Abstract
Circadian rhythms in behavior and physiology change substantially as female mammals undergo the transition from a non-pregnant to a pregnant state. Here, we examined the possibility that site-specific changes in brain regions known to regulate the sleep/wake cycle and body temperature might reflect altered rhythms in these overt functions. Specifically, we compared daily patterns of immunoreactive FOS in early pregnant and diestrous rats in the medial septum (MS), vertical and horizontal diagonal bands of Broca (VDB and HDB), perifornical lateral hypothalamus (LH), and ventrolateral, medial, and median preoptic areas (VLPO, MPA, and MnPO, respectively). In the pregnant animals, FOS expression was reduced and the daily rhythms of expression were lost or attenuated in the MS, VDB, and LH, areas known to support wakefulness, and in the MPA, a brain region that may coordinate sleep/wake patterns with temperature changes. However, despite the well-documented differences in sleep patterns between diestrous and pregnant rats, reproductive state did not affect FOS expression in the VLPO or MnPO, two brain regions in which FOS expression usually correlates with sleep. These data indicate that plasticity in sleep/wake patterns during early pregnancy may be driven by a reduction in wakefulness-promotion by the brain, rather than by an increase in sleep drive.
Keywords: Circadian rhythm, FOS, Reproduction, Lateral hypothalamus, Basal forebrain, Preoptic area
1. Introduction
Circadian rhythms have a period of roughly 24 h and are generated endogenously. When synchronized to the external environment, these rhythms allow individuals to appropriately time behavioral and physiological events in relation to predictable changes in the environment (Moore-Ede et al., 1982). These rhythms are essential to the regulation of mammalian reproduction and may be subject to change as individuals progress through various reproductive states. For example, during early pregnancy in laboratory rats, locomotor activity is reduced and becomes arrhythmic (Rosenwasser et al., 1987), whereas the body temperature rhythm shows an advance in its rising phase and a reduction in amplitude attributable to increases in the daily temperature minimum (Kittrell and Satinoff, 1988). Sleep patterns are also altered, with the total amount of both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep increased, as is the number of REM sleep bouts, during the dark phase of the day (Kimura et al., 1996), a time when non-pregnant rats are most active. While these changes in sleep/wake and body temperature rhythms during early pregnancy have been well-established, the neural mechanisms underlying them have yet to be identified.
We have documented altered rhythms in protein expression in specific components of the circadian system of the brain in early pregnant rats, as compared to diestrous females (Schrader et al., 2010, 2011). This circadian system includes a primary circadian pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Moore and Eichler, 1972; Ralph et al., 1990; Rusak, 1977; Stephan and Zucker, 1972), as well as various extra-SCN oscillators (Guilding and Piggins, 2007; Hastings et al., 2003; Reppert and Weaver, 2002; Weinert, 2005). The SCN and extra-SCN oscillators contain rhythmic cells that have a molecular transcription/translation loop that takes approximately 24 h to complete (Bell-Pedersen et al., 2005; Dunlap, 1999; Reppert and Weaver, 2002). The phase of the rhythm in expression of the protein Period2 (PER2), a key component of the molecular oscillator, is altered in both the SCN and some extra-SCN oscillators during early pregnancy in the rat (Schrader et al., 2010, 2011). Additionally, expression of FOS, the protein product of the immediate early gene cfos that often rises after neuronal activation (Kovacs, 2008), is elevated during the mid-light phase in the SCN and at other times in some extra-SCN oscillators in early pregnant rats (Schrader et al., 2010, 2011). These data indicate that circadian outputs to systems regulating overt functions, such as the sleep/wake and temperature cycles, may be changing with the transition from the non-pregnant state to pregnancy.
It is unlikely that changes in patterns of sleep, wakefulness, and body temperature rhythms that occur during early pregnancy are brought about by circadian influences alone. Non-circadian systems that more directly regulate these phenomena may also be affected by transitions in reproductive state, perhaps in ways that are not the same as those affecting the circadian system. The fact that the changes in rhythms in sleep/wakefulness and body temperature are dissociated in early pregnancy in the rat is consistent with the view that a single adjustment of a central circadian timekeeping system is unlikely to account entirely for the patterns typical of that reproductive stage. In this study, we begin to explore this issue by comparing early pregnant and diestrous rats with respect to their rhythms in expression of FOS in brain regions known to regulate these functions in a relatively direct fashion. The first group was examined on day 6 of pregnancy, as the changes in overt rhythms described above are all established by that time. Gonadal hormones, such as estrogens and progesterone, are known to influence locomotor activity, sleep, and components of the circadian system of the brain (reviewed in Mong et al. (2011)). Therefore, we selected females on day 1 of diestrus, when circulating gonadal hormones are lowest, for our non-pregnant controls.
