Abstract
Background
Increasing numbers of patients are living with multiple, chronic medical conditions and functional impairments that leave them homebound. Home-based primary and palliative care (HBPC) programs provide access to health care services for this vulnerable population. Homebound patients have high symptom burden upon program enrollment. Yet little is known as to how individual symptoms are managed at home, especially over longer time periods.
Objectives
The purpose of this study was to determine whether high symptom burden decreases following HBPC enrollment.
Methods
All patients newly enrolled in an HBPC program who reported at least one symptom on the Edmonton Symptom Assessment Scale (ESAS) were eligible for telephone ESAS follow-up. Patients received a comprehensive initial home visit and assessment by a physician with subsequent follow-up care, interdisciplinary care management including social work, and urgent in-home care as necessary. Multivariate linear mixed models with repeated measures were used to assess the impact of HBPC on pain, depression, anxiety, tiredness, and loss of appetite among patients with moderate to severe symptom levels at baseline.
Results
One hundred forty patients were followed. Patient pain, anxiety, depression, and tiredness significantly decreased following intervention with symptom reductions seen at 3 weeks and maintained at 12 weeks. (p<0.01) Loss of appetite trended toward an overall significant decrease and showed significant reductions at 12 week follow-up.
Conclusion
In a chronically ill population of urban homebound, patient symptoms can be successfully managed in the home. Future work should continue to explore symptom assessment and management over time for the chronically ill homebound.
Introduction
Symptom burden is an increasing problem for chronically ill patients despite the availability of effective palliative treatment and medication.1 It has been increasingly recognized that high-quality outpatient care includes palliative care.2 Despite the rapid growth of the palliative care field in the last decade, appropriate symptom assessment and management continues to be available primarily through inpatient palliative consultation or outpatient hospice setting. This is despite evidence showing that in home palliative care for the vulnerable elderly increases patient satisfaction and cuts costs.3 Appropriate palliative care is crucial to ensure that chronically and functionally debilitated patients can ultimately remain comfortable and satisfied in their homes or in other settings.4
As an increasing number of elderly and frail patients are homebound as a result of multiple medical conditions and functional and cognitive impairments5; the need for accessible medical and palliative care and symptom management in the home continues to grow. The majority of these patients experience significant symptom burden related to chronic illnesses, many of which go unrecognized and consequently untreated.6 Common symptoms related to their chronic illnesses include pain and depression. A reported 45% to 80% of people over the age of 65 experience serious pain with 83% reporting pain in long-term care facilities.7 A recent assessment showed that homebound patients have high symptom burden upon program entry including pain (47%) and loss of appetite (53%).8 Similarly, hospice patients in various settings, including the home, experienced severe symptom burden such as lack of energy (83%), pain (76%), and loss of appetite (63%).9
Although studies have examined interventions that have decreased pain over time in patients with conditions such as arthritis and cancer,10 few studies have evaluated symptom control over time for patients in diverse long-term settings. One recent survey found that symptom management is inadequate in long-term care facilities.11 The recognition, assessment, and management of symptoms including pain are crucial components to ensure that patients are able to receive proper medical care.12
According to the American Geriatrics Society and the American Medical Directors Association Clinical Practice Guidelines, symptom management can be improved through formalized symptom assessment.13 Numerous tools have been developed to assess the prevalence, severity, and frequency of symptoms.14 The Edmonton Symptom Assessment System (ESAS),a 10-item patient-rated symptom visual analogue scale,15 has been successfully administered to show patterns of palliative symptom control.16
Few existing studies have documented the palliative symptom burden in the growing homebound population, and none that we are aware of have examined symptom management in this vulnerable population over time. The purpose of this study was to assess whether moderate to severe symptom burden in a chronically ill homebound population is reduced following enrollment in an urban home-based primary and palliative care (HBPC) program. We conducted a longitudinal survey of newly enrolled patients in an HBPC program and assessed symptom severity at baseline, 3-week, and 12-week follow-up. We hypothesized that those patients with clinically significant baseline symptoms would have significant reduction in symptom burden that would be maintained at 12 weeks. We focused on three symptoms that our previous research found were most prevalent (approximately 50%) for homebound patients at enrollment in HBPC—pain, tiredness, and loss of appetite.8 We also included depression and anxiety as they substantially impact patient quality of life,17,18 disability,19 and health care costs,20–22 and they may be particularly amenable to in-home intervention through primary care or specialist referral.
