Abstract
Objective
To evaluate feasibility and accuracy of intraocular pressure (IOP) measurement by rebound tonometry in adult red-eared slider turtles and determine the effects of manual and chemical restraint on IOP.
Animal studied
Seventeen adult red-eared slider turtles.
Procedures
IOP was measured with TonoLab® and TonoVet® tonometers in conscious, unrestrained turtles. To evaluate the effects of manual restraint, turtles were restrained by digital pressure on the rostral head or proximal neck. The effect of two chemical restraint protocols (dexmedetomidine, ketamine, midazolam [DKM] and dexmedetomidine, ketamine [DK] subcutaneously) on IOP was evaluated. Triplicate TonoLab® and TonoVet® readings were compared to direct manometry in 3 ex vivo turtle eyes.
Results
TonoLab® correlated better with manometry at IOPs <45 mm Hg than TonoVet® (linear regression slopes of 0.89 and 0.30 respectively). Mean (±SD) IOP in unrestrained conscious turtles was significantly lower (P<0.01) with TonoLab® (10.02 ± 0.66 mmHg) than with TonoVet® (11.32 ± 1.57 mmHg). Manual neck restraint caused a significant increase in IOP (+6.31 ± 5.59 mmHg), while manual rostral head restraint did not. Both chemical restraint protocols significantly reduced IOP (DKM: −1.0 ± 0.76 mmHg,; DK: −1.79 ± 1.17) compared to measurements in conscious unrestrained turtles.
Conclusions
Chemical and manual neck restraint affected IOP. Rostral head restraint had no significant effect on IOP and is, therefore, recommended as the appropriate restraint technique in red-eared-slider turtles. TonoLab® measurements estimated actual IOP more accurately, within physiologic range, than measurements obtained using the TonoVet®.
Keywords: rebound tonometry, intraocular pressure, turtle, TonoLab®, TonoVet®, manometry, reptile
Introduction
Red-eared slider turtles (Trachemys scripta elegans) are popular companion animals worldwide. This species is also used as a laboratory animal model for basic investigation in vision, particularly for pupillary light reflex and retinal research.[1-4] Normal mean intraocular pressure (IOP) values have been reported for a number of healthy, conscious and chemically restrained exotic and wild animal species.[5-9] While limited information is available on IOP in some terrestrial reptile species,[10] in aquatic freshwater turtles, such as red-eared slider turtles, normal IOP values have not been reported [11-14] and ocular anatomy and normal ophthalmic reference data for this species are scarce. However, the establishment of normal physiological ophthalmic parameters, such as IOP, is critical for accurately diagnosing ophthalmic disease in any species.
Rebound tonometry has recently been introduced to veterinary medicine. The hand held TonoLab®, originally designed for use in rodents [15-18], has a small (1 mm diameter), round-tipped probe that is electromagnetically propelled to contact and then rebound from the corneal surface. The resulting voltage change is converted into an electrical signal which is manipulated by an internal algorithm to estimate IOP.[15] The TonoVet® utilizes similar technology, although it uses a slightly larger plastic probe tip (approximately 2 mm in diameter) and its internal algorithms are optimized for the canine and equine cornea. Both tonometers take six consecutive measurements, automatically discard the lowest and highest, and display a final reading which is the calculated mean of the four remaining measurements. Additionally, the instrument displays an indication of the standard deviation of the measurements to aid the user in determining the precision of the measurement. Due to the very light and brief contact the probe has with the cornea, rebound tonometer measurements can be acquired rapidly and without topical anesthesia.
Glaucoma has not been reported in reptiles, most likely due to the inability to accurately estimate IOP in small eyes common in this class of animals.[11, 12, 14, 19] Although TonoVet® rebound tonometry was recently described in tortoises,[10] the accuracy of these readings, when compared to true IOP as measured by a manometer and the effects of manual and chemical restraint on IOP, have not been evaluated in any reptile species. The purpose of this study was to evaluate the ability of two rebound tonometers, the TonoVet® and TonoLab®, to estimate IOP in adult red-eared slider turtles using different methods of manual and chemical restraint and to compare IOP estimates obtained with these two devices to a manometer.
