Abstract
Paying selective attention to a word in a multi-word utterance results in a decreased probability of error on that word (benefit), but an increased probability of error on the other words (cost). We ask whether excitation of the prefrontal cortex helps or hurts this cost. One hypothesis (the resource hypothesis) predicts a decrease in the cost due to the deployment of more attentional resources, while another (the focus hypothesis) predicts even greater costs due to further fine-tuning of selective attention. Our results are more consistent with the focus hypothesis: prefrontal stimulation caused a reliable increase in the benefit and a marginal increase in the cost of selective attention. To ensure that the effects are due to changes to the prefrontal cortex, we provide two checks: We show that the pattern of results is quite different if, instead, the primary motor cortex is stimulated. We also show that the stimulation-related benefits in the verbal task correlate with the stimulation-related benefits in an N-back task, which is known to tap into a prefrontal function. Our results shed light on how selective attention affects language production, and more generally, on how selective attention affects production of a sequence over time.
Keywords: Language production, cognitive control, executive functions, selective attention, transcranial direct cortical stimulation (tDCS)
1. Introduction
Selective attention can be a double-edged sword: focusing attention on one item implies not paying as much attention to other items. While selective attention has been studied extensively in visual perception (e.g. Clery, Andersson, Fonlupt, & Gomot, 2013; Corbetta, Miezin, Dobmeyer, et al., 1991; Desimone & Duncan, 1995; Fries, Reynolds, Rorie, et al. 2001, Lavie, 1995; Maris, Womelsdorf, Desimone, & Fries, 2013. Moran & Desimone, 1985; Treisman, 1969), little attention has been paid to selective attention in language production. Studies of visual attention suggest that objects in the visual input compete for processing in a system with limited capacity, such that an increase in the number of the to-be-attended items, usually makes the task more difficult (e.g. Desimone & Duncan, 1995). However, competition in the system can be quite selective and biased towards processing of the stimulus that is currently relevant to behavior. The evidence for the biased competition comes from studies showing that, unlike the number of relevant stimuli, the number of irrelevant stimuli (distractors) may have no influence on performance (Bundesen 1990, Duncan 1980).
These findings have led to the proposal of models in which attention is viewed as an emergent property of the neural systems that must resolve competition to generate the desired output (Desimone & Duncan, 1995; Miller, 2000). Detailed computational models of various levels of complexity have implemented biased competition for spatial and object-oriented attention (Deco & Lee, 2002; Lanyon & Denham, 2004; Usher & Niebur, 1996). A similar mechanism of biasing competition has been implemented to explain goal-oriented action (Cisek, 2006). More recently, the biased activation model has been used to explain top-down attentional modulation of affect (e.g. Grabenhorst, & Rolls, 2010; Rolls, 2013). While this mechanism is plausible for any system, there are clear differences between the visual system, which is predominantly perception-based, and the language production system, which is much less affected by the numerous bottom-up factors known to influence competition during visual object selection (see Desimone & Duncan, 1995 for a complete review of these factors). These differences motivate research on selective attention in the context of language production. More generally, the sequential nature of language production allows for studying the effects of selective attention in time, as opposed to space (which is the usual focus of studies of visual attention). This difference is an asset, as it makes research on selective attention in language production not only useful for understanding the interaction between the language production and executive systems, but also informative about the nature of competition-biasing mechanisms in space vs. time.
There is reason to believe that there are some parallels between selective attention in visual perception and in language production. For example, capacity limitation has also been demonstrated in production tasks requiring selective processing of one word in a sequence of words. Nozari and Dell (2012) used a verbal selective attention paradigm, in which participants had to recite 4-word tongue-twisters. Although these tongue-twisters are not coherent sentences, there is evidence that they are indeed treated as real words and not just sequences of phonemes (Oppenheim & Dell, 2008). One or none of the words was highlighted on each trial. Participants were told to avoid making errors, particularly on a highlighted word. In this, and two other experiments where participants had to either verbally emphasize, or alternatively to silently mouth the highlighted word, Nozari and Dell showed that selectively attending to one word in a sequence increased accuracy on that word, but decreased accuracy on other words in the sequence. These results suggest that while there is a benefit to focusing attention, there is a cost as well.
It is well-established that spatial attention operates through an extensive network, involving two prominent cortical areas, the prefrontal cortex (PFC) and the parietal cortex (e.g. Corbetta, 1998; Frank & Sabatinelli, 2012; Hales & Brewer, 2013; Ptak, 2012). Of the two, the role of the PFC has been extended from attention to location to other domains, such as attention to object identity (Wilson et al., 1993), although different parts of the PFC may be responsible for the two functions, reflecting extensions of dorsal and ventral streams (Mishkin et al., 1983). Similarly, a functional distinction had been made between the parietal cortex and the PFC, by suggesting that the former is involved in activating multiple responses, while the latter is responsible for selection among the competing responses (Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002). Moreover, frontal operculum is selectively activated when attentional resources are limited by temporal — as opposed to spatial — factors (Coull, 2004).
The role of PFC in biasing competition is also well-established in both comprehension and production of language. For example, in verb generation tasks, left PFC shows greater activation for generating verbs in response to nous that are associated with many possible verbs (e.g., “cat” → eat, meow, play, purr, etc.), as opposed to nouns that clearly elicit one verb (e.g., “scissors” → cut; Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997). Similarly in comprehension, when subjects are asked to judge the similarity between items, left PFC shows greater activation for judgments based on a single dimension, while ignoring other dimensions (e.g., judging whether “tooth” is more similar to “bone” or “tongue” in color), compared to global similarity judgments without selecting a single dimension (Thompson-Schill et al., 1997). Within the global judgment task too, left PFC shows stronger activation in response to items with weak associations (e.g., “candle” and “halo”) compared to items with high association (e.g., “candle” and “flame”; Wagner, Pare-Blagoev, Clark, & Poldrack, 2001). Patient and TMS studies corroborate these findings and establish a causal role for the left PFC in biasing competition (e.g., Thompson-Schill, Swick, Farah, D’Esposito, Kan, & Knight, 1998; Whitney, Kirk, O'Sullivan, Lambon Ralph, & Jefferies, 2011). Other examples of left PFC involvement in biasing competition in linguistic contexts includes processing of sentences with syntactic ambiguity (January, Trueswell, & Thompson-Schill, 2009; Keller, Carpenter, & Just, 2001; Mason, Just, Keller, & Carpenter, 2003; Novick, Kan, Trueswell, & Thompson-Schill, 2009), where top-down biasing is required for suppressing one meaning in favor of another. Recently, Rodd, Johnsrude, & Davis (2012) showed that left PFC responds to both the ambiguous word in the sentences and to the disambiguating information, clearly indicating that the role of this region is not limited to “revision” alone, but is related to operations involved in biasing towards the relevant meaning whenever the cognitive system is faced with competing alternatives.
In recent years it has been suggested that trouble with biasing competition can give rise to a clinical syndrome called dynamic aphasia (Robinson, Shallice, Bozzali, & Cipolotti, 2010; Robinson, Shallice, & Cipolotti, 2005) in which patients’ propositional speech is severely reduced, in spite of having good picture naming (at least when the name agreement is high), word repetition or comprehension skills. Robinson et al. (2010) have shown that these patients, who suffer from damage to the left inferior PFC, have a selective deficit in generating sentences in response to unconstrained prompts. For example, such patients have a much more difficult time generating a sentence from high frequency nouns, compared to low frequency and proper nouns which are much more constraining in their meaning. While consequences of a disruption in the process of biasing competition have been well documented, it remains to be seen what the consequences are for augmenting this process. This paper addresses this issue.
In this paper, we investigate the change to the cost-benefit pattern of selective attention as a function of exciting the PFC. To this end, we applied anodal transcranial direct current stimulation (tDCS) to the left PFC, and examined post-tDCS pattern of cost-benefit as subjects recited the four-word tongue-twisters. The goal of the paper is, in part, to understand the nature of selective attention in language production and, in part, to understand, more generally, the consequences of exciting the neural tissue that implements competition resolution. Note that the production sequence unfolds over time. At each time point competition must be resolved in favor of a different word. In a hypothetical cognitive system with no resource limitation, competition resolution would be perfect for each item, and the top-down bias in favor of item x at time t would not influence the bias to choose item y at time t+1. This is, however, not true for our resource-limited cognitive systems. Once attentional resources are allocated to processing of an item, either in space or in time, processing of other items will suffer. Is this because only a fraction of neuronal resources are recruited, or is this an inherent feature of the way competition resolution is implemented in the PFC? We seek answers to these questions under two opposing hypotheses: (1) The resource hypothesis: if the cost is due the insufficient recruitment of the PFC neurons, then stimulation should decrease the cost associated with selective attention. (2) The focus hypothesis: if the cost is a direct consequence of the successful biasing, then PFC stimulation could be expected to exaggerate the cost. Under both predictions, however, greater benefits (i.e. fewer errors on the attended word) would be expected.