Methods
Setting
This was a prospective longitudinal study of homebound patients newly enrolled in the Mount Sinai Visiting Doctors (MSVD) program, a large HBPC program based in New York City, between September 2008 and February 2010. Eligibility criteria for the MSVD program include living in Manhattan above 59th Street, age >18 years, and meeting the Medicare homebound definition—able to leave home only with great difficulty and for absences that are infrequent or of short duration. Patients are enrolled regardless of insurance status, comorbidities, or cognitive status.23
Design
Patients were administered a baseline ESAS as part of their routine clinical care on an initial visit by their primary care provider (PCP) to assess current symptom burden. The ESAS was completed either by the patient alone, caregiver alone, or the patient assisted by the caregiver as determined by the PCP's assessment of the patient's ability. All patients who completed the baseline ESAS with at least one reported symptom (ESAS score >0) were contacted for follow-up to assess change in symptom burden. Patients gave consent to a trained research assistant via telephone in English or Spanish for a 3-week follow-up telephone ESAS assessment and a 12-week follow-up telephone ESAS assessment. The ESAS was completed by the same person who completed the baseline ESAS (e.g., patient) at each assessment. PCPs and research assistants received extensive training on administration of the ESAS to patients, caregivers, or caregiver assisted. Patients who were not assessed at 3 weeks were still eligible to complete a 12-week follow-up. Three telephone contact attempts were made for each patient at each follow-up period.
Measures
The ESAS measures symptoms at present and consists of 10 visual analogue scales scored from 0, indicating no symptoms, to 10, the worst possible symptom burden. The symptoms assessed include pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath.16 The ESAS was chosen as it is validated in a number of care settings,24 and is validated for both patient and/or caregiver report. We categorized symptom burden ≥3 on each individual symptom as clinically significant (moderate or severe) and requiring treatment based on similar ESAS categorizations.25
Data on patient demographics, living situation, and functional status (activities of daily living [ADL] and instrumental activities of daily living [IADL]) were also collected at the initial visit by the MSVD provider. Comorbidity data at baseline were extracted from the clinical database and used to calculate a Charlson Comorbidity Index score.26 The Karnofsky Performance Scale27 was completed by the physician at baseline to classify functional impairment to reflects patients' ability to carry on normal activities and care for themselves. Scores range from 100 (Normal, no evidence of disease) to 0 (Dead).
Intervention: Home-based primary and palliative care (HBPC)
A care model previously described elsewhere,28 the MSVD is a joint program of the Division of General Internal Medicine and Department of Geriatrics and Palliative Medicine at Mount Sinai Hospital that provides care for more than 1000 homebound older patients annually. It employs 14 physicians, 2 nurse practitioners (NPs), 2 nurses, 4 social workers, and 5 clerical staff. Three of the physicians are fellowship trained in palliative and hospice medicine, whereas 6 others are board certified in the field. Patients are referred from inpatient care providers, outpatient clinics, community agencies, and family members/word of mouth. The care typically involves coordinated care involving the program's physicians, social workers, and staff; certified home health agencies; ancillary service providers; and community-based social service agencies. High priority is placed on patient comfort and minimizing unnecessary emergency room visits and hospitalizations. A comprehensive initial visit is performed for all newly enrolled patients by an assigned PCP. The initial visit consists of a complete medical history and physical exam. After the initial visit, depending on the severity of illness, the PCP sees patients every 2 to 12 weeks and, with assistance from the administrative staff, coordinates all aspects of care. In the event of an urgent medical need, patients/caregivers can contact the on-call physician 24 hours per day 7 days per week. Physicians provide ongoing chronic disease management for their patients but also provide palliative and end-of-life care with or without the involvement of hospice. When medical specialty care is required, the PCP may request a home visit by a specialist or arrange for transportation to the specialist's office. When clinically appropriate, HBPC works in conjunction with home hospice. In these situations the MSVD physician remains the PCP and collaborates with hospice nurses. Home hospice collaboration occurs in <10% of the MSVD population, largely because patients do not want to give up their existing nursing or home care services. Symptoms were assessed at each initial provider visit and treatments were made at the discretion of each provider. In general, MSVD providers can order most nonintravenous medications for symptom management in the home, oxygen if needed, and in-home laboratory and radiology services are available. PCPs take the lead in all symptom management, but social workers are also on staff and there is access to home visits by psychiatrists, neurologists, and rheumatologists on a consultative basis.
