Abstract
Objective
To analyze the effect of sociodemographic, disease, and health system characteristics and contextual features about the community of residence on the subsequent initiation of treatment with biologic agents for rheumatoid arthritis (RA).
Method
We analyzed data from the University of California, San Francisco Rheumatoid Arthritis Panel Study for the years 1999–2011. Principal data collection was by a structured annual phone survey. We estimated Kaplan-Meier curves of the time until initiation of biologic agents, stratified by age and income. We also used Cox regression to estimate the effect of individual-level sociodemographic and medical factors, contextual-level socioeconomic status measures, and density of health providers in the local community on the probability of initiating therapy with biologic agents for RA.
Results
In total, 527 persons were included in the panel in 1999, and 229 persons (44%) had initiated therapy with biologic agents by 2011. In multivariable Cox regression models, age <70 years (hazard ratio [HR] for ages 19–54 years 1.89 [95% confidence interval (95% CI) 1.24–2.87] and HR for ages 55–69 years 1.25 [95% CI 0.84–1.87]), Hispanic ethnicity (HR 2.02 [95% CI 1.05–3.86]), household income ≥$30,000/year (HR 1.61 [95% CI 1.12–2.32]), being married or with a partner (HR 1.39 [95% CI 1.00–1.92]), and residence in rural environments (HR 1.96 [95% CI 1.28–2.99]) were associated with a higher probability of initiating biologic agents. Having no (HR 0.18 [95% CI 0.08–0.40]) or only 1–4 rheumatology visits in the year prior to interview (HR 0.60 [95% CI 0.45–0.81]) and living in an area with ≥1 federally qualified health centers (HR 0.63 [95% CI 0.41–0.96]) were associated with a lower probability.
Conclusion
The probability of initiating therapy with biologic agents is affected by sociodemographic and health system characteristics as well as the nature of the community of residence, resulting in disparities in access to these medications.
Introduction
Appropriate use of disease-modifying antirheumatic drugs (DMARDs) has become a cornerstone of care for rheumatoid arthritis (RA) (1,2). Despite the universal recommendation for the use of DMARDs in RA, numerous studies have indicated that these medications are underused (3–8). Three studies identified differences in utilization of DMARDs by such characteristics as race/ethnicity, socioeconomic status (SES), and type of health plan (5,6,9), while another study observed that access to these agents, including biologic agents, was correlated with the wealth of countries (10). These differences would properly be regarded as disparities if they could not be attributed to medical need (11,12).
While the American College of Rheumatology recommends initial treatment of RA with methotrexate or another nonbiologic DMARD prior to therapy with biologic agents, biologic agents are often warranted because of incomplete disease control (2). The present study was designed to analyze whether there are disparities in the initiation of therapy with biologic agents in a community-based cohort of persons with RA. The major factors analyzed included the effect of individual-level sociodemographic and medical factors (including RA treatments), contextual-level SES measures, and density of health providers in the local community on the probability of initiating biologic agents for the treatment of RA.
Materials and Methods
Data source
The present study used the University of California, San Francisco Rheumatoid Arthritis Panel Study (RAPS). The RAPS began in 1982/1983 by taking a random sample of half of the rheumatologists then practicing in Northern California and who, in turn, maintained a log of all persons with RA presenting over a 1-month period and verified the diagnosis. The logs included both returning and new patients. Subsequent enrollments of persons with RA using the same sampling method occurred in 1989, 1995, 1999, and 2003 (to maximize the length of time to estimate time until initiation of the biologic agents, the present analysis was limited to those enrolled in 1999 or earlier). Overall, 1,447 persons entered the RAPS during one of the waves of enrollment (85% of the persons with RA listed on the logs).
The principal data collection for the RAPS is an annual structured telephone interview conducted by trained survey workers using validated batteries of items. The survey collects information on signs and symptoms of RA, the extent of comorbidity, physical and psychological health status, functional status, health care utilization information, and characteristics of health insurance plans. In the survey for each year, there is a complete inventory of all RA treatments received, including medications (name, duration, and dose). In addition, basic demographic information is collected, with updates on items such as marital and employment status and income, as warranted. Prior publications provided listings of the specific validated batteries included in the surveys (13–15).
Beginning in 1999, the annual RAPS included questions about usage in the year prior to the interview of each biologic agent that had been given a Food and Drug Administration indication for RA. Thus, the surveys from 1999 through 2011 covered usage of biologic agents from 1998 through 2011.
Over the 3 decades of the RAPS data collection, we were able to reinterview an average of 93% of those interviewed in the prior year. After excluding deaths, the reinterview rate averaged ∼95%. As a result of deaths and attrition, as of 1999, there were 527 persons enrolled in the RAPS. Data collection was approved by the University of California, San Francisco Institutional Review Board.
Statistical analysis
In the present article, we used the Kaplan-Meier method to estimate time until the first initiation of a biologic agent, including any of the following: etanercept, infliximab, abatacept, adalimumab, anakinra, and rituximab. We show a Kaplan-Meier curve for the entire RAPS sample as well as by strata selected on the basis of characteristics that were shown to be related to treatment usage in prior studies, including income and age. In the Kaplan-Meier analyses, censorship of observations could occur because of death or loss to followup. Among the 527 persons enrolled in the RAPS, 43% initiated biologic agents, 10% were censored due to death, 15% never initiated biologic agents and were interviewed through 2011, and 31% were censored because of attrition at some point. However, even among this latter 31% of persons, the median number of annual interviews after 1999 was 4.