Because employing tDCS for studying an executive process in the context of language production is new, we have implemented two controls in the design, to ensure that our results are truly due to changes in the PFC, and not task-specific processes. The first control tests whether performance in the tongue-twister task is similarly affected by the stimulation of a different brain region (primary motor cortex, or M1). This control site was chosen based on its involvement in processing phonological/phonemic elements (e.g., Schwartz et al., 2012), without involvement in attentional processes. If the changes to the cost-benefit pattern under PFC stimulation are specific to the PFC, we expect a difference between the PFC- and M1-induced stimulation patterns. The second control aims to replicate a previous finding regarding the effect of anodal stimulation of the PFC. The N-back task is known to benefit from PFC stimulation (Fregni, et al., 2005; Marshall, Molle, Siebner, & Born, 2005; Ohn et al., 2008; Zaehle, Sandmann, Thorne, Jaencke, & Herrmann, 2011). We have, therefore, had our participants complete an N-back task in the same session as they completed the tongue-twister task. Our purpose was two-fold: by replicating the finding that the N-back task benefits from PFC stimulation, we would (1) validate our stimulation protocol, and (2) create a potential index of improvement in working memory, which we could then correlate with improvement in our selective attention task. The implications of this correlation will be discussed in detail in General Discussion.
1.1. PFC stimulation
tDCS is a simple and safe (Iyer, Mattu, Grafman, Lomarev, Sato, & Wassermann, 2005) method for altering behavior by inducing changes in the resting membrane potential of neurons. These changes seem to be polarity dependent, with anode causing depolarization, and cathode, hyperpolarization (e.g. Nitsche & Paulus, 2001; Schlaug, Nair, Renga, Chakrabarti, Pascual-Leone, & Alsop, 2008; Utz, Dimova, Oppenlander, & Kerkhoff, 2010). Anodal stimulation of the PFC has shown promise by improving a variety of functions ascribed to this cortical region. Examples of such functions include - but are not limited to - working memory (Fregni, et al., 2005; Marshall et al., 2005; Ohn et al., 2008; Zaehle et al., 2011), associative verbal learning (Floel, Roesser, Miichka, Knecht, & Breitenstein, 2008), verbal fluency (Cattaneo, Pisoni, & Papagno, 2011), probabilistic learning (Kineses, Antal, Nitsche, Bartfai, & Paulus, 2004), picture naming (Fertonani, Rosini, Cotelli, Rossini, & Miniussi, 2010; Fiori et al., 2011), complex verbal associative thought (Cerruti & Schlaug, 2009) and task-switching (Leite, Carvalho, Fregni, & Goncalves, 2011). Moreover, PFC stimulation can change the performance of various patient groups. For example, improvement has been reported in working memory of stroke (Jo, Kim, Ko, Ohn, Joen, & Lee, 2009) and Parkinson (Boggio, et al., 2006) patients, recognition memory of Alzheimer’s patients (Ferrucci, et al., 2008), probabilistic associative learning of schizophrenic individuals (Vercammen, Rushby, Loo, Short, Weickert, & Weickert, 2011) and picture naming of aphasic patients (Baker, Rorden, & Fridriksson, 2010; Fridriksson, Richardson, Baker, & Rorden, 2011).
Combined tDCS and EEG studies have demonstrated that anodal tDCS over the left dorsolateral PFC induces changes in neuronal oscillations, by reducing delta band activity, or by amplifying theta and alpha frequencies (Keeser et al., 2011; Wirth et al., 2011; Zaehle, et al., 2011). Delta is a slow wave, and is observed in normal adults when alertness is reduced (Braboszcz & Delorme, 2011), as well as in patients with frontal lobe dysfunction (Spironelli, Angrilli, Calogero, & Stegagno, 2011; Winterer et al., 2000) and as such is thought to represent neural inhibition (Spironelli & Angrilli, 2009). Reduction of the delta-band frequency by anodal tDCS has thus been interpreted as “a boost of neuro-computational resources” (Wirth et al., 2011, p.3995).
In summary, there is good reason to believe that anodal tDCS excites the PFC and changes the behavior accordingly. For the reasons discussed earlier, PFC is a likely candidate for mediating selective attention in the context of our verbal task. Note, however, that selective attention in the context of language production is, to date, an understudied topic. And the handful of papers which do address the issue, are mostly concerned with how visual attention guides the choice of the to-be-produced materials (e.g. Tomlin, 1995), or where visual attention is focused during speaking (e.g. Brown-Schmidt & Tanenhaus, 2006; Griffin & Bock, 2000), and as such, address a different kind of question. To our knowledge there are no neuroimaging studies that have conclusively pinpointed the underlying neural correlates of selective attention in language production. Therefore, deciding on whether the left or the right PFC should be stimulated was a challenge. Below, we summarize the literature that we consulted to decide the appropriate stimulation side.
The verbal nature of our tongue-twister task motivates stimulation of the left hemisphere, given that, at least in the right-handed individuals, language production is heavily left-lateralized. There is also reason to believe that attentional effects in the context of verbal tasks are left-dominant. For example, in generation tasks, where subjects are required to provide as many responses as possible using a specific criterion, patients with left PFC damage show selective impairment in verbal tasks (e.g. Milner, 1964; Perret, 1974), while patients with right PFC damage do poorly on the nonverbal versions (e.g. Glosser & Goodglass, 1990). More recently, however, it has been proposed that the left PFC is important for selection guided by internal representations, while right PFC is crucial for cognitive selection through external contingencies (Podell, Lovell, & Goldberg, 2011), and directly relevant to the function addressed in this work, in competition resolution in verbal tasks (Hirshorn & Thompson-Schill, 2006; Novick et al., 2009; Thompson-Schill & Kan, 2000; Thompson-Schill et al., 1998). The role of the right PFC has been clearly shown in attention capture through external cues (Pardo, Fox, & Raichle, 1991), as well as inhibition of the stop-signal type (e.g. Jacobson, Javitt, & Lavidor, 2011).
However, when operation according to internalized rules or representations is required the left PFC is more strongly implicated (Larsen, Skinhoj, Lassen, 1978; Milner & Petrides, 1984; Petrides & Milner, 1982; Roland, 1984; Roland & Larsen, 1976; Roland & Skinhoj, 1981; Roland, Skinhoj, & Lassen, 1981). Findings of neuropsychological studies of left vs. right PFC damage are consistent with the aforementioned view. When matched for performance on the Wisconsin Card sorting Task, patients with right PFC lesions show more perseveration errors indicating their stimulus-driven behavior, while patients with left PFC damage kept switching between different categories (Robinson, Heaton, Lehman, & Stilson, 1980), reflecting their inability to hold on the internal goal.
In our task, participants complete a large part of each trial learning and reciting the words, as well as which word it is that they must attend to (if the trial is an experimental trial). However, once they reach the test phase, this information must be retrieved from internal representations, as words disappear from the screen while they recite them at a fast pace. This is more consistent with endogenous attention, which is reportedly processed by the left PFC. We, therefore, chose the left dorsolateral PFC as our anodal stimulation site. Each subject completed three sessions (prefrontal, motor and sham stimulation, described in detail under Methods), with two tasks per session; an N-back task under stimulation, and a verbal selective attention task, similar to the one used in Nozari and Dell (2012), post stimulation.
2. Materials and Methods
Subjects
We tested 24 (13 female) right-handed, native English speakers between the age of 19 and 30. One of the subjects completed only two of three sessions (PFC and sham stimulation) for reasons unrelated to the study. The data from those two sessions were used in the analyses. All subjects gave informed consent for tDCS administration in accordance with the IRB protocol of University of Pennsylvania and were compensated $20/session. Before each session, they filled out a screening questionnaire to ensure that they did not have a neurological condition (e.g. head trauma) and were not using psychotropic/anti-convulsive medications. Female participants took a pregnancy test each time. A self-reported negative pregnancy test was required for participation.
2.1. The selective attention task
Materials and Procedure
We selected 48 tongue-twisters from Nozari and Dell (2012), each comprising four words with the same vowels and an XYYX onset pattern. In the experimental trials, one of the four words in the tongue twister was printed in bold-font and was underlined (e.g. “just rum rug jump”). Each of the four words was so marked with equal probability across participants. Control trials had a similar structure but no word was singled out. For each tongue-twister in the experimental condition, there was a twin tongue-twister in the control condition (e.g. “lust rum rug lump”). Twin tongue-twisters in one pair differed only in the onset of the first and the last words (see Nozari & Dell, 2012 for more details about the materials). The appearance of the two members of a pair in the experimental and control conditions was counterbalanced across participants. The twin tongue-twister served as control for the statistical analysis. If, for example, the third word in the experimental tongue-twister (e.g. “rug”) was the target, the word in the same position in the control tongue-twister was chosen as its control (i.e. the “target” in the control trials). 10 practice trials were administered at the beginning.
On each trial, participants first enunciated the words, then rehearsed them four times, along with a metronome playing at 2 beats/sec, and finally recited them from memory at a faster pace of 3 beats/sec, with words appearing in small font on the top part of the screen in between recitations, as a reminder (See Nozari & Dell, 2012, for details). Errors were registered only during the fast recitation (test) phase. Participants were discouraged from making errors overall, and particularly on the bold and underlined words in the experimental trials, or they would hear a buzz on those words (using the buzzer from the game Taboo). Responses were transcribed offline, by the first author, and two trained assistants (native speakers of English, blind to the tDCS conditions) helped transcribe errors independently. The transcriptions were compared, and in cases of disagreement, the recording was played back. If disagreement persisted, the error was not registered.