Analysis
T test and χ2 statistics were used as appropriate to examine baseline demographic and clinical characteristics of eligible patients who did and did not participate in symptom follow-up. We used linear mixed models with repeated measures using restricted maximum likelihood estimation to account for missing data to measure the impact of time on five symptoms (pain, depression, anxiety, loss of appetite, and tiredness) in patients with moderate to severe symptom scores at baseline. Anxiety was log transformed in all analyses to conform to model specifications. Intercept only linear mixed models were examined to determine covariance structures for each of the five models. Using the Akaike information criterion (AIC), we determined the following covariance structure for each symptom: pain: autoregressive; depression: unstructured; tiredness: unstructured; loss of appetite: heterogeneous autoregressive; and anxiety: unstructured. To account for multiple comparisons, the Sidak correction for multiple comparisons29 was used to determine an alpha value of 0.01758 for all main effect models. Tukey adjusted p values were used to determine differences in individual symptom scores between baseline and 3 weeks, 3 weeks and 12 weeks, and baseline and 12 weeks. For each model we controlled for the following covariates when they were correlated with the symptom (p≤0.15): age, gender, race (white versus nonwhite), and baseline Charlson Comorbidity Index score. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC).
This study protocol was approved by the Mount Sinai School of Medicine Institutional Review Board.
Results
Of the 267 eligible patients, 140 (52%) consented to a telephone interview. Nineteen patients were discharged from the program within 30 days (due to any reason, including death, being ambulatory, or not requiring home-based care), 10 died or were placed in a nursing home with 3 months of initial assessment, 10 patients refused enrollment, and 88 could not be reached. There were no differences in the clinical or demographic characteristics of the participants or nonparticipants other than nonparticipants were more likely to have a diagnosis of congestive heart failure (p<0.05).
Of the 140 patients in the study population, the majority of patients were more than 80 years of age (73%) and female (75%). Fifty-four (39%) were white, 41 (29%) Latino, and 35 (25%) were black. A substantial portion (46%) had Medicaid and 34% lived alone. As expected, disease burden was considerable and patients were highly impaired functionally and cognitively. Ninety-two percent of patients required assistance with one or more ADLs and 99% required assistance with one or more IADLs. Fifty percent of patients had a Karnofsky Performance Scale score between 10 and 40, indicating inability to care for self (see Table 1).
Table 1.
Baseline Characteristics of Homebound Patients (n=140)
| Characteristic | Category | Number (%) |
|---|---|---|
| Gender | Female | 105 (75) |
| Male | 35 (25) | |
| Ethnicity | White | 54 (39) |
| Latino | 41 (29) | |
| Black | 35 (25) | |
| Asian | 3 (2) | |
| Other | 2 (1) | |
| Missing | 5 (4) | |
| Age | <60 | 6 (4) |
| 60–69 | 12 (9) | |
| 70–79 | 20 (14) | |
| 80–89 | 45 (32) | |
| 90–99 | 51 (37) | |
| >100 | 6 (4) | |
| Insurance | Has Medicaid | 65 (46) |
| Diagnosisa | Dementia | 64 (46) |
| CHF | 18 (13) | |
| COPD | 7 (5) | |
| Depression | 43 (31) | |
| Cancer | 19 (14) | |
| Living situation | Alone | 47 (34) |
| With family member | 46 (33) | |
| With paid caregiver | 28 (20) | |
| With family+paid caregiver | 14 (10) | |
| Unknown | 5 (3) | |
| Activities of daily living (ADL)(range 0–16) | 0–3, most independent | 32 (23) |
| 4–7 | 20 (14) | |
| 8–11 | 23 (16) | |
| 12–15 | 36 (26) | |
| 16, most dependent | 22 (16) | |
| Missing | 7 (5) | |
| Independent activities of daily living (IADL) (range 0–8) | 7–8, most independent | 2 (1) |
| 5–6 | 11 (8) | |
| 3–4 | 25 (18) | |
| 0–2, most dependent | 86 (61) | |
| Missing | 16 (12) | |
| Karnofsky Performance Scale (KPS) score (range 0–100) | 10–40 | 67 (50) |
| 50–70 | 66 (49) | |
| 80–100 | 2 (1) | |
| Missing | 5 (4) | |
| Charlson Comorbidity Index score | 0 | 15 (10.71) |
| 1 | 28 (20) | |
| 2 | 21 (15) | |
| 3 | 34 (24.29) | |
| 4 | 17 (12.14) | |
| >4 | 25 (17.86) |
Patients may have multiple diagnoses.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease.