Materials and Methods
Comparison of rebound tonometry with direct manometry ex vivo
For direct manometry, three enucleated eyes from red-eared slider turtles, euthanized for reasons unrelated to this study, were refrigerated and used within 2 - 3 hours of euthanasia. The anterior chamber was carefully cannulated with two 30-gauge needles at the 3 o’clock and 9 o’clock positions. Cyanoacrylate adhesive was used around the point of entry of the needle through the cornea to prevent leakage of aqueous humor. Leakage around the needles was not observed throughout the procedure, and corneal deformation was judged to be minimal. The eyes were kept moist throughout the readings with commercially available eyewash (purified water 99.05 %; Major®, Major Pharmaceuticals, Livonia, MI). One needle was connected via polyethylene tubing to a reservoir containing 0.9% NaCl solution; IOP was controlled by adjusting the height of the saline reservoir attached to a cannula (3-way open-stopcock method). The other needle was connected to a pressure transducer (NL108T2 Disposable Physiological Pressure Transducer, Warner Instruments, Hamden, CT) and continuous physiologic recorder (Dash® 4000 Pro, GE Healthcare, Milwaukee, WI) allowing IOP to be verified and continuously recorded. Triplicate TonoLab® followed by TonoVet® readings were acquired as described below, by the same observer (CD) at 5, 10, 15, 20, 25, 30, 35, 40, 50, 60, 70 and 80 mmHg for each eye.
Measurement of IOP in vivo
A total of 17 red-eared slider turtles (Trachemys scripta elegans) (13 males and 4 females) were used for the different experiments of this study. Mean body weight was 770 ± 130 grams (males: 719 ± 102 grams; females: 927 ± 71 grams), and mean carapace length was 19.4 ± 0.9 cm (males: 19.1 ± 0.8 cm; females: 20.4 ± 0.5 cm). All procedures and care of turtles conformed to the guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of Wisconsin-Madison and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. In all turtles, both eyes were considered to be clinically normal on the basis of slit-lamp biomicroscopy (SL-14, Kowa, Optimed Inc, Torrance, CA) and indirect ophthalmoscopy (Heine Omega 180, Heine USA Ltd, Dover, NH) by a board certified veterinary ophthalmologist (PEM). All turtles were acclimatized to the housing conditions for at least 4 weeks prior to use in this study.
Throughout the study, both the TonoVet® (Icare Oy, Helsinki, Finland) and TonoLab® (Icare Oy, Helsinki, Finland) (Fig.1 and 2) rebound tonometers were used according to manufacturers instructions. Only readings within the range of acceptable standard deviation per the manufacturer (≤ 2.5 mmHg) for the TonoVet® (as indicated by no bar or one bar ‘down’ on the instrument display) were recorded. The TonoVet® was used on the “D” setting (calibration setting for dogs and cats). For the in vivo tonometry data, the order of measurement of the right (OD) and left eye (OS) was randomly assigned.
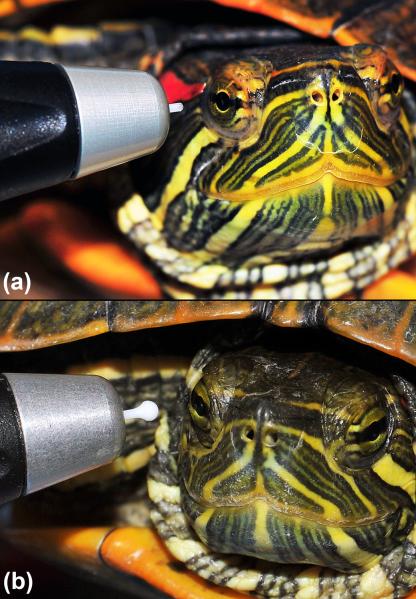
Figure 1.
IOP measurement using the TonoLab® rebound tonometry in a conscious unrestrained male red-eared slider turtle.