We then used Cox proportional hazards regression to estimate the impact of baseline characteristics of individuals and the characteristics of their communities in various years, defined at various levels of aggregation, on the rate of first initiation of any biologic agent. The individual characteristics included these sociodemographic variables: age (19–54 years, 55–69 years, or ≥70 years [reference]); female sex; race (white versus nonwhite); ethnicity (Hispanic of any race versus non-Hispanic); marital status (married or with a partner versus other); education attainment (more than high school versus high school graduate or less); annual household income (<$30,000/year versus ≥$30,000/year); residence area (rural versus urban); insurance status, including whether a health plan was fee-for-service or some form of managed care and whether the individual had insurance for medications; the number of visits to a rheumatologist in the year prior to interview (none, 1–4, or ≥5 [reference]); RA-related factors, including disease duration, number of swollen joints, number of painful joints, and functional status as measured by the Health Assessment Questionnaire score; comorbidities as measured by having a Geriatric Depression Scale score of ≥7, indicative of major depressive symptomology; the number of chronic conditions (none, 1, or ≥2 [reference]); and therapies other than biologic agents, including oral steroids, nonbiologic DMARDs, nonsteroidal antiinflammatory drugs (NSAIDs), and presence of a hospital admission for RA in the year prior to interview.
The community characteristics included the number of rheumatologists per 100,000 residents in the hospital referral region in 2006, as defined by the Dartmouth Atlas of Health Care, dichotomized as the bottom versus the top 3 quartiles (≥0.78 per 100,000 residents) (16); whether there were ≥1 federally qualified health centers in the local area in 2007, an indicator of an area underserved by health care providers, also as defined by the Dartmouth Atlas of Health Care; and whether the census block group was an area with a high concentration of persons with household incomes ≤125% of the federal poverty level, as defined by the American Community Survey for the 5-year period of 2005–2009, dichotomized as the top versus the bottom 3 quartiles (≥17% of the area's population having a family income below the target level) (17). Census block groups are roughly analogous to a small neighborhood, with approximately 400–1,000 individuals, and are relatively homogeneous. We also evaluated the interaction between living in a community in the lowest quartile of rheumatologists per capita (<0.78 per 100,000 residents) and having no or few visits to a rheumatologist. This latter analysis assessed whether the effect of visiting rheumatologists was contingent on their availability. Finally, we used the Cox regression estimates to test whether adding community characteristics to the full individual-level model resulted in an improvement in model fit, as indicated by the increase in the chi-square model.
Results
Table 1 shows the characteristics of the RAPS members in 1999, stratified by whether each initiated biologic agents at some point between 1998 and 2011. For the entire membership of the RAPS, the average age was 61 years, more than four-fifths were women, and 93% were non-Hispanic white. Approximately 2 in 5 members had household incomes <$30,000/year. Reflecting the age of the membership, the disease duration averaged 20 years. Health Assessment Questionnaire scores, averaging 1.08, were indicative of substantial limitation in activities. Approximately one-half of the RAPS members had taken oral steroids in the year prior to interview, approximately four-fifths had taken a nonbiologic DMARD, approximately three-fourths had taken an NSAID, and just under one-fourth had been hospitalized during this period. Finally, approximately one-sixth lived in an area with ≥1 federally qualified health centers (usually indicative of medically underserved areas).
Table 1. Characteristics of members of the University of California, San Francisco Rheumatoid Arthritis Panel Survey in 1999, by subsequent initiation of biologic agents through 2011*.