We characterized patterns of FOS expression in portions of the basal forebrain, preoptic area, and lateral hypothalamus that are known to be involved in the regulation of sleep, wakefulness, and/or temperature homeostasis (Fig. 1). Specifically, we examined two sleep-active brain regions, the ventrolateral preoptic area (VLPO), which has been implicated in the promotion and maintenance of sleep by inhibiting wake-promoting systems (Gaus et al., 2002; Gong et al., 2004; Saper et al., 2010; Sherin et al., 1996; Szymusiak et al., 1998; Takahashi et al., 2009), and the median preoptic nucleus (MnPO), which is more likely to keep track of building sleep pressure (Gong et al., 2004; Saper et al., 2010; Suntsova et al., 2002; Szymusiak et al., 1998; Takahashi et al., 2009). We also characterized FOS expression in several wakefulness-promoting areas, including three components of the cholinergic basal forebrain: the medial septum (MS) and vertical and horizontal diagonal bands of Broca (VDB and HDB, respectively). These basal forebrain regions promote arousal of the cerebral cortex (Greco et al., 2000; Jones, 2008; Saper et al., 2010). Additionally, we examined the perifornical region of the lateral hypothalamus (LH), which contains orexin-releasing cells that are known to promote wakefulness and inhibit REM sleep (Estabrooke et al., 2001; Peyron et al., 1998; Saper et al., 2010). Finally, we examined patterns of FOS expression in the medial preoptic area (MPA), which, along with the MnPO, plays a role in the homeostatic regulation of core body temperature (Patronas et al., 1998; Satinoff et al., 1982).
Fig. 1.
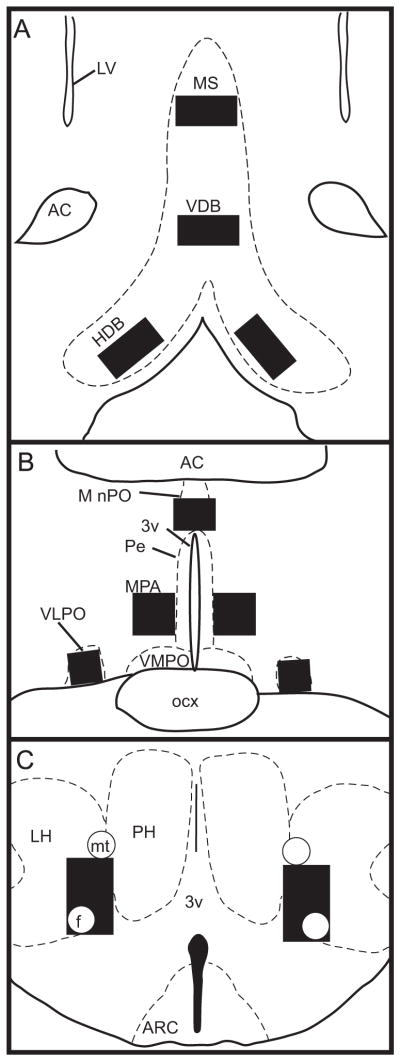
Line drawings depicting location of the MS, VDB, and HDB (A), MnPO, MPA, and VLPO (B), and LH (C). Sampling boxes (black squares) were used for cell counts in all regions as described in Section 4. Anatomical boundaries are based on Paxinos and Watson (1997). 3v: third ventricle; AC: anterior commissure; ARC: arcuate nucleus; f: fornix; LV: lateral ventricle; mt: mammillothalamic tract; Pe: periventricular hypothalamus; PH: posterior hypothalamus; ocx: optic chiasm; VMPO: ventromedial preoptic area.