Figure 2.
IOP measurement using the TonoLab® (a) and the TonoVet® (b) rebound tonometry devices in red-eared slider turtles. Note the difference in probe size and the lack of any restraint required to obtain IOP.
Comparison of rebound tonometry using TonoVet® and TonoLab® in conscious unrestrained turtles
In order to compare the two different rebound tonometry devices, triplicate IOP measurements were acquired by the same observer (CD) from both eyes with both TonoVet® and TonoLab® tonometers in 17 conscious, unrestrained adult turtles (34 eyes). All turtles were positioned horizontally with their carapace resting on a food can, without any further restraint performed during measurement of IOP (Fig. 1). The sequence of measurement of left and right eye IOP was randomized, as well for both instruments, however, measurements were obtained with TonoLab® first followed by TonoVet®.
Comparison of manual restraint methods using the TonoLab®
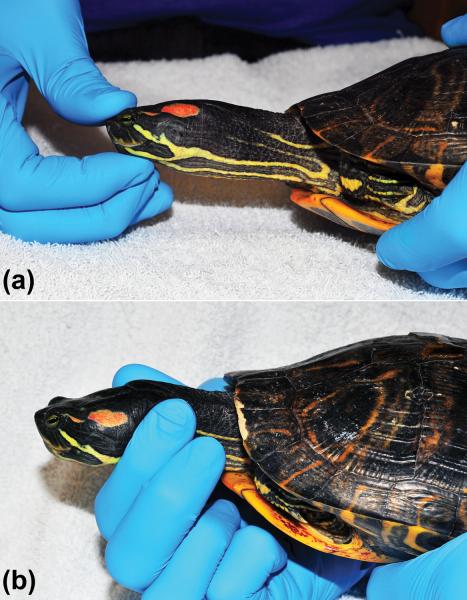
To evaluate the effects of manual restraint, 12 turtles were restrained by digital pressure on the rostral head (Fig. 3-A) or the proximal neck (Fig. 3-B). Time between measurements using the different manual restraint methods was at least 30 minutes. IOP measurements obtained with manual restraint were compared to IOP measurements obtained from the same animals without manual restraint (acquired prior to either form of manual restraint).
Figure 3.
Manual restraint of red-eared slider turtles for IOP measurement. a) Rostral head restraint. Note that during rostral head restraint, no pressure is applied to the globes or eyelids. b) Neck restraint.
Comparison of chemical restraint protocol using the TonoLab®
The effect of two different subcutaneous chemical restraint protocols was evaluated. The first used dexmedetomidine (0.1 mg/kg, Pfizer Animal Health, NY, NY 10017) + ketamine (2 mg/kg, Hospira Inc, Lake Forest, IL 60045) + midazolam (1 mg/kg, Hospira Inc, Lake Forest, IL 60045) [DKM] and the second consisted of dexmedetomidine (0.1 mg/kg) + ketamine (10 mg/kg) [DK]. The effect of the DKM protocol on IOP was evaluated in 13 out the 17 turtles used in this study, and the DK protocol was evaluated in 11 of 17 turtles. IOP measurements under chemical restraint were compared to IOP measurements from unrestrained animals obtained just prior to administration of sedative and anesthetic drugs.
Statistical analysis
Triplicate data obtained in ex vivo eyes comparing both instruments with direct manometry were analyzed by linear regression using Microsoft Excel™. Further data analysis was performed using commercial software (SigmaPlot 12.3; Systat Software, Inc. San Jose, CA). A Shapiro-Wilk W test was used to assess the data for normal distribution. Data not normally distributed were log10 transformed prior to subsequent statistical analyses. The mean of triplicate IOP measurements in vivo for the two eyes of each subject were used in subsequent statistical analyses, except for comparison between left and right eyes, in order to minimize the effects of between-eye correlation. Paired, two-tailed student’s t-tests were used to analyze the data for significant differences between left and right eye and between rebound tonometry devices. A one-way, repeated measures ANOVA was used to analyze the data for differences between manual restraint methods on IOP. Tukey’s test was used for post-hoc pair wise comparisons if significant differences between groups were found. The data are reported as means ± standard deviation. Differences were considered significant if p < 0.05.
