrices with respective elasticities of 1 kPa, 11 kPa, and 34 kPa. Results for mitomycin C treated cells are shown with diagonally-hatched bars. Scale bar is 20 μm. B) Differentiation of MSCs directed by substrate elasticity elucidated by key marker proteins. The neuronal cytoskeletal marker (β3 tubulin is expressed in branches (arrows) of initially naive MSCs (>75%) and only on soft, neurogenic matrices (first row). The muscle transcription factor MyoD is up-regulated and nuclear localized (arrow) only in MSCs on myogenic matrices (second row). The osteoblast transcription factor CBFα1 (arrow) is likewise expressed only on stiff osteogenic substrates (third row). Scale bar is 5 μm.
In committed tissue cells, important elasticity-dependent effects have been documented in the tumor-like growth of ductal epithelial cells [43], in focal adhesion dynamics in epithelial cells and fibroblasts [4], and in the contractile prestress or force-generation in primary smooth muscle cells [44]. Cell motility is also affected as observed on PA substrates with a steep rigidity gradient that has led to the term “durotaxis” [45]. Fibroblasts approaching a steep transition from a soft gel to a stiff gel will migrate across, with a concurrent increase in spreading area and traction. In contrast, cells migrating from the stiff substrate will turn around or retract, as if the cells are unable to overcome the stronger adhesion on stiffer substrates. Such processes certainly reinforce the impression that ‘cells feel their way through life’.
Traditional cell culture is done with cells on surfaces (i.e. 2D) for many reasons of convenience and accessibility, but most tissue cells in vivo are in 3D microenvironments that not only change the number of possible attachments but can also alter transport to and from the cell as well as many aspects of cell behavior, including mechanical constraints. 3D could in principle evoke new signals not utilized by cells in 2D. Cell biology research seems increasingly headed toward this added dimension [46–49]. Seminal work by Yamada et al. has shown that fibroblasts move and divide faster and are more similar to in vivo cell shapes in 3D versus 2D constructs. Softer, fibrillar 3D microenvironments also seem to stimulate matrix secretion [48], which could profoundly modulate cell phenotype [50]. Kinetic modeling by Zaman et al. has recently predicted distinct cell migration in 2D versus 3D microenvironments, consistent with processes such as durotaxis [51]. For a more systematic study of such processes, 3D models are required to mimic the native environment while still being accessible for experimental investigation. New technologies such as electro-spinning are used for the production of polymer networks with a well-defined mesh size and tunable compliance to allow for tailored 3D environments for both cell biology studies and biomedical applications [52,53].
2.4. Pharmacological effects and mechanistic insights
A large number of membrane-localized proteins and their interactions no doubt underlie the varied molecular mechanisms behind the diverse cell on gel responses (Fig. 5). Ligand–receptor binding is clearly the first step of cell-substrate or cell-cell interactions, but proteins of the integrin superfamily not only bind to ECM ligands (as mentioned above), but also establish intracellular connections with the cytoskeleton via large, multi-domain scaffolding and crosslinker proteins (talin, paxillin, etc.). Unbound integrins are relatively mobile in the cell membrane but readily form clusters and focal adhesion complexes with the cytoskeleton when they bind to the ECM in a force-dependent manner [54–56]. Integrins that are bound to the ECM induce further signaling [57,58], and cytoplasmic signaling proteins such as Rac and Rho activators are clearly involved in establishing focal complexes and maturing them to focal contacts [59,60].
Fig. 5.

Interplay of adhesion, force-sensing, and signal transduction. Integrins bind extracellular matrix ligands and link to the cytoskeleton. Signaling proteins such as Rac and Rho are up and down regulated coupled to myosin II-based contractility, thereby influencing local and global adhesion.
Cell contractility is due to molecular motors that are also needed for adhesion maturation and maintenance of focal complexes and contacts. Non-muscle myosin II (NMM II) in its three isoforms (a, b, and c) seems key to the contractility of non-muscle cells and ‘pulls the trigger’ on mechano-sensing and signal transduction [61]. The recently identified drug blebbistatin, [62] is a selective NMM IIa–c inhibitor that blocks motor activity without affecting actin binding, the drug completely shuts down the differential response of adult MSCs to matrix elasticity [42], and it does so by disabling active contraction within the cell, thereby preventing the cell from perceiving substrate elasticity. Even the initial spreading of MSCs on elastic substrates (4h after plating) that otherwise shows an increase in area of the cell body with stiffer substrates is inhibited by treatment with blebbistatin (Fig. 6A). In the presence of this drug, the main bodies of cells on all substrates remain small and round, which shows that NMM II is crucial for cell spreading. Careful titration of this drug might inhibit just enough myosin to deceive the cell into feeling a decreased “effective stiffness”, but partial inhibition might also impact the entire sensory mechanism by just ‘turning off the motor’. Such questions and others that take advantage of the reversible binding of drugs (rather than irreversible mutants) should lend important insight in the future.