2.2. The N-back task
Materials and procedure
We constructed three lists, each comprising 54 single-digit numbers (numbers 1–9). Each number appeared with equal frequency throughout the list. There were 18 critical trials in each list. For list 1, the critical trial constituted a number repeating the number preceding it (1-back). For lists 2 and 3, the number matched 2 (2-back) or 3 (3-back) numbers before respectively. Each list was paired with a practice list of 18 trials, with 6 critical trials corresponding to the rule relevant to that list. Numbers were presented in the center of the screen in Courier New font size 32, for 300 ms, at a distance of 30 inches from participants. Once the numbers disappeared, subjects could make their responses by pushing one of the two buttons (n/m) corresponding to critical (N-back) and non-critical trials, with the index and middle fingers of their right hand. The reason for the preview period before response registration was that responses faster than 300 ms would have been eliminated in the analysis anyway, due to unrealistically short latencies. Subjects had 2 seconds to respond, before the next number appeared. Before each list, subjects were reminded of the rule, and both speed and accuracy were emphasized. They then completed 18 trials during which they received feedback on their performance, followed by 54 experimental trials without feedback. Each level took about 3 minutes to complete, with 1-minute breaks between each two level and 1 minute rest at the end (total = 12 minutes).
2.3. Direct current stimulation
Saline-soaked sponge electrodes with the surface area of 25 cm2 were used to deliver single continuous direct current generated by a battery-driven continuous current stimulator (Magstim Eldith 1 Channel DC Stimulator Plus, Magstim Company Ltd., Whitland, Wales). During PFC and M1 stimulation, 1.5 mA current (with a 30 seconds ramp up and ramp down) was applied for 20 minutes, while stimulation lasted only 30 seconds during sham. For the PFC and sham stimulations, anode was placed over the F3 (e.g. Fregni et al., 2005; Gerloff, Corwell, Chen, Hallett, & Cohen, 1997; Rossi, et al., 2001), according to the 10–20 international system for EEG electrode placement (e.g. Braboszcz & Delorme, 2011), and cathode over the F4. M1 was stimulated by placing anode over C3, and cathode over C4.
Each participant completed three sessions (anodal PFC, anodal M1 and sham) in counterbalanced order. Table 1 shows the procedure within each session. The N-back task was completed under stimulation, but only the practice phase of the verbal task overlapped with stimulation. We planned this overlap during the learning of the verbal task, because it has been suggested that anodal tDCS improves learning-related NMDA receptor strengthening (Antal, Nitsche, & Paulus, 2006; Leite, et al., 2011; Liebetanz, Nitsche, Tergau, & Paulus, 2002), and helps learning last longer (Galea & Celnik, 2009). Given the past studies that have shown long-lasting behavioral/neurophysiological changes post-stimulation (Baker etal., 2010; Clark, Coffman, Trumbo, & Gasparovic, 2011; Fertonani et al., 2010; Iyer et al., 2005; Nitsche & Paulus, 2001; Ohn et al., 2008; Wirth et al., 2011), we had reason to believe that tDCS after-effects would persist over the 25 minutes after the termination of stimulation.
Table 1.
Session procedure during PFC and M1 Stimulation. Sham followed the same format, except that stimulation was terminated after 30 seconds.
| Procedure | tDCS | Duration |
|---|---|---|
| Instructions | No | Variable |
| N-Back task | Yes | 12 min |
| Break | Yes | 3 min |
| Oral-practice block | Yes | 5 min |
| Oral-blocks 1,2,3 (with breaks) | No | 25–30 min |
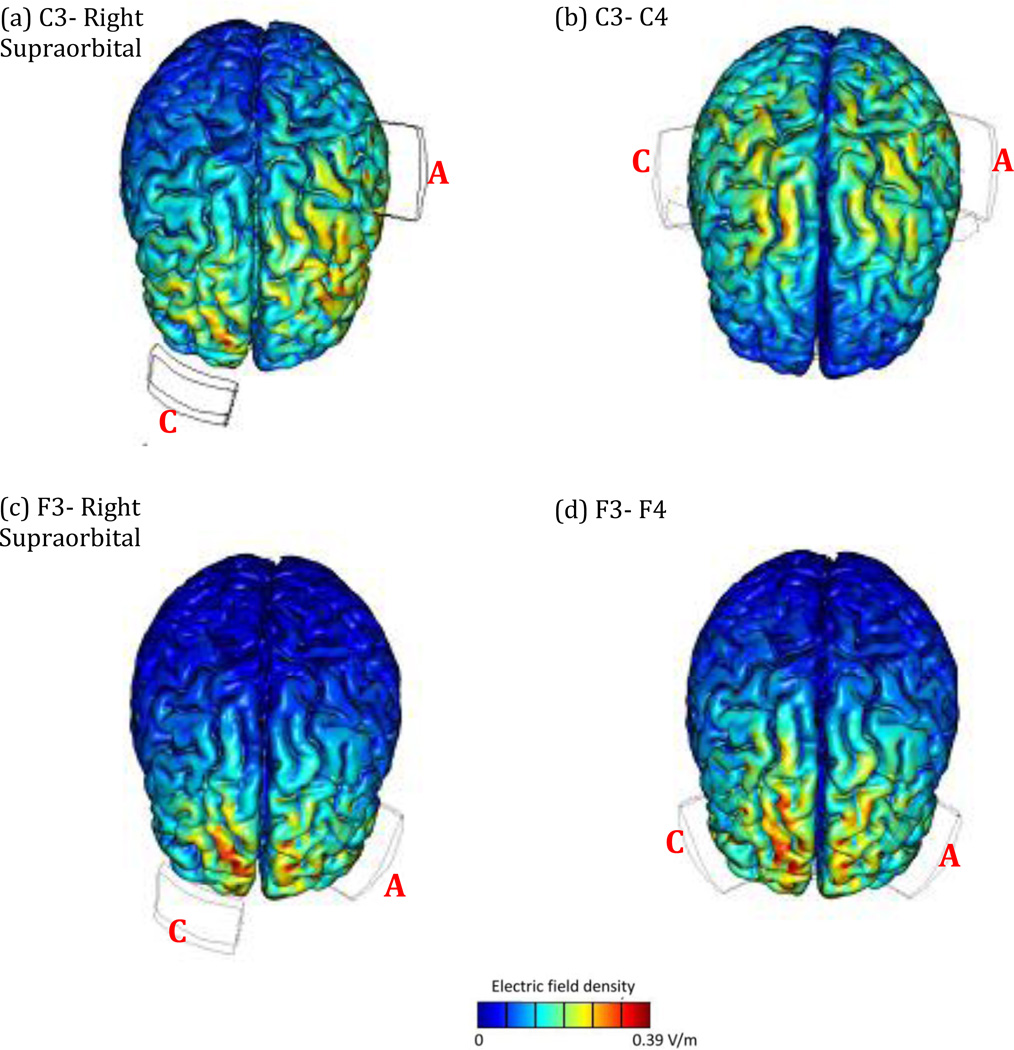
Why a symmetric, as opposed to the classic F3- right supraorbital montage? This choice was mainly dictated by the challenge we faced in picking the control site. Stimulation of temporal and visual cortices was to be avoided, due to the linguistic nature of the task and visual presentation of the stimuli respectively. We, therefore, chose to stimulate M1, by placing the anode over C3. Figure 1(a–d) shows the distribution of current in the left and right hemispheres, as a function of electrode placement on the scalp (http://bonsai.neuralengr.com). Note that these figures do not speak to the effects of the current on biological tissue, and merely demonstrate the path and density of the electric field in various brain regions. We avoided placing the reference electrode over the contralateral supraorbital, because that montage sends current to the right PFC (Figure 1a). We, therefore, placed the reference electrode over C4 to minimize the involvement of right PFC (Figure 1b). To keep the left-right balance consistent between the experimental and control stimulation conditions, we used a symmetric montage for the PFC stimulation as well, by placing the anode over F3 and cathode over F4 (Figure 1d). Note that this montage is not much different from the classic F3-Right supraorbital montage (Figure 1c), in the sense that in both montages direct current passes through the right PFC.
Fig. 1.
Electric field density in two montages for anodal stimulation of M1 (a and b) and PFC (c and d). The figure shows that the symmetric montage F3–F4 is not that different from the classic F3-Right supraorbital montage in terms of involving the right prefrontal cortex. However, C3–C4 minimizes right PFC involvement compared to the C3-Right supraorbital montage. A = Anode, C = Cathode. With permission from Marom Bikson.
2.4. Data analysis
All statistical analyses reported below are carried out by generalized linear multi-level mixed models, using the packages Ime4 (Bates, Maechler & Bolker, 2012) and languageR (Baayen, 2012) for R (R Development Core Team, 2012). When the dependent variable was error/correct, a logistic version of the model was used. Common fixed effects across the two experiments included session (to control for learning effects) and stimulation. Stimulation was coded as a categorical variable with three levels (PFC, M1 and sham), and was contrast-coded such that each stimulation condition was compared to sham. For the test of the experimental vs. the control site, this contrast compared PFC vs. M1. Session was also coded as having three levels, with contrasts of session 1 vs. session 2, and session 2 vs. session 3 to reflect the incremental nature of learning. Two random intercepts of theoretical interest were entered into the models: random intercept for subjects (do subjects have different base rates for accuracy/RTs?) and random intercept for items (are certain items associated with more errors/slower RTs?). Other task-specific fixed and random effects are discussed under each task.
3. Results
3.1. The selective attention task
Figure 2 shows the proportion of errors on the highlighted (target) and non-target words in the experimental trials that contained a highlighted word, and in control trials without such words, for sham, as well as post-PFC and post-M1 stimulation. Sham replicates the pattern observed in Nozari and Dell (2012), with fewer errors on the target and more errors on the non-target words. This is demonstrated by a significant interaction between trial type and word-status (z = 2.05; p = .039), in a subset of the data consisting of only the sham condition. This model included trial type (experimental vs. control), word status (target vs. non target), the interaction of the two, and session as fixed effects. In addition, random intercepts of subjects and items, and three random slopes of interest were also included: random slope of stimulation over subjects (do subjects respond differently to stimulation?), random slope of trial type over subjects (are the experimental trials harder for some subjects than for others?), and random slope of word-status over subjects (do subjects respond differently to the target trials?).