Results
Manometry
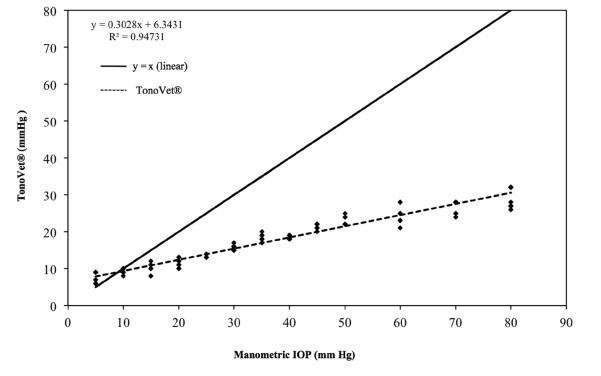
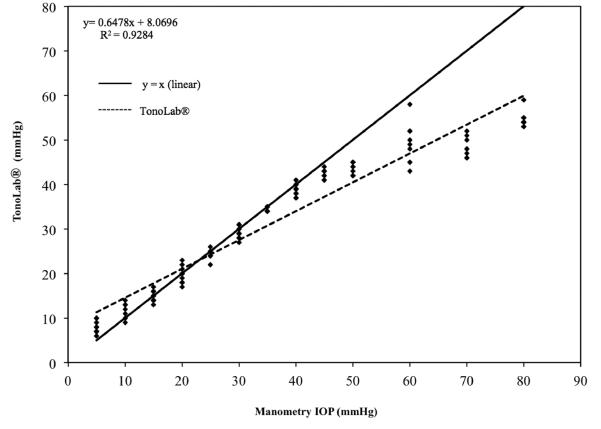
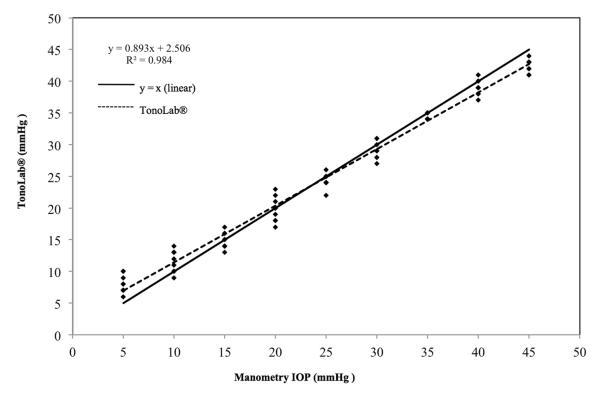
Measurements of IOP for all 3 eyes used in this phase of the study showed strong linear trends for both TonoVet® (r2 = 0.94) and for TonoLab® (r2 = 0.92). Both tonometers, however, underestimated true IOP, starting at approximately 10 mm Hg for the TonoVet® (slope = 0.303; Fig. 4) and at approximately 45 mm Hg for the TonoLab® (slope of 0.65; Fig. 5). Further data analysis using data points obtained from 5 mmHg to 45 mmHg indicated that the TonoLab® was precise and accurate over this range in IOP (regression line with r2 = 0.98 and a slope of 0.89 (Fig. 6). Closer evaluation of the TonoLab® data revealed a pronounced and progressive underestimation of IOP from 45 and 80 mmHg, which correlated with onset of corneal edema observed in all 3 ex vivo eyes at IOP of 45 mmHg and above.
Figure 4.
IOP readings for 3 turtle eyes ex vivo taken with the TonoVet® (y) versus manometric IOP (x). Note that TonoVet® consistently and progressively underestimated IOP relative to manometry between 10 and 80 mmHg. Some data points are superimposed.
Figure 5.
IOP readings for 3 turtle eyes ex vivo taken with the TonoLab® (y) and versus manometric IOP (x). TonoLab® slightly overestimates at lower IOPs and underestimates and shows greater variance at IOPs greater than 45mmHg. Some data points are superimposed.