Fig. 6.
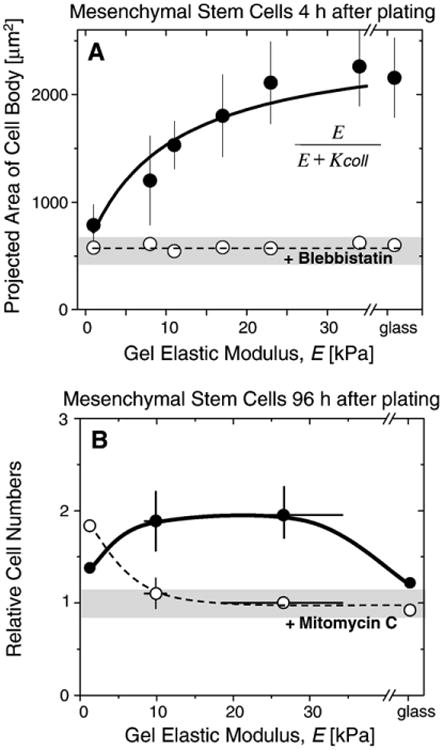
MSC spreading, proliferation, and drug responses. A) Area of MSC cell body as measured on PA gels of different stiffnesses and on glass. Cell area increases with stiffness unless treated with the myosin II inhibitor, blebbistatin. The solid line shows the best fit using the function shown as inset yielding K coll=10 kPa that agrees well with values determined for smooth muscle cells. B) MSC cell numbers relative to the number plated on elastic PA hydrogels. On soft gels, mitomycin C treated cells (open circles) seem to be more proliferative than the untreated control cells (full grey circles), whereas the behavior reverses on very rigid surfaces and on glass, proliferation is suppressed due to strong adhesion to stiffer substrates.
Inhibition of non-muscle myosin II is already known to cause disassembly of focal adhesion complexes [59]. Use of KT5926, a potent inhibitor of the myosin light chain kinase, or 2,3-butanedione monoxime (BDM), another inhibitor of myosin motors shows that focal adhesions normally seen on firm substrates disappear and are instead replaced with emerging irregular vinculin-containing structures that resemble punctates seen on very soft substrates [4]. Despite these studies, we understand very little of the mechanistic details of how NMM II together with the cytoskeleton and other proteins, collectively regulate force-generation, mechano-sensing, signal transduction, and adhesion of cells.
One key physical principle that is at least clear from single molecule studies [63,64] is that force F exponentially tends to accelerate dissociation of protein complexes. The common kinetic expression in terms of the zero-force dissociation rate ko and force-dependent rate k is:
where F* characterizes the bond sensitivity to force. In principle, this parameter F* might also be negative so that force inhibits dissociation. Such a bond has been called a ‘catch bond’ and some groups have claimed evidence for it in some examples of adhesion interactions [65–67]. Regardless, the physics highlights a generic force-dependent dynamic to all of the non-covalent interactions from matrix to ligand to receptor to actin-myosin cytoskeletal assembly. It seems most likely that force-generation – as inhibited by blebbistatin – couples to a spectrum of ko and F* to produce the wide-ranging responses found in the cell on gel effects.
Although inhibition of myosin appears to broadly prevent cell on gel responses for all substrates, other drugs could have more selective effects. Mitomycin C inhibits cell proliferation. It is therefore used as an anti-cancer drug and its effects on MSCs as a model for a ‘cancer stem cell’ or perhaps just a generic stromal cell can be informative. Surprisingly, initial data with MSCs suggests that these cells on soft gels divide somewhat more rapidly in the presence of this drug versus the controls (Fig. 6B). The proliferation of MSCs on soft gels in the presence of this drug is similar to proliferation on moderate to stiff gels. On rigid glass substrates, in comparison, adhesion seems so strong that proliferation is inhibited with or without drug. These results suggest that cells in the very soft parts of a solid tumor, which one might speculate includes the necrotic core, will divide and grow slightly more upon delivery of an anti-cancer drug. Given the potential patho-physiological relevance of matrix elasticity, such matrix-moderated effects on proliferation and on drug-induced effects on proliferation should be more seriously factored into therapeutic applications.