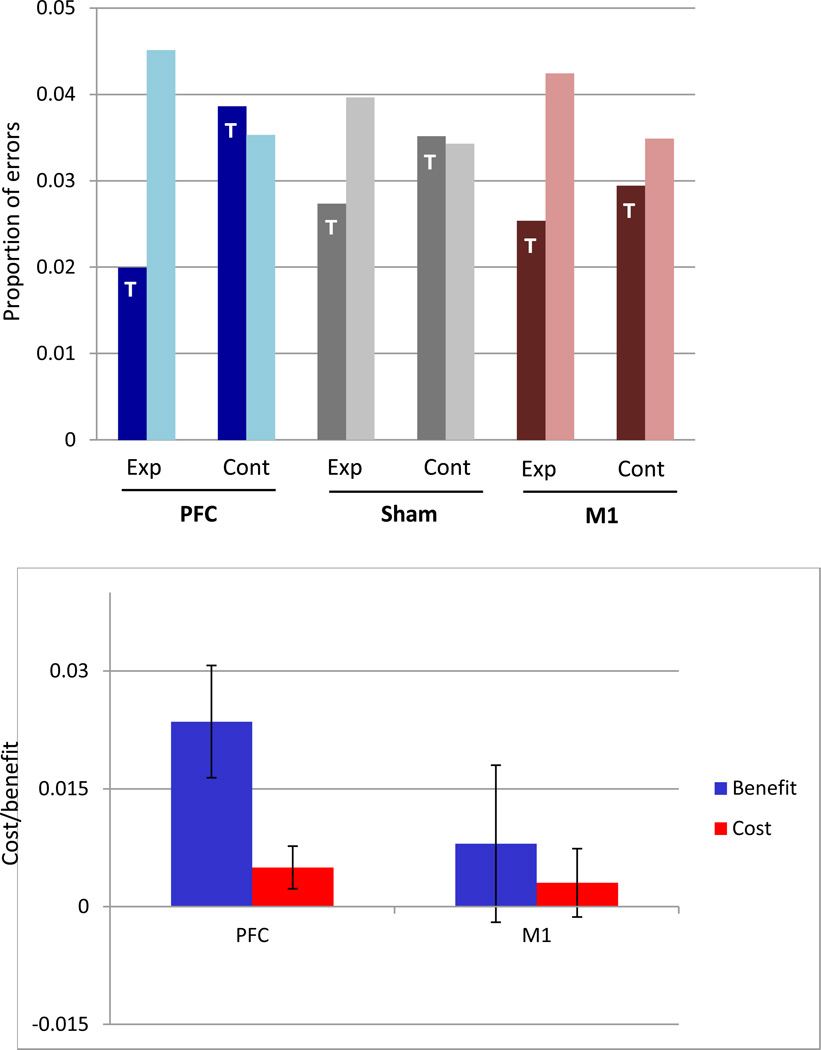
Fig. 2.
Upper panel - Proportion of errors on the target (dark bars) and nontarget (light bars) words in the experimental and control conditions, during sham and after PFC and M1 stimulation. Lower panel - Cost and benefit for PFC and M1. Benefit is calculated as (proportion of errors on experimental targets in sham - proportion of errors on control targets in sham) - (proportion of errors on experimental targets in PFC/M1 - proportion of errors on control targets in PFC/M1). Cost is calculated as (proportion of errors on experimental nontargets in PFC/M1 - proportion of errors on control nontarget in PFC/M1) - (proportion of errors on experimental nontargets in sham - proportion of errors on control nontargets in sham). Note that the graph does not represent session information.
Benefit is robust for PFC, but not for M1, and cost, although positive, is not robust for either. Exp = Experimental; Cont = Control; T = Target.
To test the effect of stimulation, the entire dataset was used. The model included the following fixed effects: trial type (experimental vs. control), word status (target vs. non-target), stimulation (PFC, M1 and sham), the three-way interaction between trial type, word status and stimulation, the two-way interaction between each pair of these three variables, and finally, session (see Data analysis for contrast coding). Random effects were as follows: random intercept for subjects and items, as well as random slopes for trial type, word status and stimulation over subjects. R requires specification of contrasts for testing levels of categorical variables. For J levels of each variable J-1 contrasts can be specified per model run. Three planned contrasts are of interest to us: the first two contrasts ask whether the pattern induced by stimulation is different from the baseline in either PFC or in M1 stimulation. These two contrasts were run in the same run of the model. The third contrast directly compares the pattern induced by PFC vs. M1 stimulation in a second run of the model with the same variables.
Appendix 1 summarizes the main fixed and random effects of the model. Although practice considerably decreased the number of errors (main effect of session, from session 1 to 2; z = −13.33; P <.001, and from session 2 to 3, z = −8.95, p < .001), a significant main effect of stimulation was not observed. However, the crucial test for whether stimulation changes the pattern observed in sham is the three-way interaction between trial-type, word-status and stimulation, which was significant when PFC was compared to sham (z = −2.58, p = .01), but not when M1 was compared to sham (z =1.59, p = .11) stimulation. PFC stimulation increased the difference between errors on the target and nontarget words in the experimental vs. control conditions by a factor of 1.29 (calculated as exp(β)* 2; where β is the coefficient for the relevant three-way interaction, and multiplication by 2 is warranted by the centered contrast coding of the PFC vs. sham condition as 1 vs. −1). Importantly, this change was specific to the stimulation of PFC, as the three-way interaction was also significant when PFC and M1 were pitted against each other (z = −2.37; p =.018).
This interaction indicates that PFC stimulation changes the pattern of performance for attended relative to unattended words, but by itself, it does not tell us the nature of these changes. Recall that we hypothesized that stimulation may modify behavior by affecting two error probabilities: (1) decreasing error probability on the target word, compared to sham (benefit), and (2) increasing error probability on the non-target words (cost). We define benefit as the number of target errors in the sham minus the number of target errors in the PFC condition. Cost is defined as the number of non-target errors in the PFC minus sham condition. (Because the number of errors in the control conditions in sham and PFC is quite close, subtracting out the control condition does not change the results. Therefore, we defined cost and benefit in more simple terms).
Using the model with the defined contrasts for cost and benefit, we found that PFC stimulation increases the benefit significantly (z = 2.00; p = .045), but cost is also prominent, even though not significant at α = 0.05 (z = 1.68; p = .09). Recall that under either PFC hypotheses, a benefit was expected. As such, the best test of site-specificity is the direct comparison of the benefit contrast between M1 and PFC/sham. After subtracting the relevant controls, a significant difference was found between the M1 and PFC (z = 2.07, p = .039), but not between M1 and sham (z = .57; p = .58). When the cost was compared, again M1 was not reliably different from sham (z = .029; p = 0.98), but was marginally different from PFC (z = 1.67; p = .09).
3.2. The N-back task
3.2.1. Analysis of errors
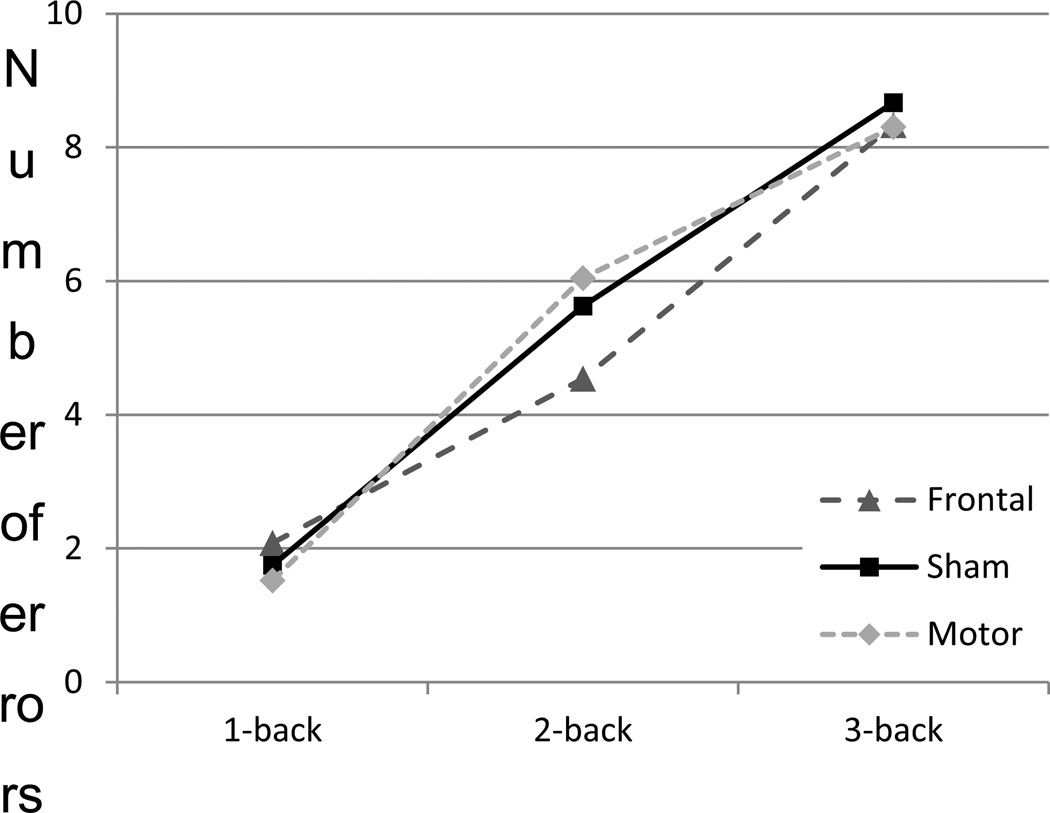
Figure 3 shows the number of errors (hits and false alarms combined) in 1, 2 and 3-back for the three stimulation conditions1. The model included stimulation, level, interaction between stimulation and level, and session as fixed effects, as well as random intercepts of subjects and items, and random slopes of stimulation and level over subjects. The level variable was coded as having 3 levels, with planned contrasts of 1 vs. 2-back, and 2 vs. 3-back to account of incremental difficulty of the task.