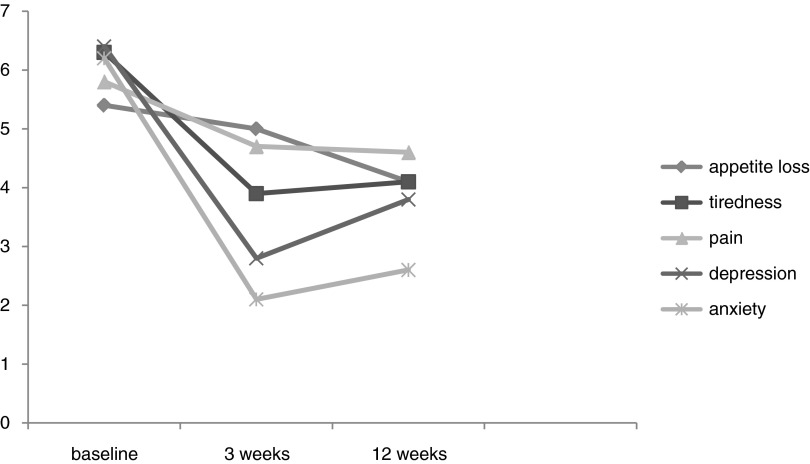
For those patients with moderate to severe pain, depression, anxiety, tiredness, or loss of appetite, we found decreased trends for all individual symptoms over time (see Fig. 1). Many patients went from having moderate to severe symptoms to no symptoms (see Table 2). For example, 58% of those with moderate to severe depression at baseline had no depressive symptoms at 3 weeks. Forty-five percent of those with moderate to severe tiredness at baseline had no tiredness symptoms at 3 weeks.
FIG. 1.
Mean score at baseline, 3 weeks, and 12 weeks for individual symptoms that were moderate to severe at admission to home-based primary care program.
Table 2.
Reduction in Moderate to Severe Symptom Burden for Patients following Admission to Home-Based Primary Care Program
| 3 weeks | 12 week | |||
|---|---|---|---|---|
| BaselineSymptom | Total patients, n | % symptom free | Total patients, n | % symptom free |
| Pain (n=63) | 52 | 25.00% | 48 | 27.08% |
| Depression (n=42) | 33 | 57.58% | 30 | 50% |
| Loss of appetite (n=66) | 58 | 20.69% | 49 | 24.49% |
| Anxiety (n=37) | 29 | 58.62% | 27 | 59.26% |
| Tiredness (n=63) | 51 | 45.10% | 40 | 47.5% |
P<0.05 compared with baseline.
Using mixed models, we found that, for patients receiving the intervention with at least moderate baseline symptoms (individual ESAS score ≥3), pain, depression, anxiety, and tiredness were significantly decreased in multivariate models controlling for age, gender, race, and comorbidities (see Table 3). Using the Tukey adjusted p values, the symptom reductions were all significant from baseline to 3 weeks and from baseline to 12 weeks but not from 3 weeks to 12 weeks. Loss of appetite trended toward significance (p=0.0252) but did not reach our corrected alpha value. Upon examining the Tukey adjusted p values, comparisons between time periods for this symptom showed significant reduction from baseline to 12 weeks but not from baseline to 3 weeks.
Table 3.