| Total (n = 527) | Started biologic agents (n = 229, 43%) | Never started biologic agents (n = 298, 57%) | P | |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age, mean ± SD years | 61.2 ± 13.9 | 56.7 ± 13.3 | 64.7 ± 13.3 | 0.00 |
| Women | 440 (83) | 194 (85) | 246 (83) | 0.51 |
| Race | 0.58 | |||
| Nonwhite | 89 (17) | 41 (18) | 48 (16) | |
| White | 438 (83) | 188 (82) | 250 (84) | |
| Ethnicity | 0.04 | |||
| Non-Hispanic | 490 (93) | 207 (90) | 285 (95) | |
| Hispanic | 37 (7) | 22 (10) | 15 (5) | |
| Marital status | 0.00 | |||
| Never married/widowed, separated, or divorced | 191 (36) | 63 (28) | 128 (43) | |
| Married/partner | 336 (64) | 166 (72) | 170 (57) | |
| Education attainment | 0.09 | |||
| High school graduate or less | 216 (41) | 84 (37) | 132 (44) | |
| More than high school | 311 (59) | 145 (63) | 166 (56) | |
| Annual household income | 0.00 | |||
| <$30,000/year | 190 (38) | 56 (26) | 134 (48) | |
| ≥$30,000/year | 337 (62) | 162 (74) | 144 (52) | |
| Rural/urban | 0.12 | |||
| Urban | 445 (84) | 187 (82) | 258 (87) | |
| Rural | 82 (16) | 42 (19) | 40 (13) | |
| Insurance status | ||||
| Managed care | 0.67 | |||
| FFS | 94 (18) | 39 (17) | 55 (18) | |
| HMO/PPO | 433 (82) | 190 (83) | 243 (82) | |
| Pay for drugs | 0.01 | |||
| No | 49 (9) | 12 (5) | 37 (12) | |
| Yes | 478 (91) | 217 (95) | 261 (88) | |
| Visits to rheumatologist | 0.00 | |||
| None | 70 (13) | 8 (4) | 62 (21) | |
| 1–4 | 238 (45) | 90 (39) | 148 (50) | |
| ≥5 | 219 (42) | 131 (57) | 88 (30) | |
| RA-related factors, mean ± SD | ||||
| Disease duration, years | 19.6 ± 10.7 | 18.4 ± 9.8 | 20.6 ± 11.2 | 0.02 |
| HAQ score | 1.08 ± 0.74 | 1.01 ± 0.70 | 1.12 ± 0.77 | 0.10 |
| No. of swollen joints | 2.0 ± 2.7 | 2.2 ± 2.5 | 1.8 ± 2.8 | 0.14 |
| No. of painful joints | 4.0 ± 4.5 | 4.4 ± 4.3 | 3.7 ± 4.6 | 0.05 |
| Comorbidities | ||||
| Geriatric Depression Scale score ≥7 | 40 (8) | 16 (7) | 24 (8) | 0.68 |
| No. of comorbidities | 0.00 | |||
| None | 279 (53) | 140 (61) | 139 (47) | |
| 1 | 168 (32) | 65 (28) | 103 (35) | |
| ≥2 | 80 (15) | 24 (10) | 56 (19) | |
| Therapies | ||||
| Nonbiologic DMARDs | 428 (81) | 207 (90) | 221 (74) | 0.00 |
| Oral steroids | 273 (52) | 133 (58) | 140 (47) | 0.01 |
| NSAIDs | 408 (77) | 196 (86) | 212 (71) | 0.00 |
| Hospitalized in prior 12 months | 119 (23) | 51 (22) | 68 (23) | 0.88 |
| Contextual variables | ||||
| Dartmouth hospital referral regions | ||||
| Rheumatologists per 100,000 residents | 0.84 | |||
| ≥0.78 | 382 (72) | 167 (73) | 215 (72) | |
| <0.78 | 145 (28) | 62 (27) | 83 (28) | |
| Dartmouth primary care service regions | ||||
| Federally qualified health centers | 0.53 | |||
| None | 438 (83) | 193 (84) | 245 (82) | |
| ≥1 | 89 (17) | 36 (16) | 53 (18) | |
| American Community Survey block group: concentrated poverty | 0.87 | |||
| Household incomes at ≤125% of federal poverty level | ||||
| <17% | 394 (75) | 172 (75) | 222 (75) | |
| ≥17% | 133 (25) | 57 (25) | 76 (25) |
Values are the number (percentage) unless indicated otherwise. FFS = fee-for-service; HMO/PPO = health maintenance organization/preferred provider organization; RA = rheumatoid arthritis; HAQ = Health Assessment Questionnaire; DMARDs = disease-modifying antirheumatic drugs; NSAIDs = nonsteroidal antiinflammatory drugs.
As of 2011, 229 RAPS members (44%) had started ≥1 biologic agents. Those who started biologic agents were younger and had slightly more involved joints, a difference that reached statistical significance with respect to painful joints. A greater proportion of those starting a biologic agent had no comorbid conditions and had been receiving nonbiologic DMARDs, oral steroids, and NSAIDs. Of note, those starting a biologic agent were more likely to report having insurance coverage for prescription medications (95% versus 88%). A smaller proportion had a household income of <$30,000/year.
In Figure 1A, we show the time until the first initiation of a biologic agent among the RAPS members. Within 2 years of the first biologic agents becoming available in 1998, approximately one-fifth of the RAPS members had initiated biologic agents; by 5 years, approximately one-third had done so. However, the rate of first initiation had slowed since then; after another 8 years, the proportion with a first initiation of biologic agents had only increased to ∼44%.
Figure 1.