Fig. 3.
Number of errors for 1, 2 and 3-back in the three stimulation conditions.
While errors decreased with practice and increased with higher levels, stimulation did not exhibit a main effect (z = .14 for PFC vs. sham, and z= −.87 for M1 vs. sham; p>.05 for both). However, there was a significant interaction between stimulation and level, such that there was a smaller increase in errors from level 1 to 2 in PFC stimulation compared to sham (z = −2.11, p = .035), but not for M1 compared to sham (z = 1.47, p = .14). A direct comparison between PFC and M1 stimulation revealed that the change in error rates from session 1 to 2 was different between the two (z = −2.039; p = .041). The results suggest that compared to sham, PFC (but not M1) stimulation decreased the error probability at 2-back.
3.2.2. Analysis of response times
Response times were analyzed with and without imposing strict cut-offs and, because the results were similar, we report the analysis using an upper cut-off point of 1300 ms (e.g. Leite et al., 2011). After imposing the cut-off, response times and their standard deviations, after 300 ms of stimulation preview, were as follows: 414(±273), 375(±252) and 383(±263) for sham, PFC and M1 stimulation respectively (See Appendix 2 for details of accuracy and RTs in the N-back task). Response times were log-transformed and the data were entered into a model with stimulation, level, the interaction of the two, and session as fixed effects, subject and item intercepts, as well as random slopes of stimulation and level over subjects, as random effects. In this model, PFC stimulation marginally decreased response times compared to sham (z = −1.76, p = .08), but M1 stimulation had a much weaker effect (z = −.36, p= .21). It is noteworthy that when the random slope of stimulation|subject is removed, the difference between becomes quite prominent: now, PFC stimulation significantly decreased response times compared to sham (z = −7.05, p < .001), but M1 stimulation had a much weaker effect (z = −1.2, p = .25). This highlights the well-known variability among subjects in responding to tDCS, which provides a good opportunity for exploring individual differences. We will return to this in the “tDCS effects across tasks”. We also tested the effect of PFC vs. M1, which revealed a significant difference between the two (z =3.35, p = .003). None of the interaction effects between stimulation and level reached significance. Putting together these results with those of the error analysis, we conclude that PFC stimulation improved the N-back performance, with a weak but robust effect on accuracy at the 2-back, and a more pervasive benefit on response times. Importantly, this effect was different from that of the M1 stimulation.
3.3. tDCS effects across tasks
Is the change to accuracy in the verbal selective attention task due to a change in the PFC or to task-specific processes? To answer this question, we looked at the correlation between the benefit in the selective attention task and the gain in speed in the N-back task. We had two reasons for choosing response times gains over accuracy benefits in the N-back task: (1) There was a positive correlation between the baseline (sham) response times in the N-back task and successfully decreasing errors on the attended word in the baseline (sham) condition (Spearman’s rho = .569, p < .005), suggesting that the underlying processes of the two measures were related. (2) Response times’ gains had a larger range than errors, and were more variable among participants. While this variability induces more noise when inferences are to be made over the mean, it is an excellent asset for exploring individual differences.
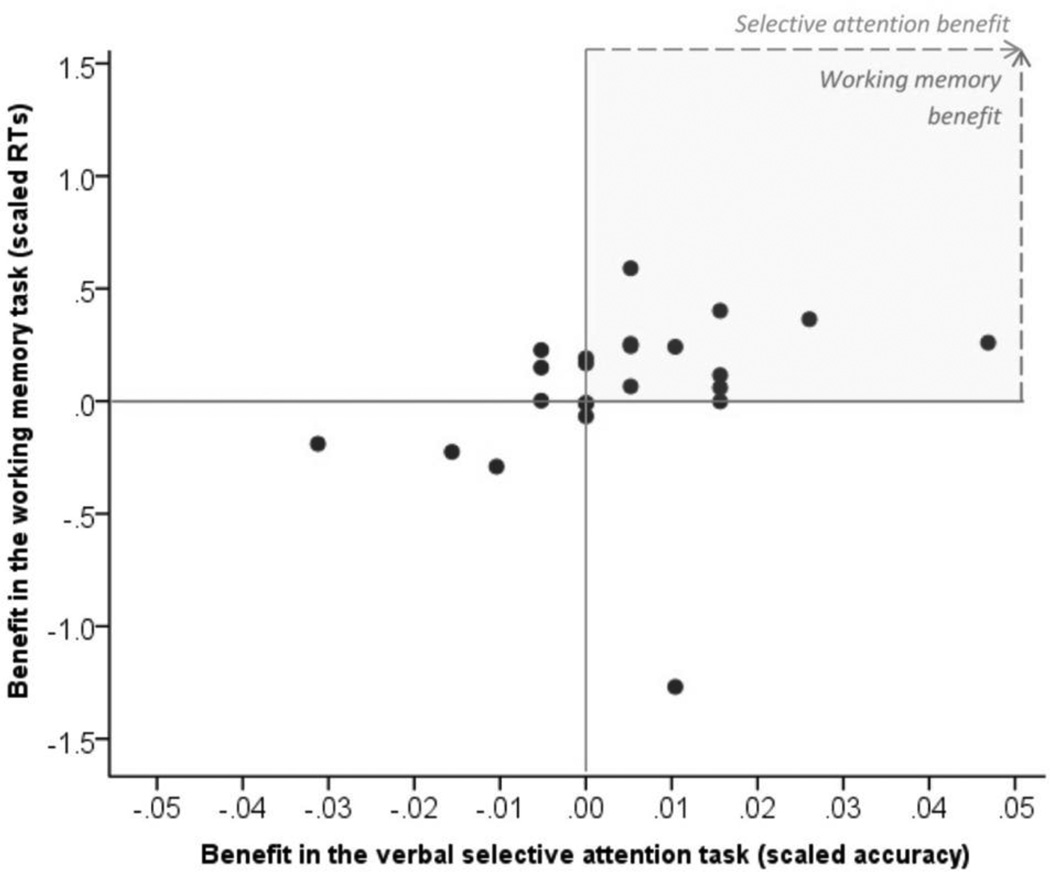
Figure 4 shows the correlation between the response-time benefits gained from PFC stimulation in the N-back task and the benefits in the verbal selective attention task. Benefit in the N-back task was calculated by subtracting the mean response times in the PFC condition from sham, and scaling the difference by subjects’ baseline mean response times in sham. We found a positive correlation between the two benefits (Spearman’s rho = .51, p = .013), with the majority of subjects clustering in the upper right quadrant, showing benefits in both tasks.
Fig. 4.
Correlation between tDCS-related benefit in the N-back and the verbal selective attention task. Subjects in the upper right quadrant show the benefit in both tasks.
4. General Discussion
In an attempt to study the effect of selective attention in language production, Nozari and Dell (2012) reported a cost-benefit pattern when participants produced four words, one of which was somehow singled out. This finding, in agreement with studies on visual attention, reflects a competition that is biased in favor of the attended item. As the result, selection of the unattended items suffers when it is their turn to be produced (resource limitation). We investigated what the source of the cost in such cases is. Is it that what manifests as resource limitation is simply due the fact that the PFC - which has been identified as a key region in implementing such biases for resolving competition - is only using a subset of its neuronal resources? If so, stimulating the PFC should deploy more resources (See Wirth et al., 2011 for a similar assertion), so that competition can be quickly and effectively resolved for each word. This is expected to result in a decreased cost. We called this the resource hypothesis. Another possibility is that greater excitation of the PFC would only exaggerate the biased competition, and hence its lingering effects. We called this the focus hypothesis.
We used anodal tDCS over the left lateral PFC to test these two predictions, in a verbal selective attention task, where we measured the number of errors as a function of stimulation on the target and non-target words. As a first check, we showed that the number of errors decreased significantly on the attended words when PFC was stimulated (benefit). This finding, which is consistent with both the resource and the focus hypotheses, was a crucial check to confirm the appropriateness of the montage for studying the effect of increased attention on language production. As an additional check, we also showed a positive correlation between the benefit in the verbal selective attention task and the benefit in the N-back task, providing further evidence that the stimulation has indeed targeted the PFC, and not task-specific processes, as the nature of the task, as well as the response mode is quite different between the tongue-twister and the N-back tasks.
To distinguish between the two hypotheses, we looked at the cost. We found that there were marginally more errors on the non-target words under PFC stimulation. This is inconsistent with the resource hypothesis that predicts reduced errors on such words. This increased cost is, however, consistent with the focus hypothesis. Stimulation did not deploy more resources, but exaggerated the bias in competition, leading to cleaner selection of the attended item, at some cost to the other items. Future research must explore the extent and this cost, and its variability among individuals. Crucially, the pattern of cost-benefit observed as a result of PFC stimulation was different from that observed in a control stimulation site (M1), providing additional support for our claim that the observed effects should not be attributed to changes in task-specific processes such as motor production.