2. Results
2.1. FOS expression in brain regions that support wakefulness
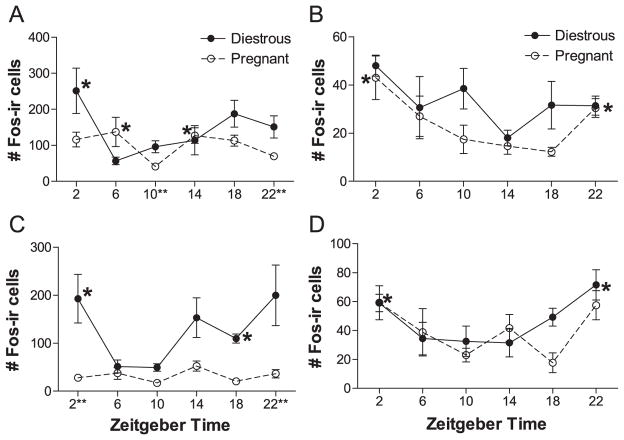
In three of the four brain regions studied that are known to support wakefulness, we found that early pregnancy was associated with tonic reduction in FOS expression and/or a reduction or loss of rhythmic FOS expression. In the MS, pregnancy induced a tonic loss of FOS expression (Figs. 2 and 3A). Diestrous rats expressed more FOS in the MS than pregnant females (Fig. 3A; Reproductive state: F=13.574, df=1, P<0.002, two-way analysis of variance (ANOVA)), but there was no significant rhythm in expression in either group or interaction between reproductive state and Zeitgeber time (ZT; F=1.638, df=5, P>0.15; interaction: F=0.948, df=5, P>0.45). Patterns of FOS expression were somewhat similar in the VDB, but here, in addition to a drop in FOS expression, the rhythm of FOS expression was also lost in pregnant females (Figs. 2 and 3B). In the VDB, FOS expression was significantly rhythmic in diestrous rats, with elevated FOS-immunoreactivity (-ir) throughout the dark phase (Fig. 3B; X2=11.052, df=5, P<0.05, Kruskal–Wallis test). In contrast, FOS expression in the VDB of pregnant rats was generally low and was not affected by time of day (X2=4.929, df=5, P>0.4, Kruskal–Wallis test). Expression was significantly higher in diestrous than pregnant females (main effect: U=177.5, P<0.003, Mann–Whitney U-test), probably due to the apparent drop in expression during the dark phase in pregnant females (Figs. 2 and 3B). Unlike the other two cholinergic basal forebrain regions, in the HDB, FOS expression was low in all animals (8.64±1.36 cells, all animals) and did not differ as a function of reproductive state (U=290.5, P>0.1, Mann–Whitney U test) or of time of day in either pregnant or diestrous rats (pregnant ZT: X2=2.437, df=5, P>0.75; diestrous ZT: X2=4.442, df=5, P>0.45, Kruskal–Wallis tests; data not shown).
Fig. 2.
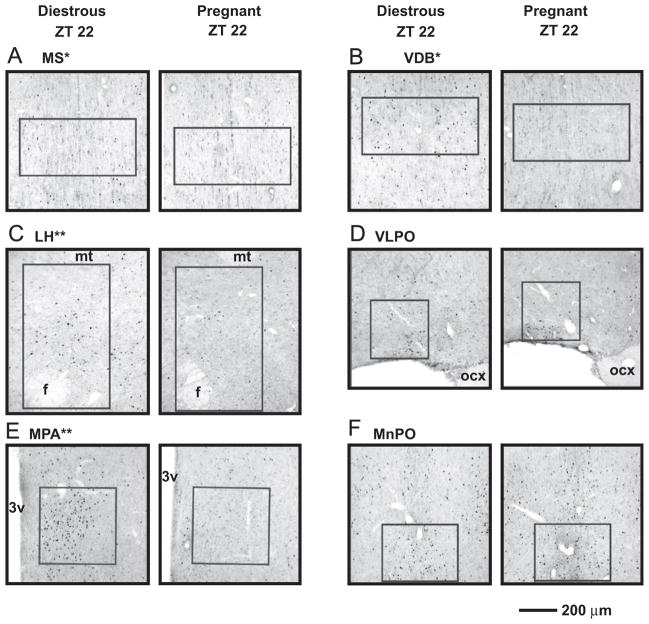
Representative photomicrographs of cells labeled for FOS in the MS (A), VDB (B), LH (C), VLPO (D), MPA (E), and MnPO (F) of diestrous (left column) and pregnant (right column) rats at ZT 22. The regions sampled are outlined (see Fig. 1). Note that the levels of FOS expression, while evident in all brain regions in the diestrous group, are most striking in the MPA and MnPO. * indicates a brain region where FOS expression is significantly higher in the diestrous group (main effect; see also Fig. 3), and ** indicates a brain region where FOS expression is significantly higher in the diestrous group at ZT 22 (specifically; see also Fig. 4). 3v: third ventricle; f: fornix; mt: mammilothalamic tract; ocx: optic chiasm. Scale bar=200 μm.
Fig. 3.