We have begun to test more directly the above hypothesis regarding cancer cells and matrix-modulated therapies. Since adhesion has a key role in matrix mechanotransduction even in cancer [43], molecularly specific therapies are being developed for cancer therapy among other conditions and diseases. Like most cells, human lung cancer derived epithelial cells (A549) spread well on rigid substrates coated with collagen I (Fig. 7). Such glass substrates might be considered more representative of fibrotic, rigidified tissue, whereas soft gels (E=4kPa) which are likewise coated and perhaps approximate compliant lung tissue promote little spreading. The A549 cells are known to express non-muscle myosins [68], which are required for cell spreading as noted above. These cells also express the cell surface receptor, Integrin Associated Protein (IAP, or CD47), that can induce apoptosis upon ligation with antibodies [69]. Such molecularly specific treatment indeed proves effective on cells growing on rigid substrates, but the antibody addition appears ineffective on cells growing on soft gels. The dye used for these studies (mitotracker) stains the mitochondria red in viable cells and then leaks into the cytoplasm of non-viable cells to become green. Such dyes thus show that the A549 cells are about as viable on soft gels as on standard rigid substrates, but they more importantly show that a molecularly specific therapy is much less effective on perfectly viable cells grown on the same ligand in the same medium but on a mechanically distinct matrix. The correlation appears simple in this case: there is 5.2-fold less spreading and 6.3-fold less cell death on soft substrates. Less adhesion leading to less effect seems very sensible for an antibody directed at an integrin associated protein such as CD47.
Fig. 7.

Substrate elasticity influences spreading and antibody-directed drug treatment of cancer cells. (A) Human lung cancer epithelial cells (A549) spread on collagen I coated rigid substrates, but 4 kPa gels induce little cell spreading. Images show viable cells are red-stained with mito-tracker dye, but the dye disperses to cytoplasm and changes to green with cell death. (B) Addition of an apoptosis inducing antibody against CD47 (B6H12) has little effect on the viability of cells on soft gels, but is effective against cells spread on rigid substrates. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Force-generation and adhesion are critical in cell spreading, but whether protein synthesis is required has not yet been adequately addressed. The question is whether simple rearrangement of existing proteins and structures is sufficient for initial cell on gel effects, or whether cells immediately synthesize and assemble new structures to adequately respond to their environment? Cycloheximide (CHX) acts on the ribosome and is an inhibitor of protein biosynthesis in eukaryotic organisms. CHX-treated cells thus lose their ability to express new proteins. Smooth muscle cells (SMCs) from rat aorta (A7r5) were allowed to spread for 4h on PA gels of different stiffness and show the usual increase in spreading on stiffer gels as reported previously with these cells [39] and also as shown above for MSCs. However, a marked decrease in spread area was observed for CHX-treated cells on gel or glass substrates that are more rigid than ∼10kPa as shown in Fig. 8 [70]. This data suggests that on stiffer substrates blocking upregulation (or denovo synthesis) of certain proteins affects cell adhesion and markedly influences the cell spreading process. Immunostaining shows that ribosomes in these cells are dispersed throughout the cytoplasm to the cell edge and could conceivably translate protein required for some or all aspects of cell spreading. Curiously, cell spreading on intermediate stiffness gels of ∼10kPa appears unaffected by CHX treatment. The results thus suggest two mechanisms or regimes of cell spreading, only one of which is drug sensitive. Such findings with smooth muscle cells may also be relevant to treatment and control of proliferation and differentiation of these cells in atherosclerotic plaques as well as in implanted stents where restenosis is a problem.
Fig. 8.