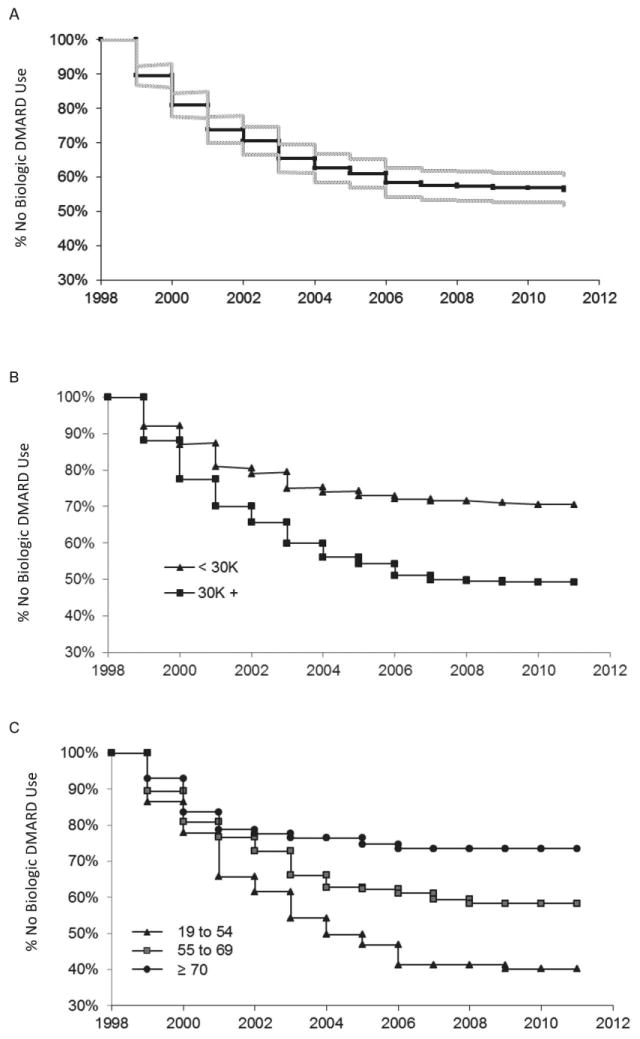
Kaplan-Meier estimates of time until first initiation of a biologic agent for all Rheumatoid Arthritis Panel Survey members (A) and by income (B) and age (C). DMARD = disease-modifying antirheumatic drug.
The proportion initiating biologic agents differed substantially by income level and age (Figures 1B and C). By 3 years after the first biologic agents became available, the proportion of persons with a household income <$30,000/year who initiated such agents was 10 percentage points lower than those with higher incomes (approximately 20% versus 30%); by the end of the period under study (2011), the proportion of the lower-income group receiving a biologic agent was 20 percentage points lower (30% versus >50%).
The probability of initiating biologic agents decreased with increasing age. By 2011, >60% of those ages 19–54 years at the outset of the study had initiated a biologic agent versus ∼40% among those ages 55–69 years and just slightly more than one-fourth among those ages ≥70 years. Furthermore, the proportion of the youngest age group with an initiation of biologic agents continued to increase substantially until 2006, whereas the proportion of the 2 older age groups with an initiation was essentially stable after 2004.
Table 2 shows the results of the Cox regression analysis of the factors affecting time until initiation of biologic agents. In addition to the 2 factors highlighted in Figure 1 (income level and age), on an unadjusted basis, the probability of initiating biologic agents was higher among Hispanics (hazard ratio [HR] 1.80 [95% confidence interval (95% CI) 1.16–2.80]), those who were married or with a partner (HR 1.69 [95% CI 1.27–2.26]), and persons with insurance coverage for prescription medications (HR 2.21 [95% CI 1.24–3.95]). On an unadjusted basis, numerous RA-related and therapeutic factors were associated with time until initiation of biologic agents, including the number of swollen and painful joints, level of comorbidity (with rates of initiation higher among those with no or 1 comorbidity), and receipt of nonbiologic DMARDs, oral steroids, and NSAIDs. Rates of initiation were higher in those members with a greater number of visits to rheumatologists in the year prior to the survey. Compared to those with ≥5 visits to rheumatologists, those with no (HR 0.13 [95% CI 0.06–0.26]) and 1–4 visits (HR 0.50 [95% CI 0.38–0.66]) were much less likely to initiate therapy.
Table 2. Probability of initiation of biologic agent use through 2011, with and without adjustment for individual and contextual factors*.
| Bivariate models | Multivariable models | ||
|---|---|---|---|
|
| |||
| Individual factors | Individual and contextual factors | ||
| Sociodemographics | |||
| Age, years | |||
| 19–54 | 2.80 (1.97-3.96)† | 1.83 (1.20-2.78)† | 1.89 (1.24-2.87)† |
| 55–69 | 1.72 (1.19–2.49) | 1.21 (0.81–1.80) | 1.25 (0.84–1.87) |
| ≥70 | 1 | 1 | 1 |
| Sex | |||
| Men | 1 | 1 | 1 |
| Women | 1.17 (0.82–1.68) | 1.06 (0.72–1.54) | 1.04 (0.71–1.53) |
| Race | |||
| Nonwhite | 0.88 (0.63–1.23) | 1.06 (0.64–1.74) | 1.07 (0.65–1.76) |
| White | 1 | 1 | 1 |
| Ethnicity | |||
| Non-Hispanic | 1 | 1 | 1 |
| Hispanic | 1.80 (1.16-2.80)† | 1.98 (1.05-3.72)† | 2.02 (1.05-3.86)† |
| Marital status | |||
| Unmarried/divorced | 1 | 1 | 1 |
| Married/partner | 1.69 (1.27-2.26)† | 1.35 (0.98–1.87) | 1.39 (1.00-1.92)† |
| Education attainment | |||
| High school graduate or less | 1 | 1 | 1 |
| More than high school | 1.27 (0.97–1.66) | 1.14 (0.85–1.52) | 1.15 (0.86–1.54) |
| Annual household income | |||
| <$30,000/year | 1 | 1 | 1 |
| ≥$30,000/year | 2.01 (1.49-2.72)† | 1.67 (1.16-2.39)† | 1.61 (1.12-2.32)† |
| Rural/urban | |||
| Urban | 1 | 1 | 1 |