Although our use of a control task and a control site make us able to conclude that the effects are surely due to changes in the PFC, an issue that the current design cannot conclusively address is whether the effects are due to stimulation of the left PFC or modulation of the balance between the left and the right PFC. The fact that PFC stimulation increased the accuracy of the target words is, however, incompatible with pure inhibition of the right PFC. There are no accounts, to our knowledge, that would predict enhancement of selective attention in verbal production as a function of inhibiting the right PFC. We are, therefore, left with two possibilities; pure effects of left PFC stimulation, or simultaneous excitation of the left PFC and inhibition of the right PFC (although the assumption of reverse polarity may not necessarily be true; Kincses, et al., 2004; Marshall et al., 2005; Rosenkranz, Nitsche, Tergau, & Paulus, 2000; Sparing, Dafotakis, Meister, Thirugnanasambandam, & Fink, 2008; Tanaka, Hanakawa, Honda, & Watanabe; 2009).
We emphasize that this issue is not limited to the current study, or even the current montage. As mentioned earlier, in both F3–F4 and F3-Right supraorbital montages, the direct current spreads in the right PFC as well as the left. Therefore, all studies in which the reference electrode is placed over the contralateral hemisphere deal with a similar confound. In spite of this confound, such montages may still be preferable to unilateral montages with an extracephalic electrode, because the magnitude and duration of stimulation after-effects have an established negative correlation with the distance between the two electrodes (Moliadze, Antal, & Paulus, 2010). For the reasons discussed in the introduction for picking the montage used in this study, we believe that the findings are better explained by modulation of the left PFC. In the same vein, we included only right-handed subjects in the study to minimize laterality difference, although we acknowledge that there might be variations even within the right-handed population.
4.1. Generalizability of cognitive improvement
Our results showed an advantage of PFC stimulation for accuracy at the 2-back, and for reaction times across the board. Fregni et al. (2005) and Ohn et al. (2008), also reported increase in the accuracy as the result of PFC stimulation (cf. Marshall et al., 2005) in the N-back task, although they found the effect at 3-back. There are a number of differences between our study and previous N-Back studies: For one, both of those studies used a go-nogo paradigm, in which participants only responded if the N-back condition was met. Second, they used only one level, the 3-back, and letter stimuli, rather than numbers. Finally, the placement of the reference electrode was on contralateral supraorbital in those two experiments. Although we did not find a significant accuracy difference between PFC stimulation and sham at the 3-back (although numerically, there were fewer errors), we did find a significant advantage of PFC stimulation on response times at this level. While the two papers mentioned above did not report benefits in terms of response times, other anodal tDCS studies have found such a benefit (e.g. Floel et al., 2008; Holland, et al., 2011). It is thus possible that the stimulation benefit at the 3-back has manifested in response times, rather than in accuracy, in our subjects. Overall, our results are in agreement with the previous studies that PFC stimulation benefits working memory.
We then correlated the tDCS-related benefits in the verbal selective attention task with those reflected in the response times in the working memory task. The greater variability in gains in response times provided an opportunity to pick those subjects who did show a tDCS-related benefit in the working memory task, vs. those who did not, acknowledging the limitation that the subjects who may have only shown a benefit in accuracy would be missed by this choice. Correlating the gain in speed in the N-back task with the gain in accuracy in the selective attention paradigm proved one to be predictive of the other. Many subjects who benefited from tDCS in the selective attention task also benefited in the working memory task, showing that our manipulations truly tapped into prefrontally-mediated processes.
We showed that improvement in one PFC function (working memory) was predictive of improvement in another (biasing competition that manifests as benefit in selective attention). We acknowledge that a positive correlation between the two could have a number of interpretations. While our design does not allow us to confirm one interpretation with certainty, we can launch a discussion of possible options. One interpretation is that in both cases it is the same function -working memory - that is being influenced by tDCS. In the context of the selective attention task, those who show the benefit may simply be more accurate at remembering which word to attend to. It is, however, unclear how this account would explain the cost. If a non-target was mistakenly remembered to be the target, then one would have expected lower error rates on non-target words.
An alternative interpretation is that there is overlap between these two PFC functions. As pointed out earlier, there is a positive correlation between response times in the N-back task and successfully decreasing errors on the attended word in the baseline (sham) condition. It has been suggested that the shared variance between working memory and other PFC functions is due to their overlap in a central executive component (e.g. Embretson, 1995; Engle, Tuholski, Laughlin, & Conway, 1999; Marshalek, Lohman, & Snow, 1983). This claim is backed up by evidence showing that the correlation between working memory measures and other PFC measures (e.g. scores on Raven) exists even under low-load conditions (e.g. Verguts & De Boeck, 2002). According to this view, differences in working memory capacity between individuals reflect individual differences in a domain-general executive component (Unsworth & Engle, 2005).
In recent years, there has been much debate about whether training one PFC function leads to improvement on other PFC functions. While some claim that such is the case (e.g. Buschkuehl, Jaeggi, 2010; Jaeggi, Buschkuehl, Jonides, & Perrig, 2008), others disagree (e.g. Owen et al., 2010; Shipstead, Redick, & Engle, 2010). Although boosting the PFC by applying direct current is inarguably different from boosting performance through practice, some of our findings may be relevant to that debate. For one thing, certain functions improved, while others did not. In the verbal task, tDCS did not cause a drop in the overall error rate, and specifically did not show much of an effect in the control condition. It seems then that for a function to improve through boosting PFC, that function must pose a clear demand on PFC.
Furthermore, individuals showed quite a bit of variability when their PFC was boosted by stimulation, but there was some systematicity to the pattern: Participants who did respond positively to tDCS in one task, were more likely to benefit from the stimulation in the other task. Recently Jaeggi, Buschkuehl, Jonides, & Shah (2011) also pointed out the importance of individual differences in generalizability of PFC training. They showed that only a subset of trained children whose performance significantly improved on the training task, showed transfer to the new task.
Finally, a positive correlation between the two effects is possible without any cognitive overlap, and simply as a function of the diffuse effects of tDCS in the PFC affecting different neuronal subpopulations that mediate distinct operations. Lack of spatial precision is a limitation of the tDCS technique that we are not able to bypass. However, this last interpretation, if true, is useful in its own right, namely that responsiveness to tDCS in one subpopulation of neurons is predictive of responsiveness in another subpopulation, at least when the PFC is concerned.
To conclude, this paper is a clear demonstration that tDCS can be used not only to change behavior, but to probe the nature of cognitive functions. Our use of the technique showed that excitation of the PFC in a verbal selective attention task exaggerates the biased competition. Behaviorally, this results in fewer errors on the attended word, but more errors on other words. More generally, these results speak to the nature of selective attention and its fine-tuning in producing a sequence over time.
Selective attention in (language) production has both benefits and costs.
PFC excitation in selective attention increases benefit reliably.
PFC excitation also causes a marginal increase in the cost.
The effect is PFC-specific, as a different pattern is observed with M1 stimulation.
The benefit correlates with improvement in another PFC-dependent task.
Acknowledgment
We would like to thank Roy Hamilton for his guidance and support on the stimulation procedure, and Marom Bikson for sharing his current density maps with us. This work was supported by R01-DC009209.
Appendix 1
Results of the main multilevel logistic regression model on the data from the verbal selective attention task. For a categorical variable with three levels (e.g. stimulation), two contrasts can be specified per each model run. In the first run, the contrasts were built to test the effect of each stimulation type vs. sham (i.e. contrast 1 = PFC vs. sham; contrast 2 = M1 vs. sham). The model was then run with the contrast PFC vs. M1. Please refer to the text for the definition of each variable. β = regression coefficient. SE (β) = standard error of the coefficient.
| (a) Report of the fixed effects | ||||
|---|---|---|---|---|
| model run 1 | ||||
| Model term | β | SE(β) | z | Pr(>|z|) |
| intercept | −3.9459 | 0.22116 | −17.842 | <0.001 |
| trial type | −0.1575 | 0.06381 | −2.468 | 0.0136 |
| word status | −0.471 | 0.11422 | −4.124 | <0.001 |
| stimulation (PFC vs. sham) | 0.08848 | 0.14137 | 0.626 | 0.5314 |
| stimulation (M1 vs. sham) | −0.1238 | 0.19985 | −0.62 | 0.5356 |
| session 1 vs. 2 | −0.7121 | 0.07382 | −9.646 | <0.001 |
| session 2 vs. 3 | −0.4512 | 0.09847 | −4.583 | <0.001 |
| trial type * word status | 0.54437 | 0.12192 | 4.465 | <0.001 |
| trial type * (PFC vs. sham) | −0.1467 | 0.13098 | −1.12 | 0.2626 |
| trial type * (M1 vs. sham) | −0.0339 | 0.13528 | −0.251 | 0.8021 |
| word status * (PFC vs. sham) | −0.4783 | 0.22167 | −2.158 | 0.0309 |
| word status * (M1 vs. sham) | −0.0967 | 0.21782 | −0.444 | 0.6572 |
| trial type * word status * (PFC vs. sham) | 0.60081 | 0.28916 | 2.078 | 0.0377 |
| trial type * word status * (M1 vs. sham) | −0.118 | 0.2974 | −0.397 | 0.6917 |
| model run 2 | ||||
| stimulation (PFC vs. M1) | 0.04971 | 0.06227 | 0.798 | 0.4246 |
| trial type * (PFC vs. M1) | 0.05642 | 0.06618 | 0.852 | 0.394 |
| word status * (PFC vs. M1) | 0.16847 | 0.10124 | 1.664 | 0.0961 |
| trial type * word status * (PFC vs. M1) | −0.3593 | 0.15143 | −2.373 | 0.0177 |
| (b) Report of the random effects | |
|---|---|
| model run 1 | |
| Random effect | Variance |
| subject intercept | 0.55493 |
| item intercept | 0.655 |
| slope of trial type|subject | 0.47378 |
| slope of PFC-sham stimulation|subject | 0.1050 |
| slope of Motor-sham stimulation|subject | 0.2460 |
| slope of word status|subject | 0.17611 |
| model run 2 | |
| slope of PFC-M1 stimulation|subject | 0.173616 |
Appendix 2
Accuracy and RTs in the N-back task for each level of the N-back. SE = standard error; SD = standard deviation.