Cell area of smooth muscle cells spread on elastic surfaces and glass, with and without ribosome inhibition with cycloheximide (CHX). Treated cells show significant (p<0.01) differences versus control cells only, when grown on stiffer surfaces (34 kPa and rigid glass). Top images show actin (red) and ribosomes (green) that are found throughout the cytoplasm and at the cell edge. Right images show the actin cytoskeleton is usually organized on rigid matrices unless CHX-treated. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Results with drug treatments of various cells on soft, stiff, and rigid matrices illustrate the broad range of possible matrix-dependent drug responses. Cells on soft gels might be relatively unaffected in spreading (Fig. 6A, 8) or apoptosis-induction (Fig. 7) while they might be stimulated to proliferate (Fig. 6B). On the other hand, cells on stiff substrates – perhaps simple models for fibrotic tissues typical in tumors – generally seem more sensitive to diverse drugs in terms of spreading (Fig. 5A, 7) and apoptosis-induction (Fig. 6), but the slowed proliferation on rigid substrates (at least for stem cells) limits anti-proliferative effects (Fig. 6B). Further differences are seen on substrates of intermediate stiffness, which underlines the key point that matrix elasticity can strongly couple to cell responses induced by soluble effectors. Thus, the ineffectiveness of in vivo drug therapies on tumors, where recurrence is common, might well reflect microenvironment heterogeneity and its direct influence on cell programs and drug susceptibility. A novel therapeutic strategy that is suggested here entails delivery of drug cocktails where each drug is effective on cells growing on one or more of the soft, stiff, or rigid substrates.
2.5. Implications for regenerative medicine scaffolds
Hard tissues such as bone as well as relatively stiff connective tissue such as cartilage have received considerable attention in tissue engineering, with the development of methods of cell-seeded polymer scaffolds. Perhaps the most common polymer for these purposes is polylactic acid (PLA), which is often crystalline but degradable over long times due to polyester bond hydrolysis. PLA is an extremely stiff polymer with a bulk elasticity of E∼1 GPa, which is ten-thousand fold stiffer than most soft tissues. As a porous scaffold for cell seeding, the macro-elasticity of PLA tends to scale with the weight fraction of the polymer but struts, webs, and plates bigger or thicker than cell-scale are likely to be perceived by cells as rigid. This assertion is based on findings that cells feel on length scales of microns or less [71]. Nanofibers which can be bent by cells might have the correct local rigidity to limit such inhomogeneous cell responses.
Engineering of soft tissue replacements also seems promising with softer polymers other than PLA, and there are certainly some widely studied examples. The previously mentioned Matrigel, a biologically derived mix of soluble matrix of collagen, laminin, and other components, has an apparent elasticity E≤1 kPa. Since this elasticity is much softer than most soft tissues cited above, seeding cells on such a soft matrix is unlikely to foster significant mechanical feedback, at least for smooth or skeletal muscle type tissue. A related but slightly stiffer cell scaffold can be made by extracting the cells without complete matrix solubilization [72]. Such a cell-free matrix derived from arteries has been seeded with stem cells and implanted to surgically reconstruct large parts of the urethra in human patients [73]. Differentiation of the stem cells toward the appropriate lineage would seem based in part on the suitably stiff substrate as well as the implanted microenvironment. Looking at tissue regeneration by the use of stem cell injection to damaged areas shows that the physical properties of damaged or impaired tissue can be dramatically different from the native one. Great care has to be taken since the microenvironment, as described above, can significantly influence the maturation of the injected stem cells. Using the experimental findings of the in vitro studies will help to face the challenges for a suitable delivery method into the body. A temporary microenvironment exhibiting suitable parameters for cell differentiation has to be designed so that as the tissue regeneration progresses, this temporary matrix will vanish after the initial preferred differentiation scheme is completed, and can therefore prevent the stem cell maturation process from taking unwanted routes.
3. Conclusions and outlook
This review has attempted to highlight only a fraction of the systematic studies that have recently emerged on the influence of the biophysical environment on cells; and further warrants studies to elucidate the signaling pathways underlying these observations. Applying current knowledge gained from mechano-sensing and force transduction while adding additional cell cues to in vitro systems seems likely to generate microenvironments of greater fidelity and enhanced applicability as therapeutic test beds. All of these findings seem likely to contribute toward optimal tailoring of the physical and chemical conditions for in vivo applications such as of tissue regeneration and drug delivery.
Acknowledgments
F.R. gratefully acknowledges the Feodor Lynen fellowship from the Alexander von Humboldt foundation. A.J.E. and D.E. D. acknowledge the NIH and NSF for the support via NRSA and R01 funding, respectively.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Natural and Artificial Cellular Microenvironments for Soft Tissue Repair”.