| Rural | 1.39 (1.00–1.94) | 1.54 (1.08-2.20)† | 1.96 (1.28-2.99)† |
| Insurance status | |||
| Managed care | |||
| FFS | 1 | 1 | 1 |
| HMO/PPO | 1.02 (0.72–1.44) | 0.69 (0.47–1.02) | 0.68 (0.46–1.00) |
| Pay for drugs | |||
| No | 1 | 1 | 1 |
| Yes | 2.21 (1.24-3.95)† | 1.16 (0.63–2.14) | 1.17 (0.63–2.16) |
| Visits to rheumatologist | |||
| None | 0.13 (0.06-0.26)† | 0.19 (0.09-0.41)† | 0.18 (0.08-0.40)† |
| 1–4 | 0.50 (0.38-0.66)† | 0.62 (0.46-0.82)† | 0.60 (0.45-0.81)† |
| ≥5 | 1 | 1 | 1 |
| RA-related factors | |||
| Disease duration, years | |||
| <25 | 1 | 1 | 1 |
| ≥25 | 0.97 (0.72–1.30) | 1.26 (0.92–1.73) | 1.23 (0.89–1.69) |
| HAQ score | |||
| <1.6 | 1 | 1 | 1 |
| ≥1.6 | 0.94 (0.70–1.25) | 1.09 (0.78–1.52) | 1.09 (0.78–1.52) |
| No. of swollen joints | |||
| <3 | 1 | 1 | 1 |
| ≥3 | 1.45 (1.11-1.91)† | 1.34 (0.98–1.84) | 1.36 (0.99–1.86) |
| No. of painful joints | |||
| <6 | 1 | 1 | 1 |
| ≥6 | 1.37 (1.04-1.80)† | 1.22 (0.89–1.69) | 1.21 (0.87–1.66) |
| Comorbidities | |||
| Geriatric Depression Scale score | |||
| <7 | 1 | 1 | 1 |
| ≥7 | 0.94 (0.57–1.56) | 1.16 (0.67–2.01) | 1.16 (0.66–2.02) |
| No. of comorbidities | |||
| None | 1.87 (1.21-2.88)† | 1.69 (1.04-2.75)† | 1.72 (1.06-2.80)† |
| 1 | 1.37 (0.86–2.18) | 1.41 (0.86–2.30) | 1.44 (0.88–2.36) |
| ≥2 | 1 | 1 | 1 |
| Therapies | |||
| Nonbiologic DMARDs | |||
| No | 1 | 1 | 1 |
| Yes | 2.62 (1.68–4.06)† | 1.27 (0.78–2.05) | 1.29 (0.79–2.10) |
| Oral steroids | |||
| No | 1 | 1 | 1 |
| Yes | 1.46 (1.13-1.90)† | 1.06 (0.79–1.42) | 1.01 (0.75–1.36) |
| NSAIDs | |||
| No | 1 | 1 | 1 |
| Yes | 2.01 (1.39-2.91)† | 1.50 (1.02-2.21)† | 1.50 (1.02-2.20)† |
| Hospitalized in prior 12 months | |||
| No | 1 | 1 | 1 |
| Yes | 1.04 (0.76–1.41) | 1.04 (0.75–1.45) | 1.03 (0.74–1.43) |
| Contextual variables | |||
| Dartmouth hospital referral regions | |||
| Rheumatologists per 100,000 residents | |||
| ≥0.78 | 1 | 1 | |
| <0.78 | 1.00 (0.75–1.34) | 1.07 (0.77–1.47) | |
| Dartmouth primary care service regions | |||
| Federally qualified health centers | |||
| None | 1 | 1 | |
| ≥1 | 0.93 (0.70–1.25) | 0.63 (0.41-0.96)† | |
| American Community Survey block group | |||
| Household incomes at ≤125% of federal poverty level | |||
| <17% | 1 | 1 | |
| ≥17% | 1.00 (0.74–1.35) | 0.83 (0.20–3.41) | |
| Interaction of individual and contextual variables | |||
| No. of visits to rheumatologist/supply of rheumatologists | |||
| None/low | 0.19 (0.08–0.46) | ||
| 1–4/low | 0.56 (0.36–0.89) | ||
| ≥5/low | 1.02 (0.69–1.52) | ||
| None/high | 0.08 (0.03–0.26) | ||
| 1–4/high | 0.49 (0.36–0.67) | ||
| ≥5/high | 1 | ||
Values are the hazard ratio (95% confidence interval). FFS = fee-for-service; HMO/PPO = health maintenance organization/preferred provider organization; RA = rheumatoid arthritis; HAQ = Health Assessment Questionnaire; DMARDs = disease-modifying antirheumatic drugs; NSAIDs = nonsteroidal antiinflammatory drugs.
Significant.
On an unadjusted basis, none of the contextual factors analyzed (number of rheumatologists per capita, presence of federally qualified health centers, and concentration of poverty in the local area) were associated with the probability of initiating biologic agents. Because of the strong effect of the number of rheumatologist visits, we evaluated whether the effect of such visits was contingent on the number of rheumatologists per capita in the local area. However, the interaction term for the conjoint effect of the number of rheumatologist visits and number of rheumatologists per capita was not significantly related to the initiation of biologic agents, suggesting that the effect of the extent of contact with rheumatologists on the initiation of biologic agents was not contingent on the supply of rheumatologists in the local area.