| (a) Accuracy | |||
|---|---|---|---|
| N-back | condition | Mean Error | SE |
| 1-back | PFC | 2.08 | 0.41 |
| M1 | 2.5 | 0.44 | |
| Sham | 1.75 | 0.45 | |
| 2-back | PFC | 4.54 | 0.43 |
| M1 | 6.04 | 0.7 | |
| Sham | 5.63 | 0.51 | |
| 3-back | PFC | 8.33 | 0.87 |
| M1 | 8.3 | 1.16 | |
| Sham | 8.67 | 1.03 | |
| (b) RTs | |||
|---|---|---|---|
| N-back | condition | Mean RT (ms) | SD |
| 1-back | PFC | 274.26 | 169.934 |
| M1 | 288.43 | 170.715 | |
| Sham | 302.36 | 175.614 | |
| 2-back | PFC | 418.75 | 257.093 |
| M1 | 421.29 | 256.918 | |
| Sham | 445.95 | 263.287 | |
| 3-back | PFC | 453.51 | 281.102 |
| M1 | 457.62 | 263.819 | |
| Sham | 479.15 | 279.444 | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The pattern of results was similar when error counts and d’s were used. To be able to use the logistic mixed model, we chose to use error counts.
References
- Antal A, Nitsche M, Paulus W. Transcranial direct current stimulation and the visual cortex. Brain Research Bulletin. 2006;68(6):459–463. doi: 10.1016/j.brainresbull.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, Fregni F. Effects of transcranial direct current stimulation on working memory in patients with parkinson's disease. Journal of the Neurological Sciences. 2006;249(1):31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- Braboszcz C, Delorme A. Lost in thoughts: Neural markers of low alertness during mind wandering. Neuroimage. 2011;54(4):3040–3047. doi: 10.1016/j.neuroimage.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Brown-Schmidt S, Tanenhaus M. Watching the eyes when talking about size: An investigation of message formulation and utterance planning. Journal of Memory and Language. 2006;54(4):592–609. [Google Scholar]
- Bundesen C. A theory of visual-attention. Psychological Review. 1990;97(4):523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Bunge S, Hazeltine E, Scanlon M, Rosen A, Gabrieli J. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17(3):1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Buschkuehl M, Jaeggi SM. Improving intelligence: A literature review. Swiss Medical Weekly. 2010;140(19–20):266–272. doi: 10.4414/smw.2010.12852. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Pisoni A, Papagno C. Transcranial direct current stimulation over broca's region improves phonemic and semantic fluency in healthy individuals. Neuroscience. 2011;183:64–70. doi: 10.1016/j.neuroscience.2011.03.058. [DOI] [PubMed] [Google Scholar]
- Cerruti C, Schlaug G. Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. Journal of Cognitive Neuroscience. 2009;21(10):1980–1987. doi: 10.1162/jocn.2008.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Integrated neural processes for defining potential actions and deciding between them: A computational model. Journal of Neuroscience. 2006;26(38):9761–9770. doi: 10.1523/JNEUROSCI.5605-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Trumbo MC, Gasparovic C. Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: A H-1 magnetic resonance spectroscopy study. Neuroscience Letters. 2011;500(1):67–71. doi: 10.1016/j.neulet.2011.05.244. [DOI] [PubMed] [Google Scholar]
- Clery H, Andersson F, Fonlupt P, Gomot M. Brain correlates of automatic visual change detection. Neuroimage. 2013;75:117–122. doi: 10.1016/j.neuroimage.2013.02.050. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems? Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin F, Dobmeyer S, Shulman G, Petersen S. Selective and divided attention during visual discriminations of shape, color, and speed - functional-anatomy by positron emission tomography. Journal of Neuroscience. 1991;11(8):2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J. fMRI studies of temporal attention: Allocating attention within, or towards, time. Cognitive Brain Research. 2004;21(2):216–226. doi: 10.1016/j.cogbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Deco G, Lee T. A unified model of spatial and object attention based on inter-cortical biased competition. Neurocomputing. 2002;44:775–781. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual-attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Duncan J. The locus of interference in the perception of simultaneous stimuli. Psychological Review. 1980;87(3):272–300. [PubMed] [Google Scholar]
- Engle R, Tuholski S, Laughlin J, Conway A. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology-General. 1999;128(3):309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, et al. Transcranial direct current stimulation improves recognition memory in alzheimer disease. Neurology. 2008;71(7):493–498. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C. Naming facilitation induced by transcranial direct current stimulation. Behavioural Brain Research. 2010;208(2):311–318. doi: 10.1016/j.bbr.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, et al. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. Journal of Cognitive Neuroscience. 2011;23(9):2309–2323. doi: 10.1162/jocn.2010.21579. [DOI] [PubMed] [Google Scholar]
- Floel A, Roesser N, Miichka O, Knecht S, Breitenstein C. Noninvasive brain stimulation improves language learning. Journal of Cognitive Neuroscience. 2008;20(8):1415–1422. doi: 10.1162/jocn.2008.20098. [DOI] [PubMed] [Google Scholar]
- Frank DW, Sabatinelli D. Stimulus-driven reorienting in the ventral frontoparietal attention network: The role of emotional content. Frontiers in Human Neuroscience. 2012;6:116. doi: 10.3389/fnhum.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Boggio P, Nitsche M, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental Brain Research. 2005;166(1):23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FAM, Nitsche MA, et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51(1):34–41. doi: 10.1016/j.appet.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia A double-blind, sham-controlled study. Stroke. 2011;42(3):819–821. doi: 10.1161/STROKEAHA.110.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds J, Rorie A, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. Journal of Neurophysiology. 2009;102(1):294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen L. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Glosser G, Goodglass H. Disorders of executive control functions among aphasic and other brain-dam-aged patients. Journal of Clinical and Experimental Neuropsychology. 1990;12(4):485–501. doi: 10.1080/01688639008400995. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Attentional modulation of affective versus sensory processing: Functional connectivity and a top-down biased activation theory of selective attention. Journal of Neurophysiology. 2010;104(3):1649–1660. doi: 10.1152/jn.00352.2010. [DOI] [PubMed] [Google Scholar]
- Griffin Z, Bock K. What the eyes say about speaking. Psychological Science. 2000;11(4):274–279. doi: 10.1111/1467-9280.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales JB, Brewer JB. Parietal and frontal contributions to episodic encoding of location. Behavioural Brain Research. 2013;243:16–20. doi: 10.1016/j.bbr.2012.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, et al. Speech facilitation by left inferior frontal cortex stimulation. Current Biology. 2011;21(16):1403–1407. doi: 10.1016/j.cub.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer M, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann E. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Javitt DC, Lavidor M. Activation of inhibition: Diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. Journal of Cognitive Neuroscience. 2011;23(11):3380–3387. doi: 10.1162/jocn_a_00020. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January D, Trueswell JC, Thompson-Schill SL. Co-localization of stroop and syntactic ambiguity resolution in broca's area: Implications for the neural basis of sentence processing. Journal of Cognitive Neuroscience. 2009;21(12):2434–2444. doi: 10.1162/jocn.2008.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo JM, Kim Y, Ko M, Ohn SH, Joen B, Lee KH. Enhancing the working memory of stroke patients using tDCS. American Journal of Physical Medicine & Rehabilitation. 2009;88(5):404–409. doi: 10.1097/PHM.0b013e3181a0e4cb. [DOI] [PubMed] [Google Scholar]
- Keeser D, Padberg F, Reisinger E, Pogarell O, Kirsch V, Palm U, et al. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: A standardized low resolution tomography (sLORETA) study. Neuroimage. 2011;55(2):644–657. doi: 10.1016/j.neuroimage.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Keller T, Carpenter P, Just M. The neural bases of sentence comprehension: A fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11(3):223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kincses T, Antal A, Nitsche M, Bartfai O, Paulus W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia. 2004;42(1):113–117. doi: 10.1016/s0028-3932(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Lanyon L, Denham S. A biased competition computational model of spatial and object-based attention mediating active visual search. Neurocomputing. 2004;58:655–662. [Google Scholar]
- Larsen B, Skinhoj E, Lassen NA. Variations in regional cortical blood flow in the right and left hemispheres during automatic speech. Brain. 1978;101:193–209. doi: 10.1093/brain/101.2.193. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology-Human Perception and Performance. 1995;21(3):451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Fregni F, Goncalves OF. Task-specific effects of tDCS-induced cortical excitability changes on cognitive and motor sequence set shifting performance. Plos One. 2011;6(9):e24140. doi: 10.1371/journal.pone.0024140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche M, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Maris E, Womelsdorf T, Desimone R, Fries P. Rhythmic neuronal synchronization in visual cortex entails spatial phase relation diversity that is modulated by stimulation and attention. Neuroimage. 2013;74:99–116. doi: 10.1016/j.neuroimage.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshalek B, Lohman D, Snow R. The complexity continuum in the radex and hierarchical-models of intelligence. Intelligence. 1983;7(2):107–127. [Google Scholar]