References
- 1.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. Cell adhesion: old and new questions. Trends in Biochemical Sciences. 1999;24(12):M33–M37. [PubMed] [Google Scholar]
- 3.Harris AK, Wild P, Stopak D. Silicone–rubber substrata—new wrinkle in the study of cell locomotion. Science. 1980;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 4.Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal. 1999;76(4):2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francius G, et al. Effect of crosslinking on the elasticity of polyelectrolyte multilayer films measured by colloidal probe AFM. Microscopy Research and Technique. 2006;69(2):84–92. doi: 10.1002/jemt.20275. [DOI] [PubMed] [Google Scholar]
- 7.Richert L, et al. Elasticity of native and cross-linked polyelectrolyte multilayer films. Biomacromolecules. 2004;5(5):1908–1916. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- 8.Engler AJ, et al. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate stiffness and cell adhesion. Surface Science. 2004;570(1–2):142–154. [Google Scholar]
- 9.Rizzi SC, et al. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II: biofunctional characteristics. Biomacromolecules. 2006;7(11):3019–3029. doi: 10.1021/bm060504a. [DOI] [PubMed] [Google Scholar]
- 10.Tan JL, et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saez A, et al. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophysical Journal. 2005;89(6):L52–L54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seliktar D. Extracellular stimulation in tissue engineering. Communicative Cardiac Cell. 2005:386–394. doi: 10.1196/annals.1341.034. [DOI] [PubMed] [Google Scholar]
- 13.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends in Cell Biology. 2003;13(5):264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman HK, et al. Basement-membrane complexes with biological-activity. Biochemistry. 1986;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 15.Kleinman HK, et al. Isolation and characterization of type-Iv procollagen, laminin, and heparan-sulfate proteoglycan from the Ehs sarcoma. Biochemistry. 1982;21(24):6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 16.Stevens MM, George JH. Exploring and engineering the cell surface interface. Science. 2005;310(5751):1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 17.Kong HJ, et al. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu XZ, et al. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3(6):1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 19.Cai SS, et al. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Liu YC, Shu XZ, Prestwich GD. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26(23):4737–4746. doi: 10.1016/j.biomaterials.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Binnig G, Quate CF, Gerber C. Atomic force microscope. Physical Review Letters. 1986;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 22.Binnig G, Quate CF, Gerber C. Atomic force microscope. Physical Review Letters. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 23.Radmacher M. Measuring the elastic properties of living cells by the atomic force microscope. Atomic Force Microscopy in Cell Biology. 2002:67–90. doi: 10.1016/s0091-679x(02)68005-7. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig M, et al. AFM, a tool for single-molecule experiments. Applied Physics A, Materials Science & Processing. 1999;68(2):173–176. [Google Scholar]
- 25.Hertz H. Über die Berührung fester elastischer Körper. Jounal für die Reine und Angewandte Mathematik. 1882;92:156–171. [Google Scholar]
- 26.von Wichert G, et al. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO Journal. 2003;22(19):5023–5035. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahaffy RE, et al. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Physical Review Letters. 2000;85(4):880–883. doi: 10.1103/PhysRevLett.85.880. [DOI] [PubMed] [Google Scholar]
- 28.Storm C, et al. Nonlinear elasticity in biological gels. Nature. 2005;435(7039):191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 29.Lu YB, et al. Viscoelastic properties of individual glial cells and neurons in the CNS. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(47):17759–17764. doi: 10.1073/pnas.0606150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georges PC, et al. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophysical Journal. 2006;90(8):3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshikawa Y, et al. Transverse elasticity of myofibrils of rabbit skeletal muscle studied by atomic force microscopy. Biochemical and Biophysical Research Communications. 1999;256(1):13–19. doi: 10.1006/bbrc.1999.0279. [DOI] [PubMed] [Google Scholar]
- 32.Bosboom EMH, et al. Passive transverse mechanical properties of skeletal muscle under in vivo compression. Journal of Biomechanics. 2001;34(10):1365–1368. doi: 10.1016/s0021-9290(01)00083-5. [DOI] [PubMed] [Google Scholar]
- 33.Engler AJ, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. Journal of Cell Biology. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collinsworth AM, et al. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. American Journal of Physiology Cell Physiology. 2002;283(4):C1219–C1227. doi: 10.1152/ajpcell.00502.2001. [DOI] [PubMed] [Google Scholar]
- 35.Goffin JM, et al. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. Journal of Cell Biology. 2006;172(2):259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry MF, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. American Journal of Physiology Heart and Circulatory Physiology. 2006;290(6):H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 37.Polllard TD, Earnshaw WC. Cell Biology. 1st. Saunders; Philadelphia: 2002. [Google Scholar]