Figure 6.
IOP measurements for all 3 turtle eyes ex vivo taken with TonoLab® between 5 - 45 mmHg. Note that the data points obtained within a physiologic IOP range more closely approximate the ideal line of y=x. Some data points are superimposed.
TonoVet® and TonoLab® in conscious unrestrained turtles
Mean IOP measurements obtained from all 17 turtles were significantly different between the two rebound tonometers. Mean IOP measured with the TonoVet® were slightly, but significantly, higher (11.32 ± 1.57 mmHg, P = 0.003, paired t-test) than the IOP measurements obtained with TonoLab® (10.2 ± 0.66 mmHg). Results obtained from this part of the study, and data from direct manometry, indicated that the TonoLab® would be more accurate than the TonoVet® tonometer, therefore all subsequent in vivo data were collected using the TonoLab® tonometer. IOP readings of left and right eyes were not statistically different using the either the TonoLab® (OS 10.16 ± 0.65 mmHg, OD 10 ± 0.7 mmHg, P = 0.23, paired t-test) or the TonoVet® (OS 11.77 ± 1.64 mmHg, OD 10.88 ± 1.98 mmHg, P = 0.065, paired t-test).
Effect of manual restraint on IOP using the TonoLab®
Compared to measurements obtained in un-restrained turtles, manual neck restraint (Fig. 3-B) caused a significant increase in IOP compared to rostral head restraint (+6.75 ± 6.01 mmHg) (Fig. 3-A) and compared to no manual restraint (+6.31 ± 5.59 mmHg, P < 0.001, 1-RM-ANOVA). Manual restraint of the rostral head had no significant effect on IOP if compared to IOP values obtained from unrestrained turtles (−0.44 ± 1.26 mmHg, P = 0.83, 1-RM-ANOVA).
Effect of chemical restraint on IOP
Both chemical restraint protocols significantly reduced IOP compared to baseline IOP values of conscious unrestrained turtles (P < 0.001, paired t-test). The IOP was significantly reduced after administration of the DK protocol by −1.79 ± 1.17 mmHg and by −1.00 ± 0.76 mmHg after administration of the DKM protocol.
Discussion
Intraocular pressure measurement by rebound tonometry was possible in conscious, unrestrained red-eared slider turtles using and TonoLab®. TonoLab® readings were found to accurately represent actual IOP across a range considered physiologically normal. However, IOPs were markedly underestimated by the TonoLab® following the onset of grossly appreciable corneal edema at higher IOPs in ex vivo eyes. Corneal edema was also reported as a plausible explanation for decreased reproducibility of IOPs in healthy canine ex vivo eyes.[20] The TonoVet® systematically underestimated IOP across the range evaluated.
Obtaining IOP values in this species, both invasively by cannulation and manometry and non-invasively by tonometry, is technically demanding due to the small size of turtle eyes. The corneal surface diameter of turtles used in this study was approximately 4 mm which is between that of a rat (approximately 5 mm) and a mouse (approximately 3 mm).[21] In normal Wistar rats and in C57BL/6 mice, an IOP range of 10-16.5 mmHg was measured, which correlated well with true IOPs obtained in cannulated normal eyes in both animal models using the TonoLab®.[22]
Theoretically, the small size of the rebound TonoVet® and TonoLab® probe tip, relative to those of commonly used applanation tonometers, should facilitate IOP measurements in veterinary patients with small eyes. However, despite the small probe size of the TonoVet®, the red-eared slider turtle’s axial cornea was found to be approximately 50% covered by the TonoVet® probe when obtaining readings. This also could explain the fact that unrestrained, conscious turtles in vivo were observed to react more to the TonoVet® than the TonoLab®, and why larger standard deviations with higher IOP readings were recorded when using the former tonometer.