Reduced Symptom Burden at Three Weeks and Twelve Weeks for Patients with Moderate to Severe Symptom Burden following Admission to Home-Based Primary Care Program
| Comparisons: change in mean score over 2 time points | ||||||
|---|---|---|---|---|---|---|
| Symptom | Predictors | F value | p value | Baseline to 3 weeks t value (p value) | Baseline to 12 weeks t value (p value) | 3 weeks to 12 weeks t value (p value) |
| 1. Pain | Time | 5.02 | 0.0084* | 2.08 (0.0397)** | 3.11 (0.0024)** | 0.71 (0.4797) |
| Gender | 1.02 | 0.3176 | ||||
| Age | 9.18 | 0.0036* | ||||
| 2. Depression | Time | 17.44 | <0.0001* | 5.74 (<0.0001)** | 3.92 (0.0004)** | −1.22 (0.2283) |
| Gender | 0.05 | 0.8227 | ||||
| Age | 0.01 | 0.9105 | ||||
| Charlson score | 0.14 | 0.7082 | ||||
| 3. Loss of appetite | Time | 3.81 | 0.0252 | 0.83 (0.4082) | 2.73 (0.0074)** | 1.85 (0.0667) |
| Gender | 1.90 | 0.1730 | ||||
| Race | 1.42 | 0.2380 | ||||
| 4. Anxiety | Time | 33.95 | <0.0001* | 7.39 (<0.0001)** | 6.46 (<0.001)** | −0.24 (0.8090) |
| Age | 10.63 | 0.0026* | ||||
| Race | 3.63 | 0.0655 | ||||
| Charlson score | 8.97 | 0.0052* | ||||
| 5. Tiredness | Time | 10.19 | 0.0002* | 4.51 (<0.0001)** | 3.97 (0.0002)** | −0.35 (0.7262) |
| Age | 0.10 | 0.7500 | ||||
| Race | 0.27 | 0.6059 | ||||
| Charlson score | 1.39 | 0.2426 | ||||
Significant p value based on 0.01758 alpha (correcting for multiple comparisons).
Significant p value based p <0.05 based on corrected Tukey.
Discussion
The present study is the first to demonstrate the impact of HBPC on symptom assessment and management in a chronically ill homebound population and represents a promising model of care in which palliative care can be integrated effectively. In this chronically ill population of the urban homebound, our data suggest that patients' symptoms can be successfully managed in the home. Our study also monitored symptoms over a clinically meaningful time period allowing symptoms, medications, and other nonmedical aspects of a patient's care plan adequate time to fully see its ultimate effects. For each individual symptom examined, among those with baseline moderate to severe symptoms, mean symptom score decreased at 3-week follow-up and remained significantly decreased at 12-week follow-up. Loss of appetite appeared to only show significant decreases at a 12-week period, suggesting that it may take longer than 3 weeks for this symptom to be addressed and effectively treated. In this vulnerable population that often does not have access to outpatient clinics and in which many are not yet hospice eligible but still have significant symptom burden, HBPC programs can contribute greatly to their care by instituting proper symptom management alongside primary care.
Although specific medical intervention strategies were not recorded in our study, MSVD practice places emphasis on holistic care and improving patient quality of life. All providers involved in the study are proficient in palliative care symptom management through training and/or continuing medical education. Patients are able to receive most available forms of nonintravenous medications for different symptom management at home as they would in an outpatient palliative care clinic. Optimal pain management is achieved through frequent opioid dosage titration alongside adjuvant therapy. Depression is aggressively treated with a variety of antidepressants along with social work support. Providers are comfortable ordering medications used commonly at the end of life including morphine, haldol, atropine, and prochlorperazine.
Although this was the first study to use ESAS to specifically monitor change in symptom burden of a homebound population receiving HBPC, there have been similar findings showing reduction in symptom burden in other populations receiving palliative care. In one study on a palliative care unit, there were reductions in mean values of ESAS scores from Day 1 to Day 7 for all symptoms apart from drowsiness and depression.30 Furthermore, Bruera et al. found similar results when assessing reduction in total ESAS score over a 21-day interval in a palliative care unit.16
There are some limitations to note. Only 52% of patients with completed baseline assessments completed at least one follow-up ESAS assessment. Almost one-fourth of those not followed were disenrolled from the HBPC program prior to follow-up assessment. In such a chronically ill population, it is expected that a significant portion of our patients would die or be unable to be managed successfully in the home, which would result in attrition.31 Our findings are therefore only generalizable to those patients who survive at least 3 months in HBPC. Other reasons for no follow-up were inability to respond verbally over the phone or absence of a caretaker or family member to assist with assessing the patient's symptom burden. Future studies should conduct in-home symptom assessment to decrease loss to follow-up. Importantly, we did not see any differences in comparing those who participated with those who did not other than the fact that they were less likely to have congestive heart failure. We used a cutoff of ≥3 in this study to indicate a clinically significant symptom, thus including a lower threshold for clinically significant symptoms than previous studies.25 We chose this cutoff to include a larger pool of homebound patients whose symptoms would be addressed by their PCP. Our findings remained unchanged using more conservative estimates of symptom burden.