- 38.Lauffenburger DA, Lindermann JJ. Receptors: Models for Binding, Trafficking, and Signaling. 2nd. Oxford University Press; London: 1996. p. 365. [Google Scholar]
- 39.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. Journal of Cellular Physiol. 2005;204(1):198–209. doi: 10.1002/jcp.20274. [DOI] [PubMed] [Google Scholar]
- 40.Engler A, et al. Substrate compliance versus ligand density in cell on gel responses. Biophysical Journal. 2004;86(1):617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaudet C, et al. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophysical Journal. 2003;85(5):3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engler AJ, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cells. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Wang N, et al. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. American Journal of Physiology Cell Physiology. 2002;282(3):C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 45.Lo CM, et al. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beningo KA, Dembo M, Wang YI. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cukierman E, et al. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 48.Mao Y, Schwarzbauer JE. Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. Journal of Cell Science. 2005;118(Pt 19):4427–4436. doi: 10.1242/jcs.02566. [DOI] [PubMed] [Google Scholar]
- 49.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 50.Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two-and three-dimensional culture. Experimental Cell Research. 2003;283(2):146–155. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 51.Zaman MH, et al. Computational model for cell migration in three-dimensional matrices. Biophysical Journal. 2005;89(2):1389–1397. doi: 10.1529/biophysj.105.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reneker DH, Chun I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology. 1996;7(3):216–223. [Google Scholar]
- 53.Matthews JA, et al. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3(2):232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 54.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 55.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84(3):371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 56.Riveline D, et al. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. Journal of Cell Biology. 2001;153(6):1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schoenwaelder SM, Burridge K. Bidirectional signaling between the cytoskeleton and integrins. Current Opinion in Cell Biology. 1999;11(2):274–286. doi: 10.1016/s0955-0674(99)80037-4. [DOI] [PubMed] [Google Scholar]
- 58.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology. 2001;3(5):466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 59.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Current Biology. 1999;9(12):640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- 60.Hinz B. Masters and servants of the force: The role of matrix adhesions in myofibroblast force perception and transmission. European Journal of Cell Biology. 2006;85(3–4):175–181. doi: 10.1016/j.ejcb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Cai Y, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophysical Journal. 2006;91(10):3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Straight AF, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299(5613):1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 63.Rief M, et al. The mechanical stability of immunoglobulin and fibronectin III domains in the muscle protein titin measured by atomic force microscopy. Biophysical Journal. 1998;75(6):3008–3014. doi: 10.1016/S0006-3495(98)77741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans E, Ritchie K. Dynamic strength of molecular adhesion bonds. Biophysical Journal. 1997;72(4):1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marshall BT, et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423(6936):190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 66.Thomas WE, et al. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109(7):913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]
- 67.Evans EA, Parsegian VA. Thermal–mechanical fluctuations enhance repulsion between biomolecular layers. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(19):7132–7136. doi: 10.1073/pnas.83.19.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jana SS, Kawamoto S, Adelstein RS. A specific isoform of nonmuscle myosin II-C is required for cytokinesis in a tumor cell line. Journal of Biological Chemistry. 2006;281(34):24662–24670. doi: 10.1074/jbc.M604606200. [DOI] [PubMed] [Google Scholar]
- 69.Mateo V, et al. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nature Medicine. 1999;5(11):1277–1284. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- 70.Eckhardt A, Engler AJ, Discher DE. Influence of protein synthesis on smooth muscle cells spreading. Molecular Biology of the Cell. 2004;15:150A. [Google Scholar]
- 71.Griffin MA, et al. Adhesion-contractile balance in myocyte differentiation. Journal of Cell Science. 2004;117(24):5855–5863. doi: 10.1242/jcs.01496. [DOI] [PubMed] [Google Scholar]
- 72.Gratzer PF, Santerre JP, Lee JM. Modulation of collagen proteolysis by chemical modification of amino acid side-chains in acellularized arteries. Biomaterials. 2004;25(11):2081–2094. doi: 10.1016/j.biomaterials.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 73.El-Kassaby AW, et al. Urethral stricture repair with an off-the-shelf collagen matrix. Journal of Urology. 2003;169(1):170–173. doi: 10.1016/S0022-5347(05)64060-8. [DOI] [PubMed] [Google Scholar]