Two common restraint methods frequently used for restraint of the head in a clinical setting were evaluated in this species. In other studies, IOP increases in a range of species were observed with jugular compression.[23-25] Thus, manual restraint method should be reported and carefully considered when interpreting IOP data in red-eared slider turtles, and rostral head or no manual restraint are preferred, due to the lack of significant effect on IOP. In addition, head and body position was shown to impact IOP in a range of species,[11, 23, 26] but both were consistent across groups in our study as turtles were consistently supported with their head and body in a horizontal position during all restraint methods evaluated.
Significant effects of anesthetic agents on IOP were reported in several species, including mice, dogs, rats and primates.[27-30] In our study, both chemical restraint protocols significantly reduced IOP, relative to conscious unrestrained measurements. Intravenous administration of dexmedetomidine alone in normal healthy dogs caused a transient decrease in IOP within 20 minutes.[28] However, when combined with butorphanol, this combination induced a transient increase and subsequent decrease of IOP relative to baseline in dogs.[31] In addition, IOP measured with TonoLab® in mice decreased less following ketamine than following administration of a ketamine-xylazine-acepromazine mixture.[29] In dogs anesthetized with a mixture of ketamine-midazolam, no effects on IOP were recorded using the Tono-Pen Vet™ [32], while IOP increased in dogs following administration of either ketamine or diazepam in another study that utilized the TonoVet® rebound tonometer.[33]
A total of 17 individual turtles were used in the different experiments of this study. However, not all animals were used in all experiments. Preliminary statistical evaluation, during the experiments revealed statistical significant differences between treatment groups. Therefore the experiments were terminated, in order to minimize the use of animals for these experiments
Conclusions
We demonstrated that IOP measurement by rebound tonometry is feasible in conscious, unrestrained red-eared slider turtles and evaluated IOP in vivo under clinically relevant conditions. Manual and chemical restraint methods commonly used when examining this species had significant effects on IOP. The TonoLab® tonometer correlated well with manometry ex vivo over a physiologic range in IOP (≤ 45 mmHg), while the TonoVet® tonometer consistently underestimated IOP.
Acknowledgements
The authors acknowledge the support for this study provided by the Companion Animal Fund, School of Veterinary Medicine, University of Wisconsin-Madison and Comparative Ophthalmic Research Laboratories (CORL), University of Wisconsin–Madison. The authors also thank Ms. Jessica Lovstad and Ms. Jessica E. McDonald for assistance with data analysis. Dr. McLellan received support from NIH grant K08EY018609.
Footnotes
Presented in part at the 42nd Annual Meeting of the American College of Veterinary Ophthalmologists, Hilton Head, SC, USA, (October 26-29, 2011), and at the International Conference on Reptile and Amphibian Medicine, Cremona, Italy, (May 13-15, 2012).
References
- 1.Dearworth JR, Jr., Sipe GO, Cooper LJ, et al. Consensual pupillary light response in the red-eared slider turtle (Trachemys scripta elegans) Vision Research. 2010;50:598–605. doi: 10.1016/j.visres.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Dearworth JR, Jr., Selvarajah BP, Kalman RA, et al. A mammalian melanopsin in the retina of a fresh water turtle, the red-eared slider (Trachemys scripta elegans) Vision Research. 2011;51:288–295. doi: 10.1016/j.visres.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Sipe GO, Dearworth JR, Jr., Selvarajah BP, et al. Spectral sensitivity of the photointrinsic iris in the red-eared slider turtle (Trachemys scripta elegans) Vision Research. 2011;51:120–130. doi: 10.1016/j.visres.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Fan TX, Scudder C, Ariel M. Neuronal responses to turtle head rotation in vitro. Journal of Neurobiology. 1997;33:99–117. [PubMed] [Google Scholar]