Furthermore, the baseline ESAS data were collected by a clinician in person during a home visit, whereas the follow-up data were collected by a research assistant over the phone. Although the research assistant was trained to administer the ESAS as consistently as possible, results may have differed when questions were asked by the patient's PCP or by telephone. Another limitation involved probable differences in physician training and practices in symptom management and techniques used. Although the practice's providers all receive similar faculty development and education in symptom management, some symptoms have more routine and widely accepted treatment plans, whereas others are less standardized depending on symptom pattern, physician, and/or patient preference. This study did not collect detailed data on specific intervention practices for patients.
Several additional challenges arose concerning the assessment of symptom burden in a population that has such a large percentage of patients with dementia or other illnesses that similarly prevent patients from reliably relaying symptom severity. Assessments were completed by the patient when possible and by family members or formal caregivers when needed. For future studies, it is worth considering other instruments that could better predict symptom burden in patients who cannot contribute the time and concentration needed for successful ESAS assessment. There are several nonverbal tools such as the Pain Assessment in Advanced Dementia (PAINAD) Scale and Pain Assessment in Noncommunicative Elderly Persons (PAINE) that require a physician or caretaker to observe behaviors that may act as indicators of certain symptoms.32 This type of tool may be more appropriate in patients with dementia and should be considered for future studies.
Conclusion
The improvements in symptom burden in homebound patients enrolled in HBPC programs underscore the importance of proper symptom assessment and management in order to improve the quality of life of chronically ill patients in the community. The number of homebound patients will increase as the population ages in the coming years, and it is imperative that they receive proper symptom management and care to avoid unnecessary institutionalizations, reduce caregiver and family burden, and improve patients' well-being and satisfaction. HBPC programs that provide the proper mix of physicians, nurses, social workers, and case management are crucial for enhancing the medical and psychological needs of homebound patients who are not also enrolled in hospice care. In the future, studies can begin to elucidate how symptoms can be most successfully managed in the home environment, and how symptom burden progresses over longer time periods.
Acknowledgments
Funding for this research was provided by the The Y. C. Ho/Helen and Michael Chiang Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jordhoy MS. Kaasa S. Fayers P. Ovreness T. Underland G. Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: Experience from a randomized controlled trial. Palliat Med. 1999;13:299–310. doi: 10.1191/026921699668963873. [DOI] [PubMed] [Google Scholar]
- 2.Meier DE. Beresford L. Outpatient clinics are a new frontier for palliative care. J Palliat Med. 2008;11:823–828. doi: 10.1089/jpm.2008.9886. [DOI] [PubMed] [Google Scholar]
- 3.Brumley R. Enguidanos S. Jamison P, et al. Increased satisfaction with care and lower costs: Results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55:993–1000. doi: 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 4.Heedman PA. Strang P. Symptom assessment in advanced palliative home care for cancer patients using the ESAS: Clinical aspects. Anticancer Res. 2001;21:4077–4082. [PubMed] [Google Scholar]
- 5.Levine S. Boal J. Home care. JAMA. 2003;290:1203–1207. doi: 10.1001/jama.290.9.1203. [DOI] [PubMed] [Google Scholar]
- 6.Kellogg FR. Brickner PW. Long-term home health care for the impoverished frail homebound aged: A twenty-seven-year experience. J Am Geriatr Soc. 2000;48:1002–1011. doi: 10.1111/j.1532-5415.2000.tb06902.x. [DOI] [PubMed] [Google Scholar]
- 7.Leone AF. Standoli F. Hirth V. Implementing a pain management program in a long-term care facility using a quality improvement approach. J Am Med Dir Assoc. 2009;10:67–73. doi: 10.1016/j.jamda.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Wajnberg A. Ornstein K. Zhang M. Smith K. Soriano T. Symptom burden in the chronically ill homebound. J Am Geriatrs Soc. 2013;61:126–131. doi: 10.1111/jgs.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutner JS. Kassner CT. Nowels DE. Symptom burden at the end of life: Hospice providers' perceptions. J Pain Symptom Manage. 2001;21:473–480. doi: 10.1016/s0885-3924(01)00281-0. [DOI] [PubMed] [Google Scholar]