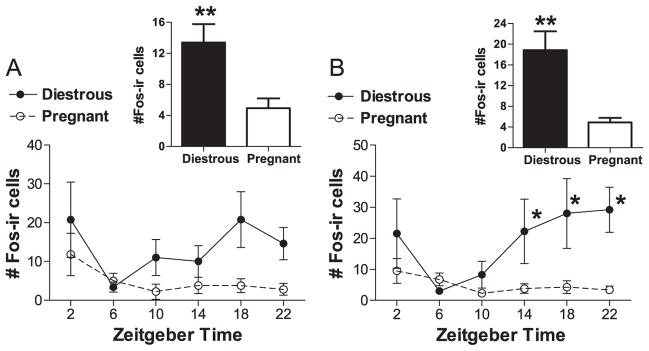
Expression of FOS in the MS (A) and VDB (B) of diestrous and pregnant rats kept in a 12:12-h LD cycle. * indicates a time point where expression is significantly elevated relative to at least 1 other ZTwithin the diestrous state (B, P<0.05, post-hoc Mann–Whitney U tests). Bar graph insets display total FOS expression for each reproductive state in each brain region. ** indicates a significant main effect of reproductive state on FOS expression (A, P<0.05, two-way ANOVA; B, P<0.05, Mann–Whitney U test).
In the perifornical region of the LH, pregnant rats maintained significant rhythmicity in FOS expression but with a dampened amplitude and reduced expression at certain times of the day (Figs. 2C and 4A). The rhythm in FOS expression significantly differed between diestrous and pregnant females (Fig. 4A; interaction: F=3.212, df=5, P<0.02, two-way ANOVA). In diestrous females, FOS expression peaked at ZT 2 and was lowest at ZT 6 (ZT: F=3.471, df=5, P<0.02, post-hoc one-way ANOVA). The rhythm of FOS-ir in pregnant rats appeared dampened (Fig. 4A), with troughs at ZT 10 and 22 but no significant peaks (ZT: F=3.696, df=5, P<0.02, post-hoc one-way ANOVA). Diestrous rats expressed significantly more FOS in the LH than pregnant ones at ZT 10 and 22, the times of lowest expression in the pregnant females (Figs. 2C and 4A).
Fig. 4.
Expression of FOS in the LH (A), VLPO (B), MPA (C), and MnPO (D) of diestrous and pregnant rats kept in a 12:12-h LD cycle. * indicates a time point where expression is significantly elevated relative to at least 2 other ZTs within the same reproductive state (A, P<0.05, post-hoc one-way ANOVA; B and C, P<0.05, post-hoc Mann–Whitney U tests) or, for the MnPO, in the combined pregnant plus diestrous rhythm (D, P<0.05, two-way ANOVA), and ** next to a ZT indicates a significant difference in expression between pregnant and diestrous rats at that time (A, P<0.05, post-hoc LSD tests; C, P<0.01, Mann–Whitney U tests).
2.2. FOS expression in temperature-regulating and sleep-active brain regions
Unlike brain regions that support wakefulness, in the VLPO, there was no significant difference in total FOS expression between diestrous and pregnant rats (Figs. 2D and 4B; main effect: U=289.5, P>0.05, Mann–Whitney U-test). However, we did find a significant rhythm in FOS expression in pregnant rats with elevated FOS-ir at both ZT 22 and ZT 2 (Fig. 4B; X2=12.525, df=5, P<0.03, Kruskal–Wallis test), whereas this was not the case in the diestrous group (X2=6.399, df=5, P>0.25, Kruskal–Wallis test). Expression did not differ significantly between the two reproductive states at any time examined (Figs. 2D and 4B). However, we found a near-significant difference at ZT 10 between the two groups, with lowered expression in the pregnant group (U=2.000, P<0.065, Mann–Whitney U-test).
In a manner similar to the effects seen in the VDB, FOS expression in the MPA of pregnant rats was reduced, and the rhythm in FOS-ir was lost (Figs. 2E and 4C). In this region, expression was significantly higher in diestrous than pregnant females (Fig. 4C, main effect: U=290.5, P<0.001, Mann–Whitney U-test). Again, FOS expression was rhythmic in the diestrous group with peak expression from the early dark phase through the early light phase (Fig. 4C; X2=14.434, df=5, P<0.02, Kruskal–Wallis test). However, there was no FOS rhythm in the MPA of pregnant rats, and expression was generally low (Fig. 4C; ZT: X2=8.812, df=5, P>0.1, Kruskal–Wallis test). FOS-ir in the MPA was significantly lower in pregnant rats than in diestrous ones at ZT 2 and 22, times of peak expression in diestrous females (Figs. 2E and 4C).