After adjustment for individual characteristics (Table 2), younger persons with RA, Hispanics, those with household incomes ≥$30,000/year, those with a greater number of visits to rheumatologists, and those taking NSAIDs remained more likely to initiate biologic agents. However, the effects of marital status, medication coverage, level of involved joints, extent of comorbidity, and receipt of nonbiologic DMARDs and oral steroids were no longer significant, while living in a rural area (HR 1.54 [95% CI 1.08– 2.20]) was associated with a higher rate of initiation. After further adjustment for contextual factors (Table 2), among the contextual factors, only the presence of a federally qualified health center was associated with the initiation of biologic agents (HR 0.63 [95% CI 0.41–0.96]), indicating that those living in an area with such a center were much less likely to initiate therapy. However, when we evaluated whether the addition of the contextual variables as a group provided an increment of explanatory power by comparing the chi-square model from the restricted and expanded models, the results indicated that the addition did not significantly improve the fit (P = 0.11).
Discussion
Among all therapies for RA, biologic agents present the greatest challenge for the goal of achieving equitable access to effective treatment and reducing disparities in outcomes. The cost of these agents, especially the out-of-pocket share borne by persons with RA, is the most tangible aspect of the challenge. However, there are other aspects, including differential access to rheumatologists who have the most familiarity with biologic agents and receiving care in health systems that have more restrictive treatment protocols, such as requiring multiple failed regimens with nonbiologic DMARDs rather than 1 or 2 before permission to prescribe a biologic agent is granted.
There are legitimate issues about the extent to which persons with RA should have biologic agents included in their regimen, especially in the absence of an adequate trial of methotrexate or another nonbiologic DMARD or a specific contraindication to the continued use of such agents. Nevertheless, there is strong evidence supporting the use of biologic agents either alone (18) or in combination with nonbiologic DMARDs (19) once ≥1 of these criteria have been met. Given this evidence, and no counter evidence that biologic agents are less effective in specific sociodemographic subgroups, it is a matter of equity that usage rates of these agents should be determined by medical need and patient preferences rather than by such characteristics as race/ethnicity, SES, the type of health system in which care is received, or the nature of the community in which one resides.
The present study took advantage of a longitudinal cohort study of persons with RA originally sampled from a random group of Northern California rheumatologists to track the usage of biologic agents after the first biologic agents were approved in 1998 and to compare the time until first initiation of biologic agents as a function of medical and sociodemographic characteristics of persons with RA, as well as characteristics of their health systems and local communities. Because many of the persons with RA had migrated away from the rheumatologist practices that initially enrolled them, but many had not, we were able to measure the impact of the extent of contact with rheumatologists on the time to initiation of a biologic agent.
In this sample of persons who had had RA for just under 2 decades on average when biologic agents first became available, 44% had initiated biologic agents by the end of the study. The rate of initiation was much greater initially; one-third of the RAPS members had initiated biologic agents in the first 5 years, but the proportion only increased another 10 percentage points after 8 more years. Consistent with the study by Chu et al (9), we observed an increased probability of initiation among Hispanics; however, we also observed some substantial disparities in the expected direction. We observed lower rates of initiation among persons with RA with household incomes of <$30,000/year, among those living alone, among those ≥70 years of age, and among those living in areas with federally qualified health centers, an indication of medically underserved areas. In addition, the probability of initiation was substantially lower among those with no visits and those with relatively few visits to rheumatologists than among those with ≥5 visits. Although the latter finding was not altered by adjustment for RA-related factors, it still may reflect in part unmeasured differences in the severity of disease. To the extent that it does not merely reflect such differences, this finding indicates that access to rheumatologists is a crucial pathway to the initiation of biologic agents. This echoes prior studies of access to DMARDs for RA (3,6,8).
The observation that the probability of initiating biologic agents was lower among those living in areas with federally qualified health centers may indicate that such areas may have fewer medical care resources available to access as a result of the poverty of many of the residents in these areas. Alternatively, this observation may indicate that physicians practicing in such centers may not be able to secure access to specialty care by rheumatologists because of a lack of insurance or, among the insured, the limits faced by those with Medicaid (20), which may exacerbate the normal difficulties primary care physicians face in gaining access to specialists for their patients (21).
Many health insurance programs in the US limit total out-of-pocket expenses; some, like Medicare, have separate limits for medications and other expenses. Few health insurance plans, however, scale the amount of the limit to incomes. Thus, although after adjustment we were able to observe only small differences in the probability of initiation of biologic agents that did not reach statistical significance by whether or not the person with RA reported having medication coverage (HR 1.17 [95% CI 0.63-2.16]), the effect of having a household income ≥$30,000/year was much larger (HR 1.61 [95% CI 1.12-2.32]) and did reach statistical significance. The effect of out-of-pocket payments would appear to be more profound for biologic agents in RA than other diseases, perhaps because of the option of continuing therapy using only nonbiologic DMARDs and because persons with RA have been shown to be more sensitive to the magnitude of out-of-pocket payments for biologic agents than persons with such conditions as cancer, kidney disease, and multiple sclerosis (22).