- 10.Follwell M. Burman D. Le LW, et al. Phase II study of an outpatient palliative care intervention in patients with metastatic cancer. J Clin Oncol. 2009;27:206–213. doi: 10.1200/JCO.2008.17.7568. [DOI] [PubMed] [Google Scholar]
- 11.Keeney CE. Scharfenberger JA. O'Brien JG. Looney S. Pfeifer MP. Hermann CP. Initiating and sustaining a standardized pain management program in long-term care facilities. J Am Med Dir Assoc. 2008;9:347–353. doi: 10.1016/j.jamda.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Hadjistavropoulos T. Herr K. Turk DC, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain. 2007;23(1 Suppl):S1–S43. doi: 10.1097/AJP.0b013e31802be869. [DOI] [PubMed] [Google Scholar]
- 13.Weissman DE. Griffie J. Muchka S. Matson S. Building an institutional commitment to pain management in long-term care facilities. J Pain Symptom Manage. 2000;20:35–43. doi: 10.1016/s0885-3924(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MP. Karoly P. Braver S. The measurement of clinical pain intensity: A comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 15.Dudgeon DJ. Harlos M. Clinch JJ. The Edmonton Symptom Assessment Scale (ESAS) as an audit tool. J Palliat Care. 1999;15:14–19. [PubMed] [Google Scholar]
- 16.Bruera E. Kuehn N. Miller MJ. Selmser P. Macmillan K. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 17.Diefenbach GJ. Tolin DF. Gilliam CM. Impairments in life quality among clients in geriatric home care: Associations with depressive and anxiety symptoms. Int J geriatr Psychiatry. 2012;27:828–835. doi: 10.1002/gps.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallegos-Carrillo K. Garcia-Pena C. Mudgal J. Romero X. Duran-Arenas L. Salmeron J. Role of depressive symptoms and comorbid chronic disease on health-related quality of life among community-dwelling older adults. J Psychosom Res. 2009;66:127–135. doi: 10.1016/j.jpsychores.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Lecrubier Y. The burden of depression and anxiety in general medicine. J Clin Psychiatry. 2001;62(Suppl 8):4–9. discussion 10–11. [PubMed] [Google Scholar]
- 20.Unutzer J. Patrick DL. Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–1623. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 21.Unutzer J. Schoenbaum M. Katon WJ, et al. Healthcare costs associated with depression in medically ill fee-for-service medicare participants. J Am Geriatr Soc. 2009;57:506–510. doi: 10.1111/j.1532-5415.2008.02134.x. [DOI] [PubMed] [Google Scholar]
- 22.Olfson M. Gameroff MJ. Generalized anxiety disorder, somatic pain and health care costs. Gen Hosp Psychiatry. 2007;29:310–316. doi: 10.1016/j.genhosppsych.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Ornstein K. Hernandez C. DeCherrie LV. Soriano T. The Mount Sinai Visiting Doctors Program: Meeting the needs of the urban homebound population. J Long Term Home Health Care. 2011;12:159–163. doi: 10.1891/1521-0987.12.4.159. [DOI] [PubMed] [Google Scholar]
- 24.Chang VT. Hwang SS. Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Selby D. Cascella A. Gardiner K, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2010;39:241–249. doi: 10.1016/j.jpainsymman.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME. Pompei P. Ales KL. MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Crooks V. Waller S. Smith T. Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139–M144. doi: 10.1093/geronj/46.4.m139. [DOI] [PubMed] [Google Scholar]
- 28.Smith KL. Ornstein K. Soriano T. Muller D. Boal J. A multidisciplinary program for delivering primary care to the underserved urban homebound: Looking back, moving forward. J Am Geriatr Soc. 2006;54:1283–1289. doi: 10.1111/j.1532-5415.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 29.Westfall P. Young S. Resampling-based Multiple Testing: Examples, Methods for P-value Adjustment. New York: Wiley; 1993. [Google Scholar]
- 30.Modonesi C. Scarpi E. Maltoni M, et al. Impact of palliative care unit admission on symptom control evaluated by the edmonton symptom assessment system. J Pain Symptom Manage. 2005;30:367–373. doi: 10.1016/j.jpainsymman.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Stromgren AS. Sjogren P. Goldschmidt D, et al. A longitudinal study of palliative care: Patient-evaluated outcome and impact of attrition. Cancer. 2005;103:1747–1755. doi: 10.1002/cncr.20958. [DOI] [PubMed] [Google Scholar]
- 32.Bjoro K. Bergen K. Tools for pain assessment in older adults with end-stage dementia. Quarterly Newsletter of the American Academy of Hospice and Palliative Medicine. 2008;9(3) [Google Scholar]