Expression of FOS in the MnPO was similar to that in the VLPO. Expression in the MnPO did not differ between diestrous and pregnant rats (Figs. 2F and 4D; reproductive state: F=1.307, df=1, P>0.25; interaction: F=1.002, df=5, P>0.40, two-way ANOVA), but FOS expression was significantly rhythmic in the combined diestrous plus pregnant groups, with peak expression at ZT 2 and 22 (Fig. 4D; ZT: F=4.653, df=5, P<0.005, two-way ANOVA).
3. Discussion
Our results indicate that the changes in sleep and wakeful-ness in early pregnancy may be due to an attenuation or loss of rhythms of neural activity in wakefulness-promoting regions of the brain, rather than stemming from alterations in the functioning of brain regions that promote sleep. FOS expression in the MS, VDB, and perifornical LH was remarkably lower in pregnant females compared to that of diestrous ones (Figs. 3 and 4A), but it was not significantly different in the VLPO or MnPO between the two groups (Fig. 4B and D).
In our pregnant animals, the rhythm in FOS expression in the LH was maintained, but its amplitude was reduced (Fig. 4A). This could reflect weaker circadian control of wakefulness by the perifornical LH due to a drop in the general tone of the output cells of this region. The area of the LH sampled here contains a high density of neurons that produce orexin and express FOS in a daily rhythmic pattern (Estabrooke et al., 2001; Martinez et al., 2002). In male rats, the pattern of expression of FOS in both orexinergic and non-orexin cells in the perifornical LH is similar (Martinez et al., 2002). Thus, it is likely that our LH sample reflects the status of orexinergic neurons of this region, which are known to promote wakefulness through projections to the cerebral cortex via the thalamus and many other wakefulness-promoting brain regions, including those of the cholinergic basal forebrain (Peyron et al., 1998; Saper et al., 2010). The overall decline in FOS expression in the LH might therefore contribute to the reduction of FOS expression in the MS and VDB during early pregnancy (Fig. 3), as well as to the loss of rhythmicity in its expression in the VDB (Fig. 3B). It should be noted, however, that the magnitude of the reduction of FOS expression in the MS and VDB renders the interpretation of a lack of rhythmicity in these regions during early pregnancy difficult.
If FOS expression reflects orexinergic tone in the perifornical LH of our females, then some aspect of early pregnancy may inhibit the synthesis and/or release of orexin by LH neurons and thus cause downstream reduction in neural activity, and potentially wakefulness-drive, in the MS and VDB. In the whole animal, the weaker rhythmic and tonic control of wakefulness could then explain why females are sleeping more. In another study, Garcia et al. (2003) found that, on day 18 of pregnancy, a time when females are also sleeping more than nonpregnant females, mRNA for the orexin precursor, preproorexin, in the rat LH is profoundly reduced to less than 10% of that of nonpregnant females. The most likely candidates for causing the change observed in their study, and potentially in ours, are lactogenic hormones, such as placental lactogens, which are produced during mid through late pregnancy by the placenta (Matthies, 1967; Robertson and Friesen, 1981), and prolactin (PRL), which is elevated during early pregnancy in the rat, with twice-daily surges around lights-on and lights-off (Butcher et al., 1972; Freeman et al., 2000). In fact, pituitary grafts that increase plasma PRL levels in female rats reduce preproorexin mRNA in the LH (Garcia et al., 2003). The pregnant animals of our study had been exposed to elevated levels of endogenous PRL compared to their diestrous counterparts, and this could thus cause the reduction of FOS expression in the LH seen here (Fig. 4A).
Prolactin may directly inhibit orexin production in the LH, or it may indirectly inhibit it by increasing the sensitivity of orexinergic cells to sleep-promoting substances. In mice, orexinergic neurons in the LH, and most likely glutamatergic inputs to them, are inhibited by adenosine, a byproduct of neuronal activity that is known to promote sleep. Specifically, in mice, adenosine inhibition of these neurons is mediated by the A1 adenosine receptor (Liu and Gao, 2007). If this holds true for the rat as well, up-regulation of A1 receptors in the LH might occur during early pregnancy, and this could lead to a drop in wakefulness-drive from the LH and any downstream brain regions, such as the MS and VDB, accounting for the drop in FOS expression seen here. Future work should test the generality of these findings by assessing the effects of pregnancy in general, and PRL in specific, on other wake-promoting regions that also receive orexinergic inputs (e.g. the dorsal raphe; Peyron et al., 1998). Alternatively, the effects seen in the MS and VDB could be directly driven by up-regulation of A1 receptors in the basal forebrain, since adenosine inhibits cholinergic activity directly and through glutamatergic inputs in the basal forebrain of mice through the same receptor (Arrigoni et al., 2006; Hawryluk et al., 2012). It is important to note that we did not phenotype the neurons that expressed FOS in this study or determine if the changes in FOS expression are associated with the release of chemical signals from these neurons. Further work identifying whether the effects seen here were in orexinergic and cholinergic neurons in the LH and basal forebrain, respectively, is needed to support the above hypotheses.