This is not the first study to observe age-related differences in RA treatment. Schmajuk and colleagues (5) observed that, even among Medicare managed care beneficiaries, those ages ≥85 years had rates of DMARD usage 30 percentage points lower than those between 65 and 69 years of age. The results of our study of the time until initiation of biologic agents and the study by Schmajuk and colleagues suggest that, even when coverage is apparently adequate or even equivalent, there may be different expectations for the expected benefit for older patients on the part of physicians, or the expected out-of-pocket payments may be too large of a burden for those of older ages, perhaps because of the depletion of their savings over time. Older patients may also differ in their preferences for specific treatments.
Our observation of higher rates of initiation of biologic agents among Hispanics and no difference in rates by race suggests that, at least in this well-insured population, access to biologic agents was at least as great among members of minority groups as the general RAPS population, and that usage may be determined more by income and other demographic characteristics, such as age, than race. The results of our study may not be generalizable to the entire population of persons with RA in the US; the study sample had had their disease for a long time, the proportion of minorities was relatively small, all persons had been to a rheumatologist at some point, and all persons had some insurance coverage. Furthermore, managed care has a high share of the health insurance market in Northern California; even by the outset of the study in 1999, 82% of the cohort was enrolled in managed care health plans. However, some of these characteristics, particularly the long disease duration, high rates of nonbiologic DMARD usage, and history of interaction with rheumatologists at some point, may have made the results a conservative assessment of the impact of sociodemographic and health system characteristics and contextual factors on time until initiation of biologic agents. For example, one would expect that disparities by age or income would be muted in well-insured populations. Another limitation is that age- and income-related disparities in usage may have been due to differences in contraindications to or preferences for treatment, neither of which were directly measured in the RAPS annual survey.
The introduction of biologic agents has had a profound effect on outcomes for RA, especially for those who have failed ≥1 nonbiologic DMARDs. In this study, we observed that those with lower incomes, older persons, and those who were not married or living with a partner experienced a longer time until initiation of biologic agents. Those with no as opposed to some or more frequent contact with rheumatologists also experienced a longer time until initiation of treatment with biologic agents. The effect of income, age, and marital status remained significant after controlling for the frequency of contact with rheumatologists, indicating that the effect of these sociodemographic characteristics was not due to differential access to rheumatologist care. There may be different reasons for each of these disparities. Individuals in low-income households may choose not to pursue treatment with biologic agents because of the out-of-pocket costs. Perhaps there was an element of age discrimination in the substantially lower rates of usage among persons ages ≥70 years, or a higher rate of clinical contraindications, such as a history of tuberculosis infection. Those who were married or with a partner may have had the benefit of being persuaded to initiate such therapy despite its costs.
Time until initiation of biologic agents was substantially lengthened for those who had low income, those who were older, and those who were living alone, indicating disparities in access to these agents that have been shown to be effective in treating RA. Clinicians must be cognizant that their ability to communicate with persons from these backgrounds does not result in these disparities (23). To reduce the chance of this happening, they might profitably initiate the use of decision aid tools designed to inform persons with RA about treatment options (24), especially when such persons are from vulnerable groups, including the poor and elderly.
Significance & Innovations.
Introduction of therapy with biologic agents in rheumatoid arthritis (RA) has dramatically improved outcomes for RA, but differential access to these agents may create disparities in outcomes.
Few studies have longitudinal followup of a sufficient duration to study the initiation of therapy with biologic agents and have a wide range of risk factors prospectively measured, including characteristics of disease, sociodemographics, health systems, and local communities.
The extent of access to rheumatologists and living in areas with federally qualified health centers, an indicator of medical underservice, substantially reduced the probability of initiating therapy with biologic agents.
Acknowledgments
Supported by the NIH (grant R01-AR-056215).
Dr. Yelin's and Ms Tonner's work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Multidisciplinary Clinical Research Center grant P60-A-053308). Dr. Kim's work was supported by the NIH (grant K23-AR-059677). Dr. Brookhart's work was supported by investigator-initiated grants from Amgen, the Agency for Healthcare Research and Quality, the National Institute on Aging (grants R01-AG-023178 and R01-AG-042845), and the Patient-Centered Outcomes Research Institute (funding announcement 12001). Dr. Solomon's work was supported by the NIH (grants K24-AR-055989 and P60-AR-047782).
Dr. Yelin has received research support from Amgen (formerly Immunex) prior to 2004 and Aspreva. Dr. Kim has received research support from Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health, funded by Pfizer, Millennium Pharma, and Asisa. Dr. Brookhart has been an unpaid member of scientific advisory boards for Amgen, Merck, and Pfizer. Dr. Solomon has received research support from Amgen and Lilly and serves in unpaid roles on trials sponsored by Novartis, Lilly, Pfizer, and Bristol-Myers Squibb.
Footnotes
Author Contributions: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Yelin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Yelin, Tonner, Kim, Ayanian, Solomon.
Acquisition of data. Yelin.
Analysis and interpretation of data. Yelin, Tonner, Kim, Katz, Ayanian, Brookhart, Solomon.