- 5.Ofri R, Horowitz IH, Kass PH. Tonometry in three herbivorous wildlife species. Veterinary Ophthalmolgy. 1998;1:21–24. doi: 10.1046/j.1463-5224.1998.00004.x. [DOI] [PubMed] [Google Scholar]
- 6.Ofri R, Horowitz IH, Raz D, et al. Intraocular pressure and tear production in five herbivorous wildlife species. Veterinary Record. 2002;151:265–268. doi: 10.1136/vr.151.9.265. [DOI] [PubMed] [Google Scholar]
- 7.Ofri R, Horowitz IH, Kass PH. How low can we get? Tonometry in the Thomson gazelle (Gazella thomsoni) Journal of Glaucoma. 2000;9:187–189. doi: 10.1097/00061198-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Sapienza JS, Porcher D, Collins BR, et al. Tonometry in clinically normal ferrets (Mustela putorius furo) Progress in Veterinary and Comparative Ophthalmology. 1991;1:291–294. [Google Scholar]
- 9.Montiani-Ferreira F, Mattos BC, Russ HH. Reference values for selected ophthalmic diagnostic tests of the ferret (Mustela putorius furo) Veterinary Ophthalmolgy. 2006;9:209–213. doi: 10.1111/j.1463-5224.2006.00475.x. [DOI] [PubMed] [Google Scholar]
- 10.Selleri P, Di Girolamo N, Andreani V, et al. Evaluation of intraocular pressure in conscious Hermann’s tortoises (Testudo hermanni) by means of rebound tonometry. American Journal of Veterinary Research. 2012;73:1807–1812. doi: 10.2460/ajvr.73.11.1807. [DOI] [PubMed] [Google Scholar]
- 11.Chittick B, Harms C. Intraocular pressure of juvenile loggerhead sea turtles (Caretta caretta) held in different positions. Veterinary Record. 2001;149:587–589. doi: 10.1136/vr.149.19.587. [DOI] [PubMed] [Google Scholar]
- 12.Selmi AL, Mendes GM, McManus C, et al. Intraocular pressure determination in clinically normal red-footed tortoise (Geochelone carbonaria) Journal of Zoo and Wildlife Medicine. 2002;33:58–61. doi: 10.1638/1042-7260(2002)033[0058:IPDICN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker CJ, Heaton-Jones TG, Kubilis PS, et al. Intraocular pressure variation associated with body length in young American alligators (Alligator mississippiensis) American Journal of Veterinary Research. 1995;56:1380–1383. [PubMed] [Google Scholar]
- 14.Selmi AL, Mendes GM, MacManus C. Tonometry in adult yellow-footed tortoises (Geochelone denticulata) Veterinary Ophthalmolgy. 2003;6:305–307. doi: 10.1111/j.1463-5224.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 15.Kontiola AI. A new induction-based impact method for measuring intraocular pressure. Acta Ophthalmologica Scandinavica. 2000;78:142–145. doi: 10.1034/j.1600-0420.2000.078002142.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang WH, Millar JC, Pang IH, et al. Noninvasive measurement of rodent intraocular pressure with a rebound tonometer. Investigative Ophthalmology & Visual Science. 2005;46:4617–4621. doi: 10.1167/iovs.05-0781. [DOI] [PubMed] [Google Scholar]
- 17.Goldblum D, Kontiola AI, Mittag T, et al. Non-invasive determination of intraocular pressure in the rat eye. Comparison of an electronic tonometer (TonoPen), and a rebound (impact probe) tonometer. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2002;240:942–946. doi: 10.1007/s00417-002-0571-y. [DOI] [PubMed] [Google Scholar]