In addition to the changes in the LH, MS, and VDB, we also found a loss of rhythmic expression of FOS and general decline in FOS-ir in the MPA of pregnant rats (Fig. 4C). The MPA plays a role in the regulation of multiple behaviors and physiological parameters, including proceptive and parental behaviors, sleep, and core body temperature (for reviews, see Boulant (2000), Kumar et al. (2007), Numan (2007) and Sakuma (2008)). The patterns of FOS expression we found in the MPA contrast with those reported by Lee et al. (1998), who found a significant rhythm in FOS expression in the MPA on day 6 of pregnancy in the rat. The reason for the discrepancy is unclear, though perhaps it is due to the fact that Lee et al. (1998) sampled a more caudal portion of the MPA than we did. Normal proceptive behavior depends upon a functional MPA (Guarraci et al., 2004; Hoshina et al., 1994; Whitney, 1986; Yang and Clemens, 2000), so the reduced expression of FOS in the MPA in pregnant rats may be related to the protracted absence of proceptive sexual behaviors that accompanies early pregnancy. Although diestrous females are also not normally proceptive, they differ from pregnant ones, as diestrous females are a few days away from becoming proceptive on proestrus again. The MPA also plays a critical role in the coordination of sleep and the associated decrease in core body temperature (Kumar et al., 2007). During early pregnancy in the rat, the sleep/wake cycle and rhythms in core body temperature are dissociated (Kimura et al., 1996; Kittrell and Satinoff, 1988; Rosenwasser et al., 1987). Perhaps the loss of rhythmic expression of FOS in the MPA of pregnant rats reflects reduced activity in cells in this region that coordinate sleep/wake behavior with core body temperature.
The findings of the present study, along with those from our previous work (Schrader et al., 2010, 2011), are consistent with the hypothesis that the suite of changes in overt rhythms during early pregnancy in the rat are due to differential changes in rhythmic activity in different brain regions. Thus, pregnancy appears to induce a re-organization of the circadian system of the brain and how it interacts with brain regions that are responsible for different behavioral and homeostatic functions. It is unclear whether the changes we observed here are driven by changes in the circadian timekeeping system or are instead influenced independently by pregnancy-associated stimuli acting on targets of the circadian system, or by a combination of both influences.
4. Experimental procedures
4.1. Animals
Adult female and male Sprague-Dawley laboratory rats (Harlan Laboratories, Indianapolis, IN, USA) housed in polypropylene cages (48×27×20 cm) were kept in a 12 h:12 h light/dark (LD) cycle with lights on at Zeitgeber Time (ZT) 0 and provided ad libitum access to food (Teklad 8640 rodent diet, Harlan) and water; a dim red light (<5 lx) was on constantly. All experiments were performed in compliance with guidelines established by the Michigan State University Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their discomfort.
4.2. Tissue collection and immunocytochemistry
The estrous cycles were monitored by vaginal cytology. Females that were randomly selected to be part of the pregnant group were paired with males on the morning of proestrus, and mating success was later determined through vaginal smearing to confirm the presence of sperm. Females (n=4–6/group) were then perfused at one of six time points (ZT 2, 6, 10, 14, 18, or 22) on either day 1 of diestrus (n=27) or day 6 of pregnancy (n=28); these were the same animals used in Schrader et al. (2010), where further details are described. Brains were collected and sectioned coronally at 30 μm into three alternate series using a freezing microtome. One series of tissue was processed using an anti-cFOS antibody (made in rabbit, Santa Cruz Biochemistry, Santa Cruz, CA, USA; 1:25,000). Further details of tissue processing are described in Schrader et al. (2010).
4.3. Quantitative and statistical analysis
Counts of labeled cells in the MS, VDB, HDB, MnPO, MPA, VLPO, and LH were taken from three sections of each region using a charge-coupled device video camera (CX900, MBF Bioscience, Williston, VT, USA) attached to a light microscope (Zeiss, Göttingen, Germany). These images were processed using Adobe Photoshop 7 (Adobe Systems, Mountain View, CA, USA). FOS-immunoreactive (FOS-ir) cells were counted from these images with the NIH ImageJ program (NIH, Bethesda, MD, USA). Sampling boxes were used for counts in each region as follows (Fig. 1): MS and VDB (600 μm × 300 μm, midline), HDB (600 μm × 300 μm, bilaterally), MnPO (400 μm × 300 μm, midline), MPA (400 μm × 400 μm, bilaterally), VLPO (300 μm × 300 μm, bilaterally), and LH (perifornical region: 450 μm × 750 μm, bilaterally). All counts were made by an investigator unaware of the reproductive state or time of perfusion of animals.