References
- 1.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 2.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaCaille D, Anis A, Guh D, Esdaile JM. Gaps in care for rheumatoid arthritis: a population study. Arthritis Rheum. 2005;53:241–8. doi: 10.1002/art.21077. [DOI] [PubMed] [Google Scholar]
- 4.Schmajuk G, Schneeweiss S, Katz JN, Weinblatt ME, Setoguchi S, Avorn J, et al. Treatment of older adult patients diagnosed with rheumatoid arthritis: improved but not optimal. Arthritis Rheum. 2007;57:928–34. doi: 10.1002/art.22890. [DOI] [PubMed] [Google Scholar]
- 5.Schmajuk G, Trivedi AN, Solomon DH, Yelin E, Trupin L, Chakravarty EF, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305:480–6. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken) 2012;64:184–9. doi: 10.1002/acr.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavares R, Pope JE, Tremblay JL, Thorne C, Bykerk VP, Lazovskis J, et al. Early management of newly diagnosed rheumatoid arthritis by Canadian rheumatologists: a national, multicenter, retrospective cohort. J Rheumatol. 2011;38:2342–5. doi: 10.3899/jrheum.110249. [DOI] [PubMed] [Google Scholar]
- 8.Tavares R, Pope JE, Tremblay JL, Thorne C, Bykerk VP, Lazovskis J, et al. Time to disease-modifying antirheumatic drug treatment in rheumatoid arthritis and its predictors: a national, multicenter, retrospective cohort. J Rheumatol. 2012;39:2088–97. doi: 10.3899/jrheum.120100. [DOI] [PubMed] [Google Scholar]
- 9.Chu LH, Portugal C, Kawatkar AA, Stohl W, Nichol MB. Racial/ethnic differences in the use of biologic disease-modifying antirheumatic drugs among California Medicaid rheumatoid arthritis patients. Arthritis Care Res (Hoboken) 2013;65:299–303. doi: 10.1002/acr.21798. [DOI] [PubMed] [Google Scholar]
- 10.Putrik P, Ramiro S, Kvien T, Sokka T, Pavlova M, Uhlig T, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73:198–206. doi: 10.1136/annrheumdis-2012-202603. [DOI] [PubMed] [Google Scholar]
- 11.Kawachi I, Subramanian SV, Almeida-Filho N. A glossary for health inequalities. J Epidemiol Community Health. 2002;56:647–52. doi: 10.1136/jech.56.9.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braveman PA. Monitoring equity in health and healthcare: a conceptual framework. J Health Popul Nutr. 2003;21:181–92. [PubMed] [Google Scholar]
- 13.Yelin E, Henke C, Kramer J, Nevitt MC, Shearn M, Epstein WV. A comparison of the treatment of rheumatoid arthritis in health maintenance organizations and fee-for-service practices. N Engl J Med. 1985;312:962–7. doi: 10.1056/NEJM198504113121506. [DOI] [PubMed] [Google Scholar]
- 14.Yelin E, Criswell L, Feigenbaum P. Health care utilization and outcomes among persons with rheumatoid arthritis in fee-for-service and prepaid group practices. J Am Med Assoc. 1996;276:1048–53. [PubMed] [Google Scholar]
- 15.Katz PP, Morris A, Yelin EH. Prevalence and predictors of disability in valued life activities among individuals with rheumatoid arthritis. Ann Rheum Dis. 2006;65:763–9. doi: 10.1136/ard.2005.044677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Center for the Evaluative Clinical Sciences at Dartmouth Medical School. Dartmouth Atlas of Health Care. Lebanon (NH): Dartmouth Medical School; 2004. [Google Scholar]
- 17.U.S. Bureau of the Census Design and methodology. American Community Survey. Washington, DC: U.S. Government Printing Office; 2009. [Google Scholar]
- 18.Venkateshan SP, Sidhu S, Malhotra S, Pandhi P. Efficacy of biologicals in the treatment of rheumatoid arthritis: a metaanalysis. Pharmacology. 2009;83:1–9. doi: 10.1159/000165777. [DOI] [PubMed] [Google Scholar]
- 19.Kuriya B, Arkema EV, Bykerk VP, Keystone EC. Efficacy of initial methotrexate monotherapy versus combination therapy with a biological agent in early rheumatoid arthritis: a metaanalysis of clinical and radiographic remission. Ann Rheum Dis. 2010;69:1298–304. doi: 10.1136/ard.2009.118307. [DOI] [PubMed] [Google Scholar]
- 20.Cook NL, Hicks LS, O'Malley AJ, Keegan T, Guadagnoli E, Landon BE. Access to specialty care and medical services in community health centers. Health Aff (Millwood) 2007;26:1459–68. doi: 10.1377/hlthaff.26.5.1459. [DOI] [PubMed] [Google Scholar]
- 21.Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89:39–68. doi: 10.1111/j.1468-0009.2011.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman D, Joyce G, Lawless G, Crown WH, Willey V. Benefit design and specialty drug use. Health Aff (Millwood) 2006;25:1319–31. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D. Patient-physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:857–64. doi: 10.1002/acr.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraenkel L, Peters E, Charpentier P, Olsen B, Errante L, Schoen RT, et al. Decision tool to improve the quality of care in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:977–85. doi: 10.1002/acr.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]