- 18.Filippopoulos T, Matsubara A, Danias J, et al. Predictability and limitations of non-invasive murine tonometry: comparison of two devices. Experimental Eye Research. 2006;83:194–201. doi: 10.1016/j.exer.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Yin J, Huang J, Chen C, et al. Corneal complications in streptozocin-induced type I diabetic rats. Investigative Ophthalmology & Visual Science. 2011;52:6589–6596. doi: 10.1167/iovs.11-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorig C, Coenen RT, Stades FC, et al. Comparison of the use of new handheld tonometers and established applanation tonometers in dogs. American Journal of Veterinary Research. 2006;67:134–144. doi: 10.2460/ajvr.67.1.134. [DOI] [PubMed] [Google Scholar]
- 21.Remtulla S, Hallett PE. A schematic eye for the mouse, and comparisons with the rat. Vision Research. 1985;25:21–31. doi: 10.1016/0042-6989(85)90076-8. [DOI] [PubMed] [Google Scholar]
- 22.Kontiola AI, Goldblum D, Mittag T, et al. The induction/impact tonometer: a new instrument to measure intraocular pressure in the rat. Exp Eye Res. 2001;73:781–785. doi: 10.1006/exer.2001.1088. [DOI] [PubMed] [Google Scholar]
- 23.Broadwater JJ, Schorling JJ, Herring IP, et al. Effect of body position on intraocular pressure in dogs without glaucoma. American Journal of Veterinary Research. 2008;69:527–530. doi: 10.2460/ajvr.69.4.527. [DOI] [PubMed] [Google Scholar]
- 24.Klein HE, Krohne SG, Moore GE, et al. Effect of eyelid manipulation and manual jugular compression on intraocular pressure measurement in dogs. Journal of the American Veterinary Medical Association. 2011;238:1292–1295. doi: 10.2460/javma.238.10.1292. [DOI] [PubMed] [Google Scholar]
- 25.Pauli AM, Bentley E, Diehl KA, et al. Effects of the application of neck pressure by a collar or harness on intraocular pressure in dogs. Journal of the American Animal Hospital Association. 2006;42:207–211. doi: 10.5326/0420207. [DOI] [PubMed] [Google Scholar]
- 26.Komaromy AM, Garg CD, Ying GS, et al. Effect of head position on intraocular pressure in horses. American Journal of Veterinary Research. 2006;67:1232–1235. doi: 10.2460/ajvr.67.7.1232. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Sapp HL, Plummer CE, et al. IOP Change Undergoing Anesthesia in Rhesus Macaques (Macaca mulatta) with Laser-Induced Ocular Hypertension. Journal of Veterinary Medical Science. 2012;74:1359–1361. doi: 10.1292/jvms.12-0059. [DOI] [PubMed] [Google Scholar]
- 28.Artigas C, Redondo JI, Lopez-Murcia MM. Effects of intravenous administration of dexmedetomidine on intraocular pressure and pupil size in clinically normal dogs. Veterinary Ophthalmolgy. 2012;15(Suppl 1):79–82. doi: 10.1111/j.1463-5224.2011.00966.x. [DOI] [PubMed] [Google Scholar]
- 29.Danias J, Kontiola AI, Filippopoulos T, et al. Method for the noninvasive measurement of intraocular pressure in mice. Investigative Ophthalmology & Visual Science. 2003;44:1138–1141. doi: 10.1167/iovs.02-0553. [DOI] [PubMed] [Google Scholar]
- 30.Jia L, Cepurna WO, Johnson EC, et al. Effect of general anesthetics on IOP in rats with experimental aqueous outflow obstruction. Investigative Ophthalmology & Visual Science. 2000;41:3415–3419. [PubMed] [Google Scholar]
- 31.Rauser P, Pfeifr J, Proks P, et al. Effect of medetomidine-butorphanol and dexmedetomidine-butorphanol combinations on intraocular pressure in healthy dogs. Veterinary Anaesthesia and Analgesia. 2012;39:301–305. doi: 10.1111/j.1467-2995.2011.00703.x. [DOI] [PubMed] [Google Scholar]
- 32.Ghaffari MS, Rezaei MA, Mirani AH, et al. The effects of ketamine-midazolam anesthesia on intraocular pressure in clinically normal dogs. Veterinary Ophthalmolgy. 2010;13:91–93. doi: 10.1111/j.1463-5224.2010.00762.x. [DOI] [PubMed] [Google Scholar]
- 33.Kovalcuka L, Birgele E, Bandere D, et al. The effects of ketamine hydrochloride and diazepam on the intraocular pressure and pupil diameter of the dog’s eye. Veterinary Ophthalmology. 2013;16:29–34. doi: 10.1111/j.1463-5224.2012.01015.x. [DOI] [PubMed] [Google Scholar]