Counts were subjected to a two-way ANOVA (reproductive state X ZT); FOS-ir counts from the MS were first square-root transformed to equalize variances amongst treatment groups. Post-hoc least significant difference (LSD) tests were conducted when significant main effects were found. Nonparametric analyses were performed on data sets in which square root transformations did not equalize the variances, which was the case for FOS-ir counts in the VDB, HDB, MPA, and VLPO. In these cases, we compared the effects of ZT within each reproductive state separately (Kruskal–Wallis tests, followed by post-hoc pairwise Mann–Whitney U tests). We also tested for a main effect of reproductive state (all ZTs combined, Mann–Whitney U tests) and compared reproductive states at select ZTs with Mann–Whitney U tests where the means of the pregnant and diestrous groups were separated by more than twice the larger standard error (VDB at ZT 18 and 22; MPA at ZT 2, 10, 14, 18, and 22; VLPO at ZT 10); Bonferroni corrections were applied within each brain region. All data analyses were conducted with SPSS 17 software (SPSS Inc., Chicago, IL, USA). All differences were considered significant when P<0.05, except where Bonferroni corrections were applied. Details of statistics are omitted from the text when they are presented in figure legends. For all figures, data are presented as means±standard errors.
Acknowledgments
We thank B. Cavanaugh, E. Walaszczyk, A. Stowie, A. Baumgras, A. Castillo-Ruiz, and C. Ramanathan for technical assistance; and R. Figueira, T. Lee, L. Yan, and S. Sakai for technical and/or statistical advice. This work was supported by NIH (RO1 MH53433).
Abbreviations
- 3v
third ventricle
- AC
anterior commissure
- ANOVA
analysis of variance
- ARC
arcuate nucleus
- f
fornix
- HDB
horizontal diagonal band of Broca
- ir
immunoreactivity
- LD
light/dark
- LH
lateral hypothalamus
- LSD
least significant difference
- LV
lateral ventricle
- MnPO
median preoptic nucleus
- MPA
medial preoptic area
- MS
medial septum
- mt
mammillothalamic tract
- NREM
non-rapid eye movement
- ocx
optic chiasm
- Pe
periventricular hypothalamus
- PER2
Period2
- PH
posterior hypothalamus
- PRL
prolactin
- REM
rapid eye movement
- SCN
suprachiasmatic nucleus
- VDB
vertical diagonal band of Broca
- VLPO
ventrolateral preoptic area
- VMPO
ventromedial preoptic area
- ZT
Zeitgeber time
Contributor Information
Jessica A. Schrader, Email: SchraderJ@brevardcc.edu.
Laura Smale, Email: smale@msu.edu.
Antonio A. Nunez, Email: nunez@msu.edu.
References
- Arrigoni E, Chamberlin NL, Saper CB, McCarley RW. Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience. 2006;140:403–413. doi: 10.1016/j.neuroscience.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31 (Suppl 5):S157–161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Fugo NW, Collins WE. Semicircadian rhythm in plasma levels of prolactin during early gestation in the rat. Endocrinology. 1972;90:1125–1127. doi: 10.1210/endo-90-4-1125. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Lopez M, Gualillo O, Seoane LM, Dieguez C, Senaris RM. Hypothalamic levels of NPY, MCH, and preproorexin mRNA during pregnancy and lactation in the rat: role of prolactin. FASEB J. 2003;17:1392–1400. doi: 10.1096/fj.02-0933com. [DOI] [PubMed] [Google Scholar]
- Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco MA, Lu J, Wagner D, Shiromani PJ. c-Fos expression in the cholinergic basal forebrain after enforced wakefulness and recovery sleep. Neuroreport. 2000;11:437–440. doi: 10.1097/00001756-200002280-00002. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Megroz AB, Clark AS. Paced mating behavior in the female rat following lesions of three regions responsive to vaginocervical stimulation. Brain Res. 2004;999:40–52. doi: 10.1016/j.brainres.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Hawryluk JM, Ferrari LL, Keating SA, Arrigoni E. Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons. J Neurophysiol. 2012;107:2769–2781. doi: 10.1152/jn.00528.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina Y, Takeo T, Nakano K, Sato T, Sakuma Y. Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behav Brain Res. 1994;61:197–204. doi: 10.1016/0166-4328(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Jones BE. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann N Y Acad Sci. 2008;1129:26–34. doi: 10.1196/annals.1417.026. [DOI] [PubMed] [Google Scholar]