- Marshall L, Molle M, Siebner H, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. Bmc Neuroscience. 2005;6:23. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R, Just M, Keller T, Carpenter P. Ambiguity in the brain: What brain imaging reveals about the processing of syntactically ambiguous sentences. Journal of Experimental Psychology-Learning Memory and Cognition. 2003;29(6):1319–1338. doi: 10.1037/0278-7393.29.6.1319. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex: complex neural properties for complex behavior. Neuron. 1999;22:15–17. doi: 10.1016/s0896-6273(00)80673-x. [DOI] [PubMed] [Google Scholar]
- Miller E. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Milner B, Petrides M. Behavioral-effects of frontal-lobe lesions in man. Trends in Neurosciences. 1984;7(11):403–407. [Google Scholar]
- Moliadze V, Antal A, Paulus W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clinical Neurophysiology. 2010;121(12):2165–2171. doi: 10.1016/j.clinph.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229(4715):782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nozari N, Dell GS. Feature migration in time: Reflection of selective attention on speech errors. Journal of Experimental Psychology-Learning Memory and Cognition. 2012;38(4):1084–1090. doi: 10.1037/a0026933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick JM, Kan IP, Trueswell JC, Thompson-Schill SL. A case for conflict across multiple domains: Memory and language impairments following damage to ventrolateral prefrontal cortex. Cognitive Neuropsychology. 2009;26(6):527–567. doi: 10.1080/02643290903519367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohn SH, Park C, Yoo W, Ko M, Choi KP, Kim G, et al. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport. 2008;19(1):43–47. doi: 10.1097/WNR.0b013e3282f2adfd. [DOI] [PubMed] [Google Scholar]
- Oppenheim GM, Dell GS. Inner speech slips exhibit lexical bias, but not the phonemic similarity effect. Cognition. 2008;106(1):528–537. doi: 10.1016/j.cognition.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, et al. Putting brain training to the test. Nature. 2010;465(7299) doi: 10.1038/nature09042. 775-U6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J, Fox P, Raichle M. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349(6304):61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behavior. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal-lobe and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3) doi: 10.1016/0028-3932(82)90100-2. 249-&. [DOI] [PubMed] [Google Scholar]
- Podell K, Lovell M, Goldberg E. lateralization of Frontal Lobe Functions. In: Salloway SP, Malloy PE, Duffy JD, editors. The Frontal Lobes and Neuropsychiatric Illness. Washington, DC: American Psychiatric Press; 2011. pp. 83–99. [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: Action, saliency, and a priority map of the environment. Neuroscientist. 2012;18(5):502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Robinson A, Heaton R, Lehman R, Stilson D. The utility of the Wisconsin card sorting test in detecting and localizing frontal-lobe lesions. Journal of Consulting and Clinical Psychology. 1980;48(5):605–614. doi: 10.1037//0022-006x.48.5.605. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Bozzali M, Cipolotti L. Conceptual proposition selection and the LIFG: Neuropsychological evidence from a focal frontal group. Neuropsychologia. 2010;48(6):1652–1663. doi: 10.1016/j.neuropsychologia.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Robinson G, Shallice T, Cipolotti L. A failure of high level verbal response selection in progressive dynamic aphasia. Cognitive Neuropsychology. 2005;22(6):661–694. doi: 10.1080/02643290442000239. [DOI] [PubMed] [Google Scholar]
- Rodd JM, Johnsrude IS, Davis MH. Dissociating frontotemporal contributions to semantic ambiguity resolution in spoken sentences. Cerebral Cortex. 2012;22(8):1761–1773. doi: 10.1093/cercor/bhr252. [DOI] [PubMed] [Google Scholar]
- Roland P. Metabolic measurements of the working frontal-cortex in man. Trends in Neurosciences. 1984;7(11):430–435. [Google Scholar]
- Roland E, Larsen B. Focal increase of cerebral blood-flow during stereognostic testing in man. Archives of Neurology. 1976;33(8):551–558. doi: 10.1001/archneur.1976.00500080029005. [DOI] [PubMed] [Google Scholar]
- Roland P, Skinhoj E. Extrastriate cortical areas activated during visual-discrimination in man. Brain Research. 1981;222(1):166–171. doi: 10.1016/0006-8993(81)90953-7. [DOI] [PubMed] [Google Scholar]
- Roland P, Skinhoj E, Lassen N. Focal activations of human cerebral-cortex during auditory-discrimination. Journal of Neurophysiology. 1981;45(6):1139–1151. doi: 10.1152/jn.1981.45.6.1139. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A biased activation theory of the cognitive and attentional modulation of emotion. Frontiers in Human Neuroscience. 2013;7:74. doi: 10.3389/fnhum.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz K, Nitsche M, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neuroscience Letters. 2000;296(1):61–63. doi: 10.1016/s0304-3940(00)01621-9. [DOI] [PubMed] [Google Scholar]
- Rossi S, Cappa S, Babiloni C, Pasqualetti P, Miniussi C, Carducci F, et al. Prefontal cortex in long-term memory: An "interference" approach using magnetic stimulation. Nature Neuroscience. 2001;4(9):948–952. doi: 10.1038/nn0901-948. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Nair D, Renga V, Chakrabarti A, Pascual-Leone A, Alsop D. Relating diffusion tensor imaging to fugl-meyer impairment scores in chronic stroke patients with incomplete recovery. Neurology. 2008;70(11):A233–A234. [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Does working memory training generalize? Psychologica Belgica. 2010;50(3–4):245–276. [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparing R, Dafotakis M, Meister IG, Thirugnanasambandam N, Fink GR. Enhancing language performance with non-invasive brain stimulation - A transcranial direct current stimulation study in healthy humans. Neuropsychologia. 2008;46(1):261–268. doi: 10.1016/j.neuropsychologia.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A. EEG delta band as a marker of brain damage in aphasic patients after recovery of language. Neuropsychologia. 2009;47(4):988–994. doi: 10.1016/j.neuropsychologia.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A, Calogero A, Stegagno L. Delta EEG band as a marker of left hypofrontality for language in schizophrenia patients. Schizophrenia Bulletin. 2011;37(4):757–767. doi: 10.1093/schbul/sbp145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Experimental Brain Research. 2009;196(3):459–465. doi: 10.1007/s00221-009-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill S, D'Esposito M, Aguirre G, Farah M. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill S, Kan I. Relationship between response set size and prefrontal activity during verbal fluency. Journal of Cognitive Neuroscience. 2000 114-114. [Google Scholar]
- Thompson-Schill S, Swick D, Farah M, D'Esposito M, Kan I, Knight R. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin RS. Focal attention, voice, and word order. In: Downing P, Noonan M, editors. Word order in discourse. Amsterdam, Netherlands: John Benjamins; 1995. pp. 517–552. [Google Scholar]
- Treisman A. Strategies and models of selective attention. Psychological Review. 1969;76(3) doi: 10.1037/h0027242. 282-&. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Engle R. Working memory capacity and fluid abilities: Examining the correlation between operation span and raven. Intelligence. 2005;33(1):67–81. [Google Scholar]
- Usher M, Niebur E. Modeling the temporal dynamics of IT neurons in visual search: A mechanism for top-down selective attention. Journal of Cognitive Neuroscience. 1996;8(4):311–327. doi: 10.1162/jocn.1996.8.4.311. [DOI] [PubMed] [Google Scholar]
- Utz KS, Dimova V, Oppenlaender K, Kerkhoff G. Electrified minds: Transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology-A review of current data and future implications. Neuropsychologia. 2010;48(10):2789–2810. doi: 10.1016/j.neuropsychologia.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Vercammen A, Rushby JA, Loo C, Short B, Weickert CS, Weickert TW. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophrenia Research. 2011;131(1–3):198–205. doi: 10.1016/j.schres.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Verguts T, De Boeck P. On the correlation between working memory capacity and performance on intelligence tests. Learning and Individual Differences. 2001;13(1):37–55. [Google Scholar]
- Wagner A, Pare-Blagoev E, Clark J, Poldrack R. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31(2):329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O'Sullivan J, Ralph MAL, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex. 2011;21(5):1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, Herrmann W. Frontal dysfunction in schizophrenia - a new electrophysiological classifier for research and clinical applications. European Archives of Psychiatry and Clinical Neuroscience. 2000;250(4):207–214. doi: 10.1007/s004060070026. [DOI] [PubMed] [Google Scholar]
- Wirth M, Rahman RA, Kuenecke J, Koenig T, Horn H, Sommer W, Dierks T. Effects of transcranial direct current stimulation (tDCS) on behaviour and electrophysiology of language production. Neuropsychologia. 2011;49(14):3989–3998. doi: 10.1016/j.neuropsychologia.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Sandmann P, Thorne JD, Jaencke L, Herrmann CS. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: Combined behavioural and electrophysiological evidence. Bmc Neuroscience. 2011;12:2. doi: 10.1186/1471-2202-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]