- Kimura M, Zhang SQ, Inoue S. Pregnancy-associated sleep changes in the rat. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1063–1069. doi: 10.1152/ajpregu.1996.271.4.R1063. [DOI] [PubMed] [Google Scholar]
- Kittrell EM, Satinoff E. Diurnal rhythms of body temperature, drinking and activity over reproductive cycles. Physiol Behav. 1988;42:477–484. doi: 10.1016/0031-9384(88)90180-1. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. Measurement of immediate-early gene activation—c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Kumar VM, Vetrivelan R, Mallick HN. Noradrenergic afferents and receptors in the medial preoptic area: neuroanatomical and neurochemical links between the regulation of sleep and body temperature. Neurochem Int. 2007;50:783–790. doi: 10.1016/j.neuint.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Lee Y, Arbogast LA, Voogt JL. Semicircadian rhythms of c-Fos expression in several hypothalamic areas during pregnancy in the rat: relationship to prolactin secretion. Neuroendocrinology. 1998;67:83–93. doi: 10.1159/000054302. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypothalamus: a possible sleep-promoting effect. J Neurophysiol. 2007;97:837–848. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GS, Smale L, Nunez AA. Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res. 2002;955:1–7. doi: 10.1016/s0006-8993(02)03264-x. [DOI] [PubMed] [Google Scholar]
- Matthies DL. Studies of the luteotropic and mammotropic factor found in trophoblast and maternal peripheral blood of the rat at mid-pregnancy. Anat Rec. 1967;159:55–67. doi: 10.1002/ar.1091590109. [DOI] [PubMed] [Google Scholar]
- Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM, Fuller CA. The Clocks That Time Us: Physiology of the Circadian Timing System. Harvard University Press; Cambridge Massachussetts: 1982. [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Patronas P, Horowitz M, Simon E, Gerstberger R. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res. 1998;798:127–139. doi: 10.1016/s0006-8993(98)00405-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, Compact. 3. Academic Press; San Diego, California: 1997. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Robertson MC, Friesen HG. Two forms of rat placental lactogen revealed by radioimmunoassay. Endocrinology. 1981;108:2388–2390. doi: 10.1210/endo-108-6-2388. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Hollander SJ, Adler NT. Effects of pregnancy and parturition on free-running circadian activity rhythms in the rat. Chronobiol Int. 1987;4:183–187. doi: 10.3109/07420528709078524. [DOI] [PubMed] [Google Scholar]
- Rusak B. The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster, Mesocricetus auratus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1977;118:145–164. [Google Scholar]
- Sakuma Y. Neural substrates for sexual preference and motivation in the female and male rat. Ann N Y Acad Sci. 2008;1129:55–60. doi: 10.1196/annals.1417.009. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satinoff E, Liran J, Clapman R. Aberrations of circadian body temperature rhythms in rats with medial preoptic lesions. Am J Physiol Regul Integr Comp Physiol. 1982;242:R352–357. doi: 10.1152/ajpregu.1982.242.3.R352. [DOI] [PubMed] [Google Scholar]
- Schrader JA, Nunez AA, Smale L. Changes in and dorsal to the rat suprachiasmatic nucleus during early pregnancy. Neuroscience. 2010;171:513–523. doi: 10.1016/j.neuroscience.2010.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader JA, Nunez AA, Smale L. Site-specific changes in brain extra-SCN oscillators during early pregnancy in the rat. J Biol Rhythms. 2011;26:363–367. doi: 10.1177/0748730411409714. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Characterization and mapping of sleep-waking specific neurons in the basal fore-brain and preoptic hypothalamus in mice. Neuroscience. 2009;161:269–292. doi: 10.1016/j.neuroscience.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Weinert D. Ontogenetic development of the mammalian circadian system. Chronobiol Int. 2005;22:179–205. doi: 10.1081/cbi-200053473. [DOI] [PubMed] [Google Scholar]
- Whitney JF. Effect of medial preoptic lesions on sexual behavior of female rats is determined by test situation. Behav Neurosci. 1986;100:230–235. doi: 10.1037//0735-7044.100.2.230. [DOI] [PubMed] [Google Scholar]
- Yang LY, Clemens LG. MPOA lesions affect female pacing of copulation in rats. Behav Neurosci. 2000;114:1191–1202. [PubMed] [Google Scholar]